Abstract
Background:
Non-small cell lung cancer (NSCLC) has a poor prognosis despite conventional treatments of surgery, radiotherapy, and chemotherapy. Small-molecule tyrosine kinase inhibitors acting on epidermal growth factor receptor (EGFR) have shown high efficacy and low toxicity for NSCLC. In particular, combining erlotinib with the VEGF antibody bevacizumab has therapeutic value in NSCLC, but the drugs’ separate effects as monotherapy and any adverse outcomes of combination therapy remain unclear.
Objectives:
To determine the efficacy and safety of erlotinib and bevacizumab for NSCLC, we conducted a meta-analysis and systematic review of randomized controlled trials.
Data sources:
PubMed, Embase, Web of Science, and Cochrane databases were searched using keywords and manual review.
Study eligibility criteria, participants, and interventions:
We reviewed randomized controlled trials on the use of erlotinib combined with bevacizumab in adult patients with NSCLC, including data on outcome measures of overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and adverse events.
Study appraisal and synthesis methods:
After quality assessment, datasets were evaluated for heterogeneity. In the event of significant heterogeneity, a random-effects model was used to assess the overall outcome measures as a result of treatments. Subgroup analysis was conducted to evaluate the source of heterogeneity on PFS.
Results:
Compared with erlotinib or bevacizumab alone, the combined treatment did not significantly prolong OS (95% confidence interval [CI] = 0.84–1.11; P = .62) or increase the ORR (95% CI = 0.91–1.20; P = .52), but significantly improved PFS (95% CI = 0.58–0.73; P < .001). This improvement was especially notable in patients with the following characteristics: Eastern Cooperative Oncology Group Performance Status score of 0 or 1, female, no smoking history, adenocarcinoma, and EGFR Exon19 deletion or Exon21 Leu858Arg mutation. Combination therapy significantly increased incidence of grade 1–2 hypertension (20.3% vs 6.3%, 95% CI 1.73–5.88; P < .01) and severe diarrhea (10% vs 3.2%, 95% CI 1.36–6.60; P = .01).
Limitations:
The low number of available randomized controlled trials could influence interpretation.
Conclusions:
Compared with erlotinib or bevacizumab monotherapy, their combination effectively prolongs PFS but increases incidence of adverse events in NSCLC patients.
Keywords: bevacizumab, erlotinib, metaanalysis, non-small cell lung cancer, systematic review
1. Introduction
With a poor overall prognosis, lung cancer is the leading cause of cancer-related deaths worldwide,[1,2] and less than 15% of patients survive for 5 years.[3] Non-small cell lung cancer (NSCLC) accounts for over 85% of all lung cancer cases, and approximately 75% of NSCLCs are diagnosed at a terminal stage (unresectable or metastatic).[4] Current NSCLC treatments mainly include surgery and chemotherapy,[5,6] although targeted drugs are preferred if traditional treatment is ineffective.
The targeted drug bevacizumab is reported to significantly extend progression-free survival (PFS) and overall survival (OS) in patients with NSCLC; thus, it has been approved for treating advanced NSCLC without hemoptysis.[7,8] The drug is an antibody specific to vascular endothelial growth factor (VEGF), a key signaling molecule for promoting angiogenesis, critical to endothelial cell survival and neovascularization. Additionally, the targeted drug erlotinib is a small-molecule inhibitor of epidermal growth factor receptor (EGFR). Used to treat patients with advanced or metastatic NSCLC who are not responding to chemotherapy regimens,[9–11] erlotinib is particularly effective in improving survival rate of patients without prior treatment.[12]
Although current treatment regimens typically involve single targeted drugs as monotherapy, combination therapy may have improved effects on patients with advanced or metastatic disease.[13] However, 1 study showed that patients with advanced NSCLC had no significant response to combination therapy, leading to controversy on its advantages.[14] In addition, targeted drugs are associated with a high risk of adverse events such as hypertension, rash, paronychia, diarrhea, neutropenia, and fatigue.[15] Therefore, substantial attention has been paid to potential increases in incidence of adverse side-effects when applying a combined therapy.
The extensive research on these targeted drugs for NSCLC[16,17] have not thus far made a distinction between first-line and second-line treatment. Moreover, little research is available on adverse events associated with combining erlotinib and bevacizumab. To resolve these issues, we conducted a meta-analysis and systematic review of randomized control trials (RCTs). We compared the effects of erlotinib+bevacizumab combination therapy with the respective monotherapies, specifically examining OS, PFS, objective response rate (ORR), as well as incidence and severity of adverse events. We also conducted subgroup analyses on the specific clinical and demographic factors affecting PFS and adverse events.
2. Materials and methods
All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
2.1. Study selection
Two researchers independently conducted a literature screen, assessed the quality of retrieved studies, then extracted and cross-checked data according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.[18] Disagreement between the 2 researchers was resolved through consulting a third researcher.
2.2. Search strategy
On June 2, 2019, 2 researchers independently retrieved articles published before June 2019 from the PubMed, Embase, Web of Science, and Cochrane databases for all RCTs on the combined use of erlotinib and bevacizumab to treat NSCLC. Keywords were “Non-Small Cell Lung Cancer” [MeSH], “Carcinoma, Non-Small Cell Lung,” “Lung Carcinoma, Non-Small-Cell,” “Erlotinib” [MeSH], “Hydrochloride, Erlotinib,” “Gefitinib” [MeSH], and “Iressa.” All references in the relevant articles were manually reviewed for appropriate studies.
2.3. Inclusion and exclusion criteria
Inclusion criteria for literature retrieval included:
-
1.
patients aged 18 years or older;
-
2.
histologically or cytologically confirmed NSCLC;
-
3.
assessment of erlotinib vs erlotinib combined with bevacizumab, or bevacizumab vs erlotinib combined with bevacizumab;
-
4.
RCTs; (5) data on OS, PFS, or ORR, and incidence of adverse events.
Exclusion criteria included:
-
1.
animal or cadaver studies;
-
2.
studies without extractable or valid data;
-
3.
comments and conference papers without full text;
-
4.
systematic reviews, meta-analyses, case reports, and retrospective studies.
2.4. Data extraction
Two researchers independently extracted baseline data from RCTs that met the inclusion criteria, including: study date, number of patients, sex ratio, ethnicity, smoking history, Eastern Cooperative Oncology Group Performance Status (ECOG-PS) score, histology, clinical stage, regional therapy, lines of therapy, and outcome measures. Any discrepancies were resolved through discussions with a third researcher. Researchers requested original data or relevant information from study authors via email if data were unavailable in the paper.
2.5. Quality assessment
Risks of bias among the included studies were assessed using the Cochrane Intervention System Review Manual,[19] including random sequence generation, allocation concealment, double blinding of researchers and participants, blinding of outcome assessment, incomplete outcome data, and selective reporting. Each study was qualified as high, low, or unclear risk of bias.[20]
2.6. Outcome measures
Primary outcome variables were
-
1.
OS (time from randomization to death, considered as the best therapeutic endpoint in cancer clinical trials),
-
2.
PFS (time from randomization to tumor progression or death),
-
3.
ORR (proportion of patients whose symptoms were relieved to a predetermined value within the minimum time limit), and
-
4.
PFS in patient subgroups.
Analyses aimed to determine whether combination therapy increased these variables compared with monotherapy. Specifically, subgroup analyses were performed to determine the effects of age (>65 or ≤65 years),[21–24] disease stage (IIIB, IV, and other stages),[22–24] ethnicity (Caucasian, Asian, or Pacific Islanders),[21,22] ECOG-PS score (PS0, PS1, or PS2),[21–24] sex (male or female),[21–24] smoking history (none, currently smoking, or former smokers),[21–24] medical history, pathological classification (large cell carcinoma, adenocarcinoma, squamous cell carcinoma, and other diseases),[21–23] and EGFR mutation (Exon19 deletion, Exon21 Leu858Arg mutation, EGFR FISH-positive, EGFR-FISH negative, and EGFR wild type),[21,23–25] and adverse events (rash, diarrhea, hypertension, and bleeding) on PFS. Adverse events were rated as levels 1–2 and levels 3–5 (serious) according to the National Cancer Institute's Common Toxicity Criteria for Adverse Events (version 3.0).[26]
2.7. Statistical analysis
Statistical analysis was performed in Stata Version 11.0 and Review Manager (Revman) Version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Heterogeneity was assessed by the Chi-Squared test.[27] If significant heterogeneity was detected (I2 > 50% or P < .1),[28] a random-effects model was used; otherwise, a fixed-effect model was used. Significance was set at P < .05. Time-event variables, including OS and PFS, were assessed according to the hazard ratio (HR). Dichotomous variables, including ORR and incidence of adverse events, were assessed as risk ratios (RR) with 95% confidence interval (CI) estimates. Hypothetical test results for each variable were listed in a forest map. For outcome indicators with significant heterogeneity, a sensitivity analysis was performed, eliminating the included studies 1 at a time to determine the source of heterogeneity. Subgroup analyses were performed on factors that could influence PFS, i.e., age, ethnicity, sex, ECOG, and smoking history. If outcome measures of >10 primary documents, the funnel figure shall be used for publication bias test. And the publication bias test was performed using Egger test, when outcome measures of >20 primary documents.
3. Results
3.1. Literature retrieval
We retrieved 166 studies from the 4 databases that fit our initial inclusion criteria, then excluded 31 duplicate studies and 108 ineligible studies. Of the remaining 27 articles, reviews, meta-analysis, and topic-independent studies were excluded. The final meta-analysis thus used 6 studies (Fig. 1). Evaluation of the quality of the reports is shown in Figure 2.
Figure 1.
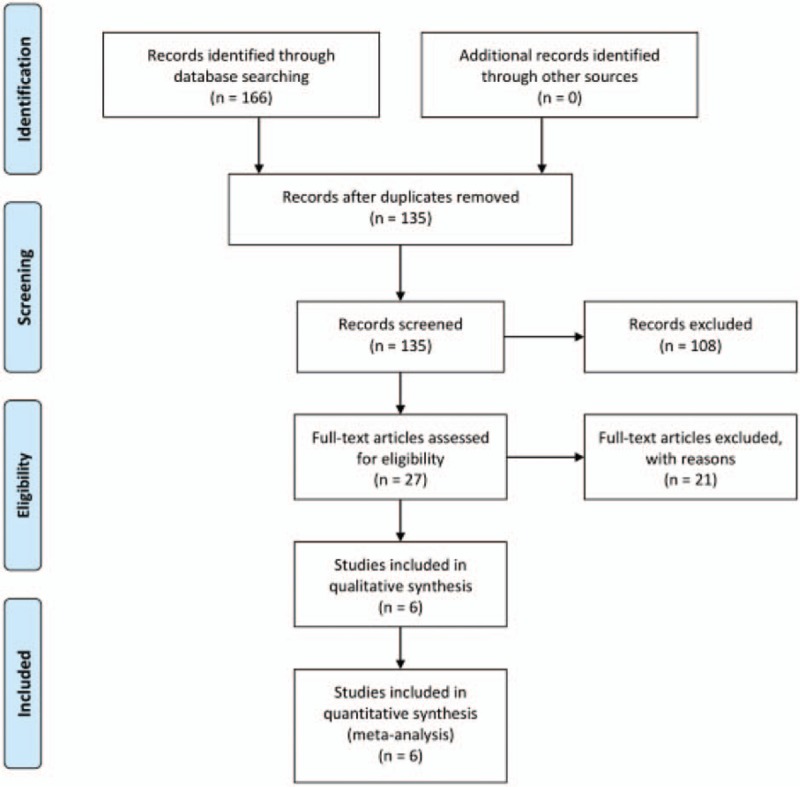
Flowchart of literature retrieval and selection.
Figure 2.
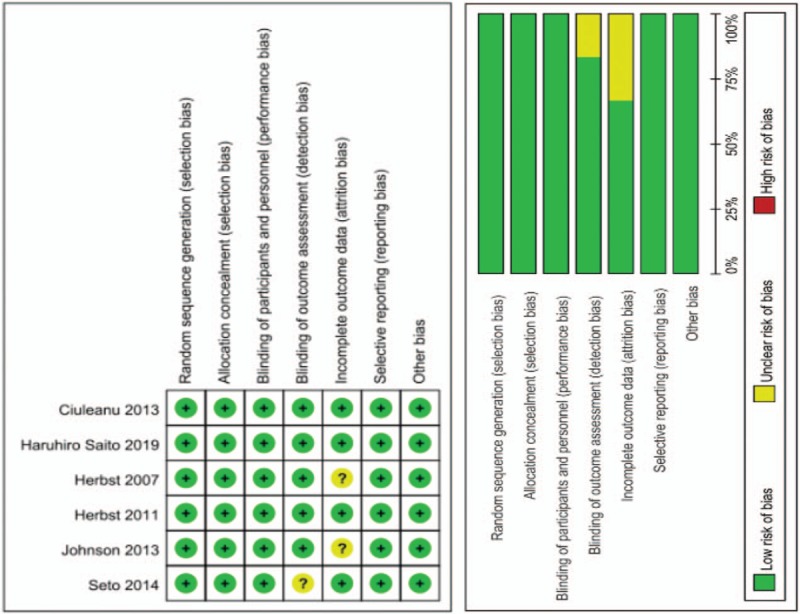
Methodological quality of studies included in meta-analysis.
3.2. Study characteristics
The 6 included studies[21–26] involved 1960 participants and were published from October 2007 to April 2019 (Table 1). Four studies[21,23,24,26] compared erlotinib with combination therapy, and 2 studies[22,25] compared bevacizumab with combination therapy. Three studies[23–25] explored the role of erlotinib+bevacizumab as first-line therapy, while others[21,22,26] focused on second-line therapy. Three studies[22–24] provided data on disease stage (IIIB, IV, and other stages). Five studies[21,23–26] described EGFR status, and 2[28,29] elaborated on the specific EGFR mutation detected (Exon19 deletion or Exon21 Leu858Arg mutation) (Table 2). Tables 3 to 5 summarizes the different levels of adverse events.
Table 1.
Summary of studies included in the final meta-analysis.
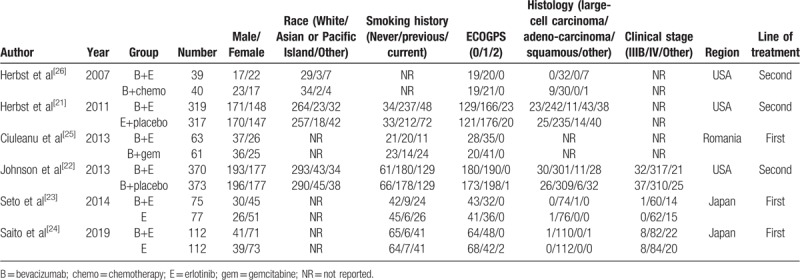
Table 2.
Number of patients with different epidermal growth factor receptor mutation status.
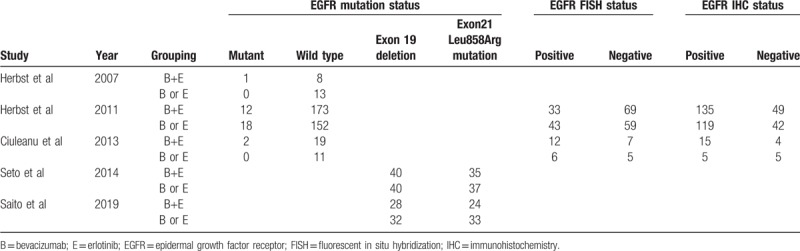
Table 3.
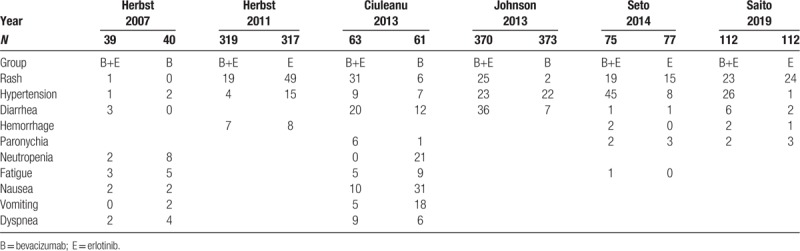
Incidence of level 1–2 adverse events in 2 studies.
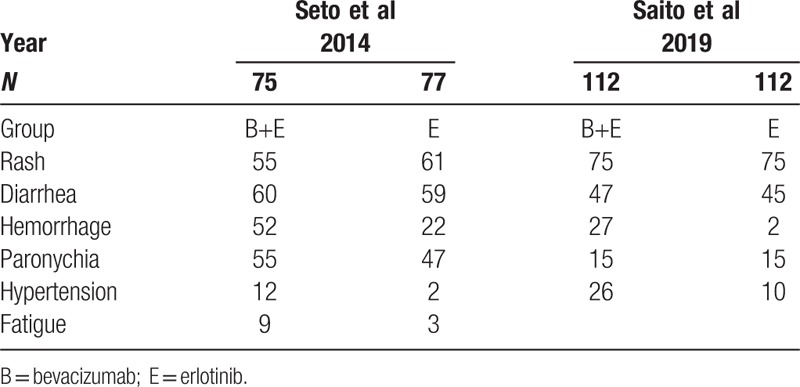
Table 5.
Incidence of overall adverse events in four studies.
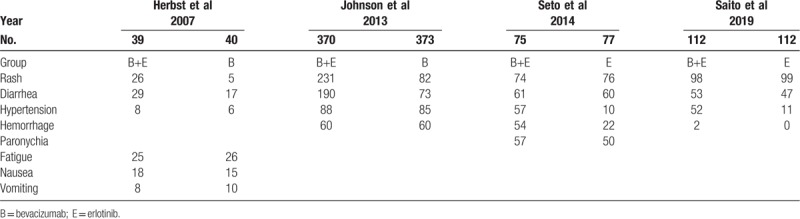
Table 4.
Incidence of serious adverse events in all studies.
3.3. Outcome measures
3.3.1. OS
Four studies[21,22,25,26] reported OS. We selected the fixed-effects model because heterogeneity was low (I2 = 0%). Combination therapy as either first-line or second-line treatment did not significantly improve OS (HR = 1.24, 95% CI = 0.75–2.05, P = .40; HR = 0.94, 95% CI = 0.81–1.10, P = .44) (Fig. 3 A).
Figure 3.
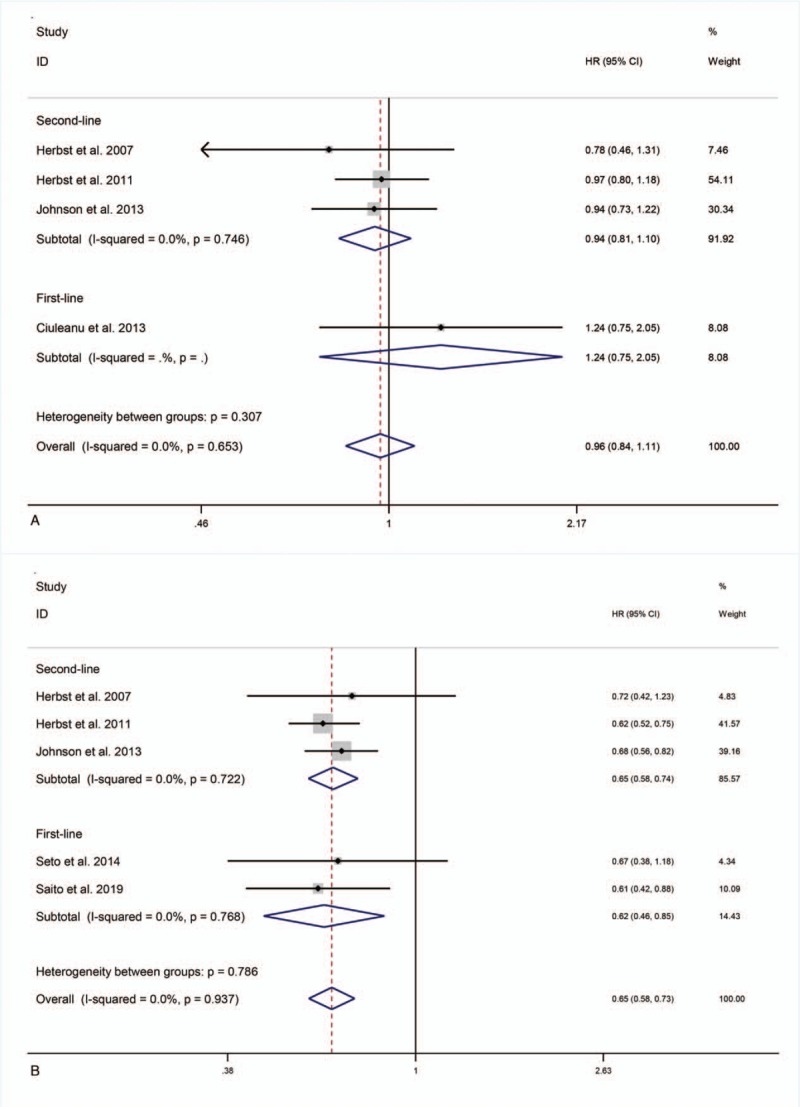
OS, PFS or ORR for combination therapy of bevacizumab plus erlotinib with bevacizumab or erlotinib alone.
3.3.2. PFS
All 6 studies reported PFS. The study by Ciuleanu et al[22] resulted in significant heterogeneity (I2 = 64.1%) and was removed after sensitivity analysis. Removal reduced I2 to 0%, allowing the use of a fixed-effects model. Compared with erlotinib or bevacizumab alone, first-line and second-line combination therapy prolonged PFS (HR = 0.62, 95% CI = 0.46–0.85, P < .01; HR = 0.65, 95% CI = 0.58–0.74, P < .01) (Fig. 3 B).
3.3.3. ORR
Five studies[21,23–26] reported ORR. The study by Herbst et al[21] led to I2 = 72% and was removed after sensitivity analysis, reducing I2 to 0%. The fixed-effects model revealed that compared with erlotinib or bevacizumab alone, first-line and second-line combination therapy did not elevate ORR (RR = 1.03, 95% CI = 0.90–1.19, P = .65; RR = 1.44, 95% CI = 0.50–4.14, P = .50) (Fig. 3 C).
3.3.4. PFS in subgroup analyses
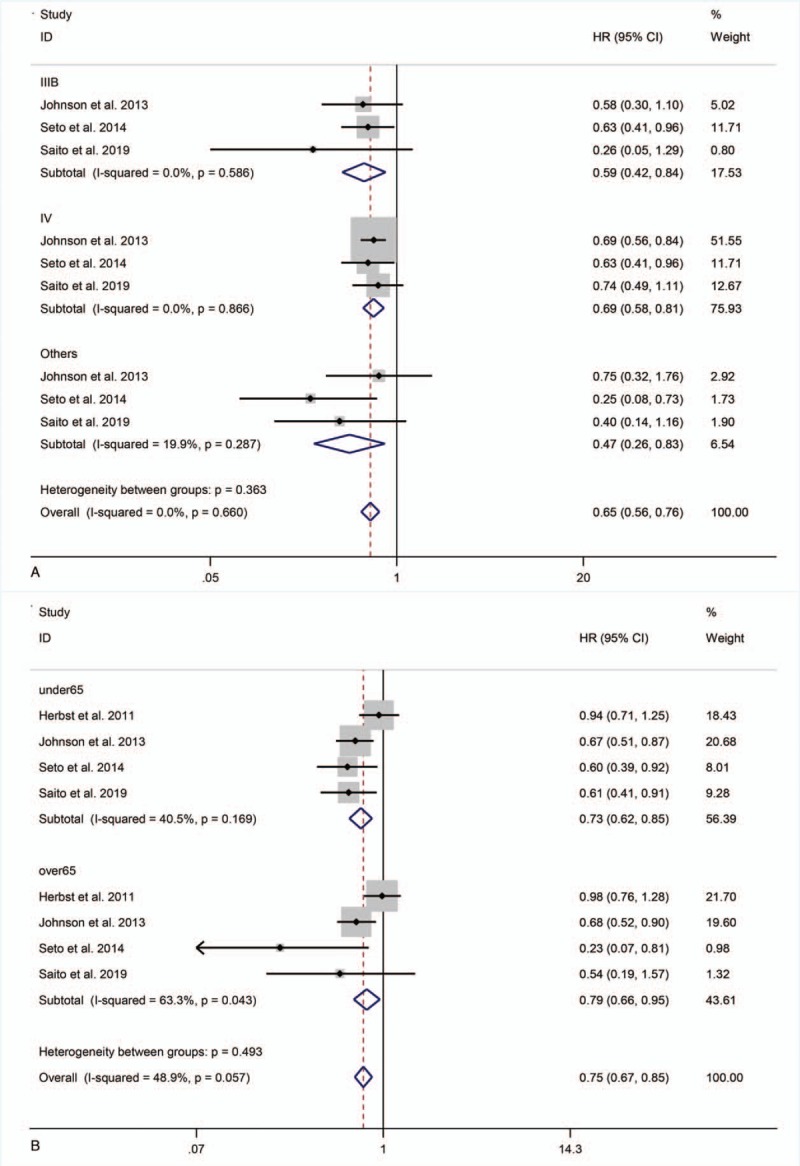
Three studies[22–24] reported subgroup data for disease staging. Compared with monotherapy, combination therapy significantly prolonged PFS in patients with stage IIIB disease (HR = 0.59, 95% CI = 0.42–0.84; P < .01), stage IV disease (HR = 0.69, 95% CI = 0.58–0.81; P < .001), and at other stages (HR = 0.47, 95% CI = 0.26–0.83; P = .01) (Fig. 4 A).
Figure 3 (Continued).
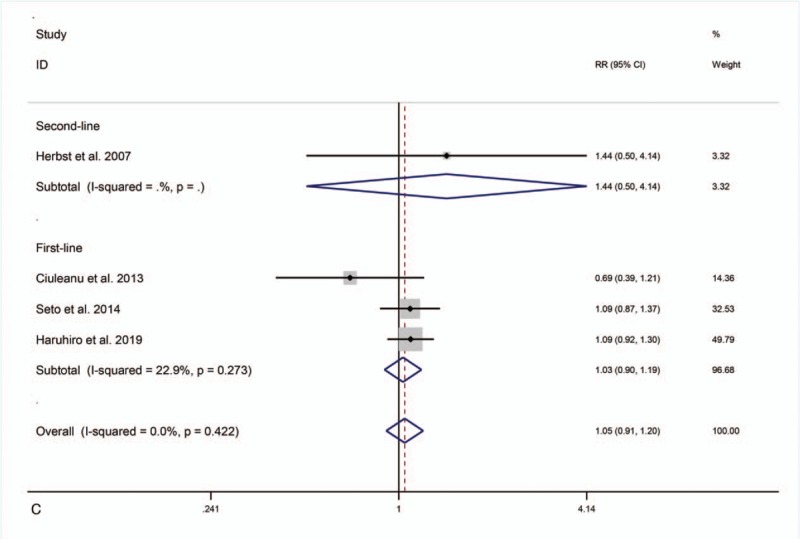
OS, PFS or ORR for combination therapy of bevacizumab plus erlotinib with bevacizumab or erlotinib alone.
Figure 4 (Continued).
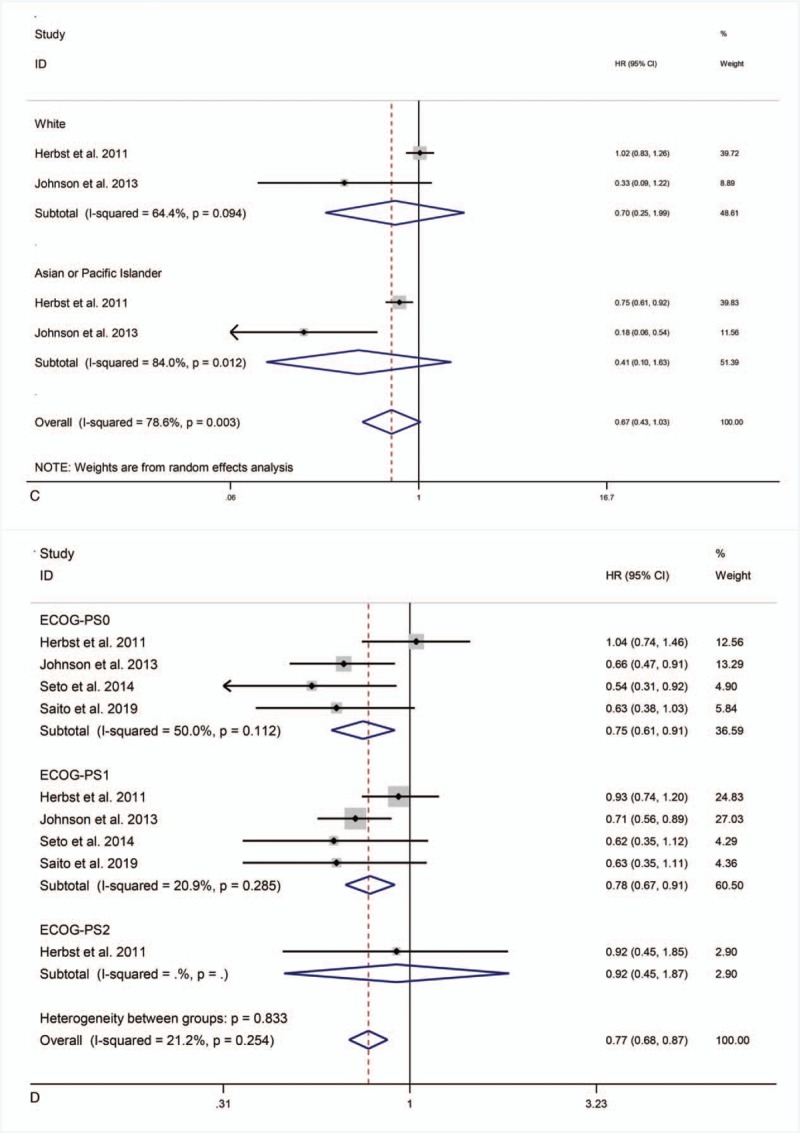
PFS in subgroup analyses.
Figure 4 (Continued).
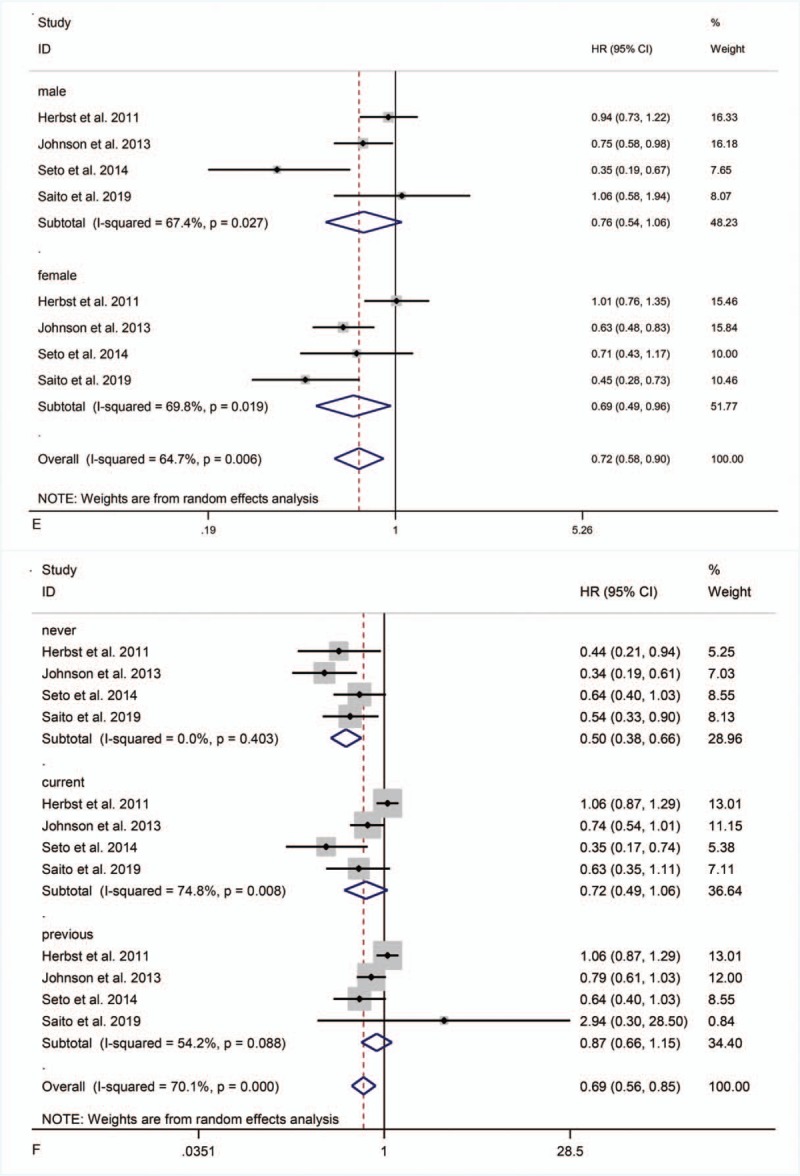
PFS in subgroup analyses.
Figure 4 (Continued).
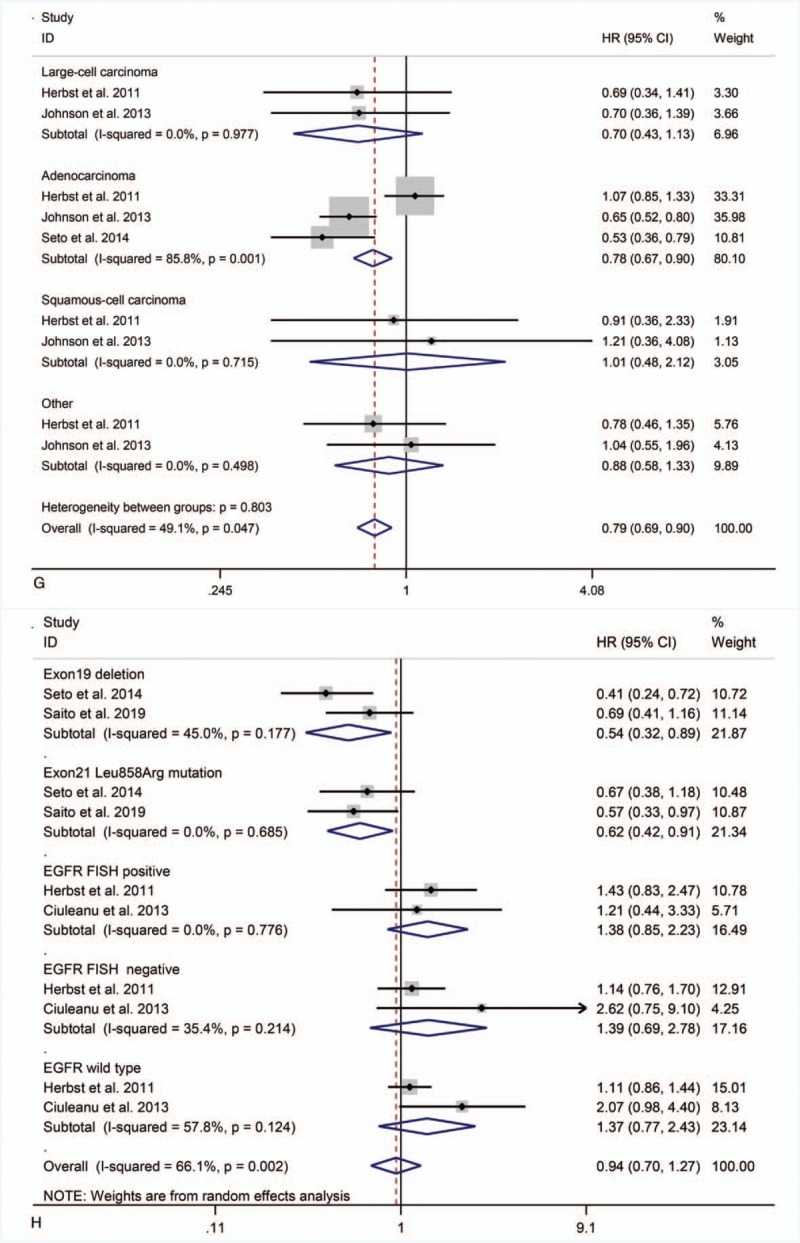
PFS in subgroup analyses.
Four studies[21–24] reported subgroup data for age. Combination therapy extended PFS in patients <65 and ≥65 years old (HR = 0.73, 95% CI = 0.62–0.85, P < .001; HR = 0.79, 95% CI = 0.66–0.95, P = .01) (Fig. 4 B).
Two studies[21,22] reported subgroup data for ethnicity. Combination therapy did not significantly prolong PFS in Caucasian or Asian and Pacific Islander patients (HR = 0.70, 95% CI = 0.25–1.99, P = .51; HR = 0.41, 95% CI = 0.10–1.63, P = .21) (Fig. 4 C).
Four studies[21–24] reported subgroup data for ECOG-PS. Combination therapy significantly improved PFS in patients with ECOG-PS0 (HR = 0.75, 95% CI = 0.61–0.91; P < .01) and ECOG-PS1 (HR = 0.78, 95% CI = 0.67–0.91; P < .01), but had no significant effect on patients with ECOG-PS2 (HR = 0.92, 95% CI = 0.45–1.87; P = .82) (Fig. 4 D).
Four studies[21–24] reported subgroup data for sex. Combination therapy did not significantly prolong PFS in male patients but did in female patients (HR = 0.76, 95% CI = 0.54–1.06, P = .10; HR = 0.69, 95% CI = 0.49–0.96, P = .03) (Fig. 4 E).
Four studies[21–24] reported subgroup data for smoking. Combination therapy significantly prolonged PFS in patients with no smoking history (HR = 0.50, 95% CI = 0.38–0.66, P < .001), but not in those currently smoking or former smokers (HR = 0.72, 95% CI = 0.49–1.06, P = .19; HR = 0.87, 95% CI = 0.66–1.15, P = .33) (Fig. 4 F).
Three studies[21–23] reported subgroup data for pathological typing. Combination therapy did not significantly prolong PFS in patients with large cell carcinoma (HR = 0.70, 95% CI = 0.43–1.13; P = .15), squamous cell carcinoma (HR = 1.01, 95% CI = 0.48–2.12; P = .98), or other diseases (HR = 0.88, 95% CI = 0.58–1.33; P = .54), but significantly prolonged PFS in patients with adenocarcinoma (HR = 0.78, 95%CI = 0.67–0.90; P < .01) (Fig. 4 G).
Four studies[21,23–25] reported subgroup data for EGFR mutations. Combination therapy significantly prolonged PFS in patients with EGFR Exon19 deletion and Exon21 Leu858Arg mutation (HR = 0.54, 95% CI = 0.32–0.89, P = .02; HR = 0.62, 95% CI = 0.42–0.91, P = .02), but did not do so in patients with EGFR FISH-positive (HR = 1.38, 95% CI = 0.85–2.23; P = .20), EGFR FISH-negative (HR = 1.39, 95% CI = 0.69–2.78; P = .35), or EGFR wild-type (HR = 1.37, 95% CI = 0.77–2.43; P = .29) tumors (Fig. 4 H).
3.3.5. Adverse events
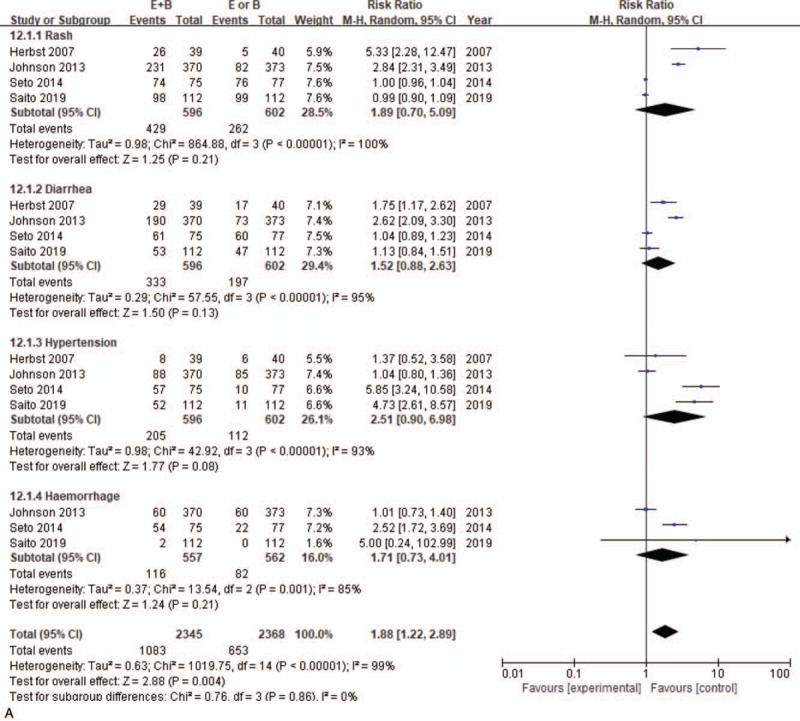
Patients who received monotherapy vs combined bevacizumab+erlotinib did not significantly differ in incidence of rash (72% vs 43.5%, 95% CI = 1.51–1.82; P = .21), diarrhea (55.9% vs 32.7%, 95% CI = 0.88–2.63; P = .13), hypertension (34.4% vs 18.6%, 95% CI = 0.90–6.98; P = .08), or hemorrhage (20.8% vs 14.6%, 95% CI = 0.73–4.01; P = .21) (Fig. 5 A).
Figure 4.
PFS in subgroup analyses.
Figure 5.
Adverse events for combination therapy of bevacizumab plus erlotinib with bevacizumab or erlotinib alone.
Figure 5.
Adverse events for combination therapy of bevacizumab plus erlotinib with bevacizumab or erlotinib alone.
Figure 5.
Adverse events for combination therapy of bevacizumab plus erlotinib with bevacizumab or erlotinib alone.
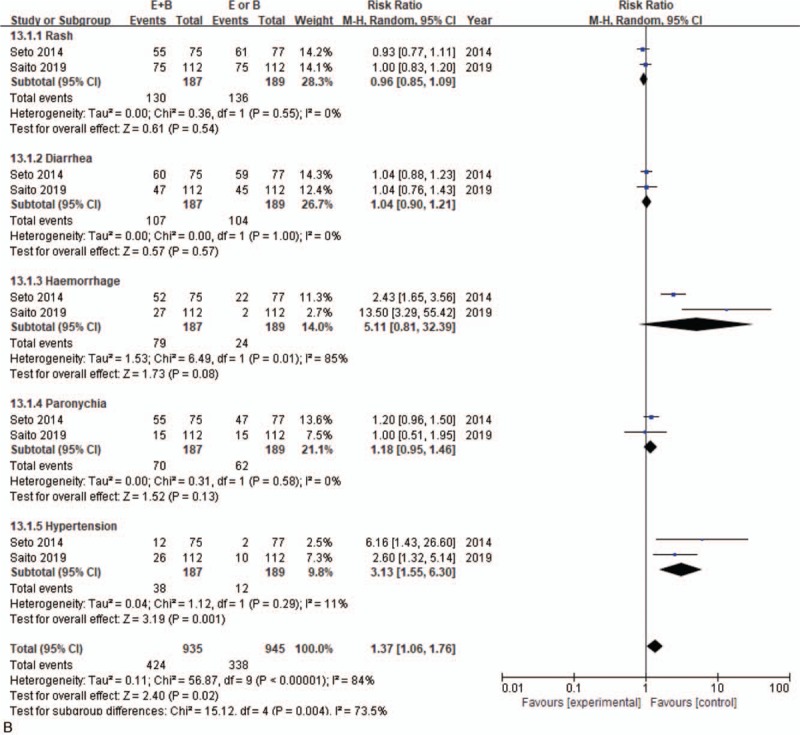
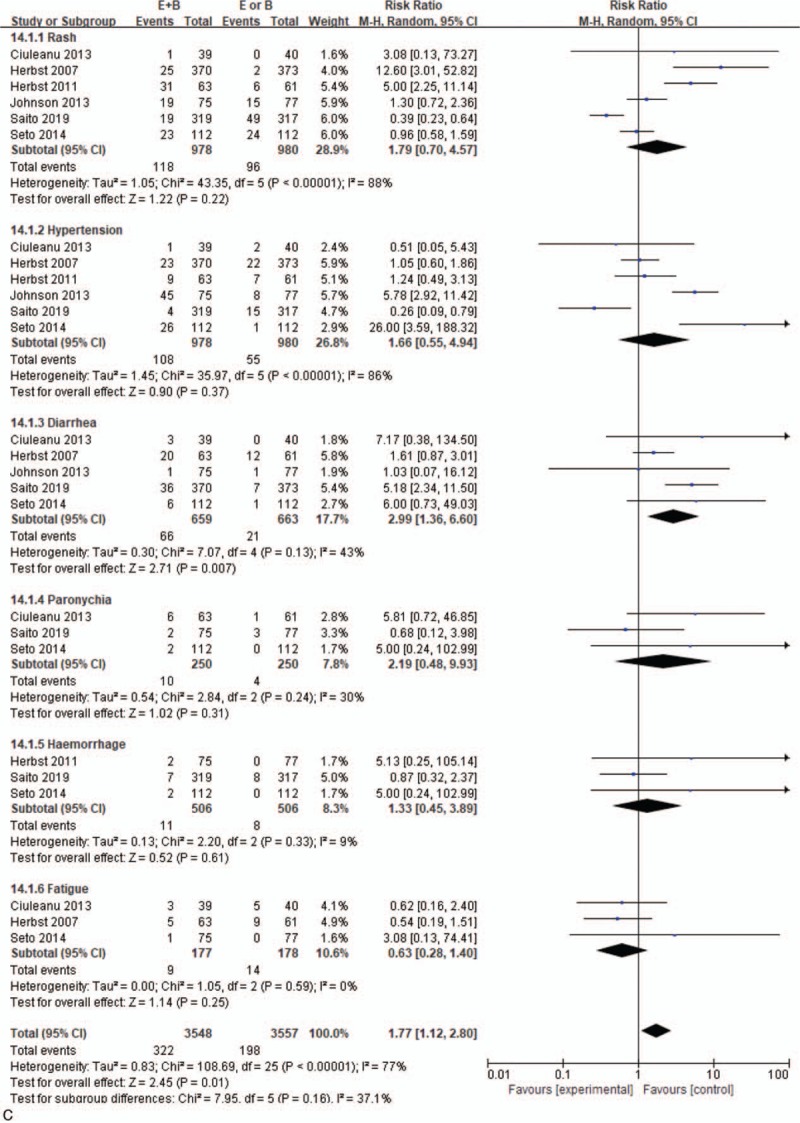
Monotherapy and combined therapy groups did not differ significantly in levels 1–2 adverse events of rash (69.5% vs 72%, 95% CI = 0.85–1.09; P = .54), diarrhea (57.2% vs 55%, 95% CI = 0.90–1.21, P = .13), hemorrhage (42.2% vs 12.7%, 95% CI = 0.81–32.39, P = .08), or paronychia (37.4% vs 32.8%, 95% CI = 0.95–1.46, P = .13), but differed significantly in hypertension incidence (20.3% vs 6.3%, 95% CI = 1.73–5.88, P = .001) (Fig. 5 B). For severe adverse events, the 2 groups differed significantly in diarrhea incidence (10% vs 3.2%, 95% CI = 1.36–6.60; P = .01), but not in incidence of rash (12.1% vs 9.8%, 95% CI = 0.70–4.57; P = .22), hypertension (11% vs 5.6%, 95% CI = 0.55–4.94; P = .37), fatigue (5.1% vs 7.9%, 95% CI = 0.28–1.40; P = .25), paronychia (4% vs 1.6%, 95% CI = 0.48–9.93; P = .31), or hemorrhage (2.2% vs 1.6%, 95% CI = 0.45–3.89; P = .61) (Fig. 5 C).
4. Discussion
4.1. Findings and interpretations
Our meta-analysis indicates that compared with monotherapy, erlotinib+bevacizumab combination therapy prolongs PFS of patients with NSCLC, but cannot extend OS or elevate ORR. Prolongation of PFS was not associated with disease stage, age, or ethnicity. However, female patients and those with ECOG-PS0 or ECOG-PS1, no smoking history, adenocarcinoma, EGFR Exon19 deletion, or Exon21 Leu858Arg mutation all experienced prolonged PFS under combination therapy. Moreover, combination therapy increased incidence of common complications, such as rash, diarrhea, hypertension, hemorrhage, and severe diarrhea in levels 1–2 adverse events.
This study provides new insight to help resolve existing controversies surrounding the combined use of erlotinib and bevacizumab for NSCLC treatment. Although we clearly demonstrated a benefit for PFS, the lack of an effect on OS requires further investigation. Our finding is similar to a previous meta-analysis, where the authors suggested that a low number of studies and small sample size resulted in limited statistical power to detect effects on OS.[17] However, although we increased sample size and merged data, we still found that combination therapy failed to prolong OS, indicating that sample size is not the issue. Moreover, combination therapy could not improve ORR, consistent with previous studies.[29–31] Heterogeneity was high in studies on ORR, largely due to the report by Herbst et al[21] Therefore, combination therapy might improve ORR with the use of a pre-specified fixed sequence test.
We also detected high heterogeneity (stemming from Ciuleanu et al[25]) among studies evaluating PFS. The randomized follow-up design of Ciuleanu et al did not allow for effective evaluation in some patients. Previous studies have found that kinase plays an important role in normal and malignant biology,[32,33] chemotherapy can affect the therapeutic effects of EGFR and tyrosine kinase inhibitors,[34–37] resulting in a lower response rate.[38] Therefore, we also compared first- and second-line treatments, but neither influenced the beneficial effect of combination therapy on prolonging PFS.
Our stratification analysis indicated that combination therapy differentially affected certain patient subgroups but not others. Specifically, erlotinib+bevacizumab prolonged PFS regardless of disease stage, age, or ethnicity. Additionally, while combination therapy extended PFS in patients with ECOG-PS0 and ECOG-PS1, patients with ECOG-PS2 did not see such benefits. However, this result requires further verification because only one study reported patients with ECOG-PS2.[21] Combination therapy also improved PFS in female patients but not in male patients. Furthermore, patients who currently or formerly smoked did not see the same benefits from combination therapy as non-smokers. Patients would thus benefit from quitting smoking to increase the likelihood of a positive response to treatment. Combination therapy also appears to be appropriate for patients experiencing pathological NSCLC, as the outcomes were either beneficial or neutral; individuals with adenocarcinoma had significantly longer PFS under combination therapy than patients with other pathological NSCLC types. Previous studies[39–41] found that erlotinib was more effective in patients with the Exon19 deletion than in those with the Exon21 Leu858Arg mutation, similar to our results here. Combination therapy extended PFS in patients with EGFR gene mutations, but was not effective for patients with other mutations. Therefore, pre-therapy genetic testing is necessary to avoid unnecessary treatment while targeting those most likely to receive benefits.
Previous studies have suggested unsatisfactory outcomes after combination therapy, pointing to increased incidence of adverse events such as rash, diarrhea, hypertension, hemorrhage, paronychia, and fatigue.[15] In this study, combination therapy increased incidence of rash, diarrhea, hypertension, and bleeding. When we examined subgroups based on severity of adverse events, we found that combination therapy did not significantly increase level 1–2 rash, diarrhea, hemorrhage, and paronychia incidence, but significantly increased level 1–2 hypertension. Additionally, severe hypertension, rash, and paronychia were not significantly elevated, while severe diarrhea was. These outcomes suggest that combination therapy only increases minor adverse events that can be treated with proper control of patient blood pressure and administration of antidiarrheal drugs. However, we note the importance of considering differences in individual responses to combined drugs. We should also consider the costs of combination therapy vs monotherapy when assessing treatment appropriateness, with the aim of minimizing medical waste.
4.2. Strengths and limitations
The main strengths of this study are that we performed a comprehensive database and literature search, including recent studies that had not been considered in previous meta-analyses. Moreover, we performed a detailed stratification analysis of subgroups and adverse events, allowing a detailed examination of factors contributing to variation in patient responses under combination therapy for NSCLC.
Nevertheless, our study has one major limitation: is a low number of available RCTs, resulting in an insufficient sample to evaluate different outcome measures. Therefore, high-quality RCTs with large sample sizes are necessary to verify our conclusions and to further explore the efficacy and adverse events of erlotinib+bevacizumab combination therapy.
5. Conclusions
Combining erlotinib and bevacizumab did not improve OS and ORR of patients with NSCLC but did prolong PFS. Subgroup analysis confirmed that combination therapy prolonged PFS without causing severe incurable complications in female patients, as well as those with ECOG-PS0 or ECOG-PS1, no smoking history, adenocarcinoma, and an EGFR Exon19 deletion or Exon21 Leu858Arg mutation. Therefore, we particularly recommend combination therapy for these patients. Our findings can help resolve existing controversies surrounding the benefits of erlotinib+bevacizumab therapy, thus further improving and personalizing patient selection for this treatment.
Author contributions
KZ searched the literatures, drew charts and wrote the article. SZ searched the literatures and drew charts. WG searched the literatures and revised the article. LD designed the experiment and provided guidance.
Footnotes
Abbreviations: CI = confidence interval, ECOG-PS = Eastern Cooperative Oncology Group Performance Status, EGFR = epidermal growth factor receptor, HR = hazard ratio, NSCLC = non-small cell lung cancer, ORR = overall response rate, OS = overall survival, PFS = progression-free survival, RCT = randomized controlled trial, RR = risk ratio.
How to cite this article: Zhou K, Zhao S, Guo W, Ding L. Efficacy and safety of erlotinib combined with bevacizumab in the treatment of non-small cell lung cancer: A systematic review and meta-analysis. Medicine. 2020;99:3(e18771).
The authors report no conflicts of interest.
References
- [1].Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300. [DOI] [PubMed] [Google Scholar]
- [2].Gelatti A, Drilon A, Santini F. Optimizing the sequencing of tyrosine kinase inhibitors in epidermal growth factor receptor mutation-positive non-small cell lung cancer. Lung Cancer 2019;137:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].National Cancer Institute: Surveillance, Epide-miology, and End Results (SEER) Program: SEER Stat Database, National Cancer Institute, Surveillance Research Program, Cancer Statistics Branch. http://www.seer.cancer.gov/. [Google Scholar]
- [4].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [5].Tavares e Castro A, Clemente J, Carvalho L, et al. Small-cell lung cancer in never-smokers: a case series. Lung Cancer 2016;93:82–7. [DOI] [PubMed] [Google Scholar]
- [6].Jin Y, Xu H, Zhang C, et al. Combined effects of cigarette smoking, gene polymorphisms and methylations of tumor suppressor genes on non small cell lung cancer: a hospital-based case-control study in China. BMC Cancer 2010;10:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542–50. [DOI] [PubMed] [Google Scholar]
- [8].Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: Results from a randomised phase III trial (AVAiL). Ann Oncol 2010;21:1804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 2011;377:1846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bonomi P. Erlotinib: a new therapeutic approach for non-small cell lung cancer. Expert Opin Investig Drugs 2003;12:1395–401. [DOI] [PubMed] [Google Scholar]
- [11].Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123–32. [DOI] [PubMed] [Google Scholar]
- [12].Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013;31:4105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Spigel DR, Burris HA, 3rd, Greco FA, et al. Randomized, double-blind, placebo-controlled, phase II trial of sorafenib and erlotinib or erlotinib alone in previously treated advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2582–9. [DOI] [PubMed] [Google Scholar]
- [14].Ohgami M, Kaburagi T, Kurosawa A, et al. Drug interaction between erlotinib and phenytoin for brain metastases in a patient with nonsmall cell lung cancer. Lung Cancer 2016;101:9–10. [DOI] [PubMed] [Google Scholar]
- [15].Takeshita J. Erlotinib plus bevacizumab is effective in EGFR-mutant NSCLC. Cancer Discov 2014;11:OF18. [DOI] [PubMed] [Google Scholar]
- [16].Dingemans AM, de Langen AJ, van den Boogaart V, et al. First-line erlotinib and bevacizumab in patients with locally advanced and/or metastatic non-small-cell lung cancer: a phase II study including molecular imaging, Ann. Oncol 2011;22:559–66. [DOI] [PubMed] [Google Scholar]
- [17].Zhang S, Mao X-dong, Wang H-tao, et al. Efficacy and safety of bevacizumab plus erlotinib versus bevacizumab or erlotinib alone in the treatment of non-small-cell lung cancer: a systematic review and meta-analysis. BMJ Open 2016;6:e011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].David M, Alessandro L, Jennifer T, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Revista Española De Nutrición Humana Y Dietética 2009;18:e123. [Google Scholar]
- [19].BMJ Publishing Group, Jüni P, Altman DG, Egger M. Assessing the Quality of Randomised Controlled Trials. 2001;87–108. [Google Scholar]
- [20].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind,placebo-controlled, phase 3 trial. Lancet 2011;377:1846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: Randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2013;31:3926–34. [DOI] [PubMed] [Google Scholar]
- [23].Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236–44. [DOI] [PubMed] [Google Scholar]
- [24].Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019;20:625–35. [DOI] [PubMed] [Google Scholar]
- [25].Ciuleanu T, Tsai CM, Tsao CJ, et al. A phase II study of erlotinib in combination with bevacizumab versus chemotherapy plus bevacizumab in the first-line treatment of advanced non-squamous non-small cell lung cancer. Lung Cancer 2013;82:276–81. [DOI] [PubMed] [Google Scholar]
- [26].Herbst RS, O’Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007;25:4743–50. [DOI] [PubMed] [Google Scholar]
- [27].Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101–29. [Google Scholar]
- [28].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567):an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236–44. [DOI] [PubMed] [Google Scholar]
- [30].Herbst RS, O’Neill VJ, Fehrenbacher L, et al. Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007;25:4743–50. [DOI] [PubMed] [Google Scholar]
- [31].Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2013;31:3926–34. [DOI] [PubMed] [Google Scholar]
- [32].Pankaj D, Muench DE, Michael W, et al. Time resolved quantitative phospho-tyrosine analysis reveals Bruton's Tyrosine kinase mediated signaling downstream of the mutated granulocyte-colony stimulating factor receptors. Leukemia 2019;33:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dwivedi P, Muench DE, Wagner M, et al. Phospho serine and threonine analysis of normal and mutated granulocyte colony stimulating factor receptors. Scientific Data 2019;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol 2012;30:3002–11. [DOI] [PubMed] [Google Scholar]
- [35].Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med 2006;355:2542–50. [DOI] [PubMed] [Google Scholar]
- [36].Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiL. J Clin Oncol 2009;27:1227–34. [DOI] [PubMed] [Google Scholar]
- [37].Niho S, Kunitoh H, Nokihara H, et al. For the JO19907 Study Group. Randomized phase II study of first-line carboplatin–paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer 2012;76:362–7. [DOI] [PubMed] [Google Scholar]
- [38].Chin TM, Quinlan MP, Singh A, et al. Reduced Erlotinib sensitivity of epidermal growth factor receptor-mutant non-small cell lung cancer following cisplatin exposure: a cell culture model of second-line erlotinib treatment. Clin Cancer Res 2008;14:6867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation–positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735–42. [DOI] [PubMed] [Google Scholar]
- [40].Rosell R, Carcereny E, Gervais R, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46. [DOI] [PubMed] [Google Scholar]
- [41].Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958–67. [DOI] [PubMed] [Google Scholar]


