Supplemental Digital Content is available in the text
Keywords: cause of death, cohort study, follow-up studies, mortality, radius fractures
Abstract
The various harmful impacts of distal radius fractures (DRFs) may cause adverse effects. Although previous studies have reported the adverse effects of DRFs on mortality, most studies were performed in adults of advanced age and paid little attention to confounding factors of mortality. Furthermore, most of these studies investigated the overall impact of DRFs on mortality without differentiating the specified causes of death.
The purpose of the present study was to estimate the risk of mortality in DRF patients according to the cause of death.
Data from the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC) from 2002 to 2013 were collected. A total of 27,295 DRF participants who were 50 years or older were 1:4 matched with control participants for age, sex, income, and region of residence. The causes of death were grouped into 12 classifications.
DRFs were not associated with increased overall mortality. The adjusted hazard ratio (HR) of mortality was 1.04 (95% confidence interval [CI] = 0.98–1.11, P = .237). The adjusted HR for mortality was not significantly different according to age. The odds ratio of overall mortality was 1.03 (95% CI = 0.97–1.11, P = .329).
DRFs were not associated with a significant increase in mortality.
1. Introduction
Distal radius fracture (DRF) is one of the most common types of fractures, and the incidence of this fracture appears to be increasing.[1] Between 2008 and 2011, the number of osteoporosis-related DRFs increased by 31.6%, and the annual total healthcare costs increased by 45.0% among Koreans.[2] DRFs can be a significant source of mortality and cause the loss of independence in elderly individuals. Wrist fractures contribute to clinically important functional decline in women of advanced age.[3] Additionally, a previous study reported that wrist fractures are associated with increased risk for another wrist fracture,[4] vertebral fracture,[5] and hip fracture[6,7] incidence.
Previously, hip and spine fracture were associated with mortality in both sexes in many studies. However, there are inconsistencies among studies on the effect of wrist fractures on mortality. Several studies reported no significant association between wrist fractures and mortality.[8,9,10,11,12,13,14,15,16] Another study reported that patients who sustained wrist fractures had similar or improved early and medium-term survival compared to the general population,[17] and medicare beneficiaries had significantly lower mortality after DRFs than the general population.[18] In contrast, Rozental et al found a 14% decrease in the survival of elderly DRF patients compared with the general population,[19] and Oyen et al reported increased standard mortality ratios (SMR) in women aged >70 years 5 years after DRFs.[20] Recently, Kwon et al reported that the average SMR for a patient age over 50 years is 1.12 (95% confidence interval [CI] = 1.08–1.15), indicating increased mortality associated with DRFs.[21] The variations in the cross-sectional study designs, the study populations, and overlooked confounders could explain the conflicting results of prior studies. The definitions of wrist and forearm fractures also influenced the inconsistent results in prior studies.
Because there are various direct and indirect effects of DRFs on mortality, they are predicted to be different in accordance with each cause of death. For instance, the direct effect of DRFs could be more influential on the risk of death due to physical trauma than on the risks of other causes of death. However, indirect effects, including functional level, feeding quality, and hygiene, could have adverse impacts on many medical diseases. Moreover, the causes of death could be different according to population characteristics, such as age. Most of the previous studies estimated the risk of all-cause mortality but did not specify each cause of death.[8,9,10,11,13,15,16,17,18,19,20,21]
Although previous studies have reported adverse effects of DRFs on mortality, most studies were performed in elderly adult patients and paid little attention to confounding factors of mortality. Furthermore, most of these studies investigated the overall impact of DRFs on mortality without differentiating the specified cause of death. DRFs could increase the risk of trauma or falls due to balance deficits. The hypothesis of the present study was that DRFs could increase the risk of mortality through the direct effects of balance, such as death due to physical trauma, as well as through other indirect effects. To evaluate this hypothesis, each cause of death was separately analyzed for its association with DRFs. The possible confounders of age, sex, income, region of residence, hypertension, diabetes, dyslipidemia, ischemic heart disease, and stroke histories were adjusted to analyze the association between DRFs and mortality.
2. Materials and methods
2.1. Study population and data collection
The Ethics Committee of Hallym University (2014-I148) approved the use of these data. Written informed consent was exempted by the Institutional Review Board. Data from the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC) were collected and classified as in a previous study.
2.2. Participants selection
Out of the 1,125,691 cases with 114,369,638 medical claim codes, we included participants who were diagnosed with DRFs from 2002 through 2013 (n = 27,295). DRFs were defined as fractures of the lower end of the radius (International Statistical Classification of Diseases and related health problems [ICD]-10 codes: S525). The control participants were extracted from 1,098,396 patients who were never diagnosed with DRF from 2002 through 2013.
The DRF patients were matched 1:4 with the control group. The matches were processed for age; group; sex; income group; region of residence; and past medical histories of hypertension, diabetes mellitus, or dyslipidemia. To prevent selection bias when selecting the matched participants, the control group participants were sorted using a random number order and then selected from top to bottom. It was assumed that the matched control participants were medically involved at the same time as each matched DRF patient. Those in the control group who died before the involvement time of their matched DRF patients were excluded. DRF patients for whom we could not identify enough matching participants were excluded (n = 99). Patients who were diagnosed with DRFs who were under 50 years old were excluded (n = 14,032). Finally, 1:4 matching resulted in the inclusion of 13,164 DRF patients and 52,656 control participants (Fig. 1). However, they were not matched for the past medical histories of ischemic heart disease and stroke because these events were relatively rare.
Figure 1.
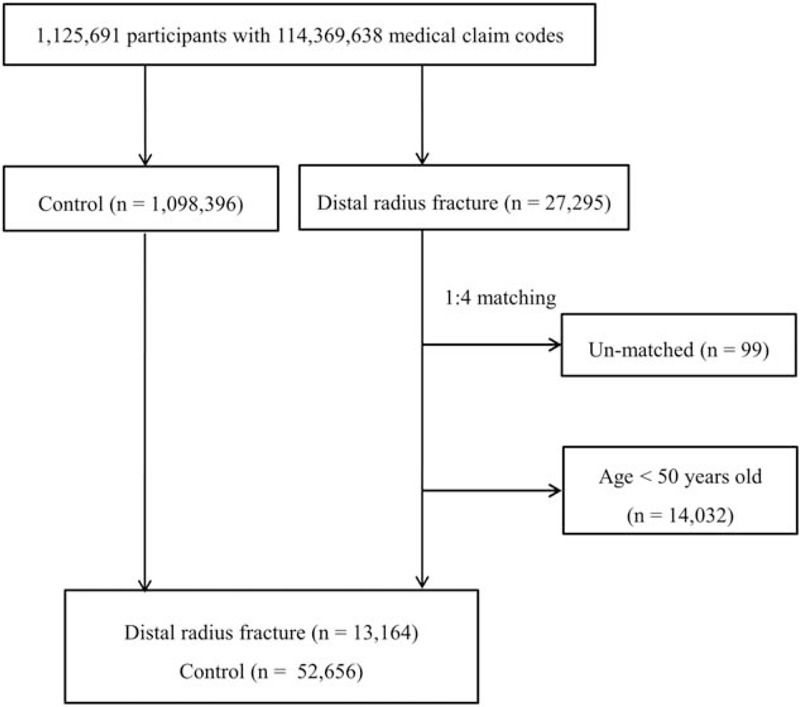
A schematic illustration of the participant selection process that was used in the present study. Out of a total of 1,125,691 participants, 27,295 DRF participants were selected. The DRF participants were matched 1:4 with a control group that were not diagnosed with DRF. Unmatched and <50-year-old patients were excluded (n = 14,131). Finally, 13,164 DRF patients and 52,656 control participants were included. DRF = distal radius fracture.
2.3. Variables
The patients’ age, sex, income, and location were specified as variables. The causes of death were classified and categorized. Additionally, the past medical histories of the participants were evaluated using ICD-10 codes. Details are provided in the supplement file.
2.4. Statistical analyses
A chi-square test or Fisher exact test was used to compare the rate of mortality between the DRF and control groups according to the cause of death.
To analyze the hazard ratio (HR) of DRF on mortality, a Cox-proportional hazard model was used. In this analysis, crude (simple) and adjusted (age, sex, income, region of residence, hypertension, diabetes, hyperlipidemia, ischemic heart disease, and stroke histories) models were used. The 95% CI was calculated. Two-tailed analyses were conducted, and P values less than .05 were considered to indicate significance. The results were statistically analyzed using SPSS v. 21.0 (IBM, Armonk, NY).
3. Results
The mean follow-up was 57.8 (standard deviation [SD] = 40.3) months in the DRF group and 58.0 (SD = 40.4) months in the control group.
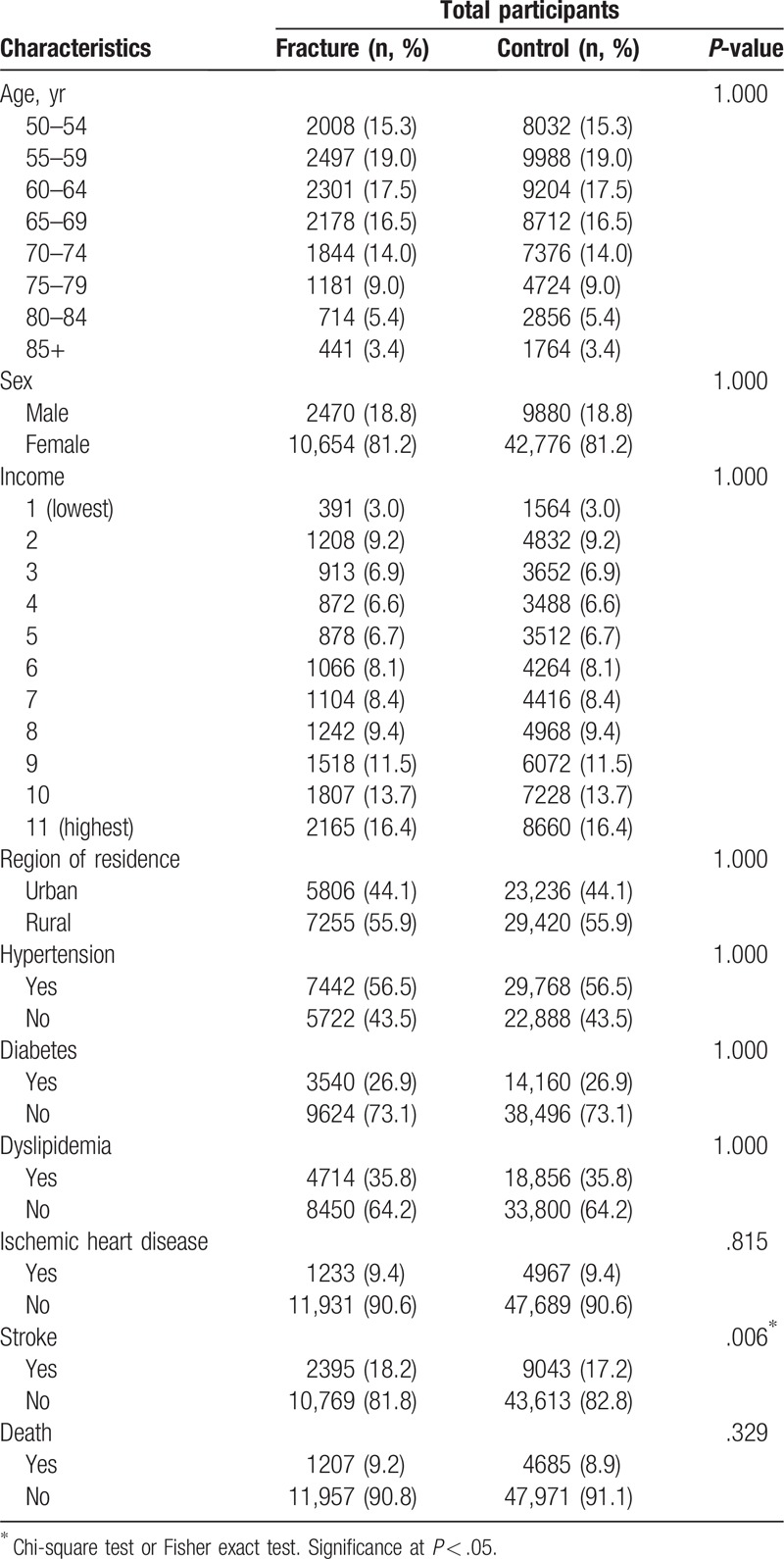
Age, sex, level of income, and region of residence were matched between DRF patients and the control participants (Table 1). The DRF group showed a significantly higher ratio of stroke (P = .006) than the control group.
Table 1.
General characteristics of the participants.
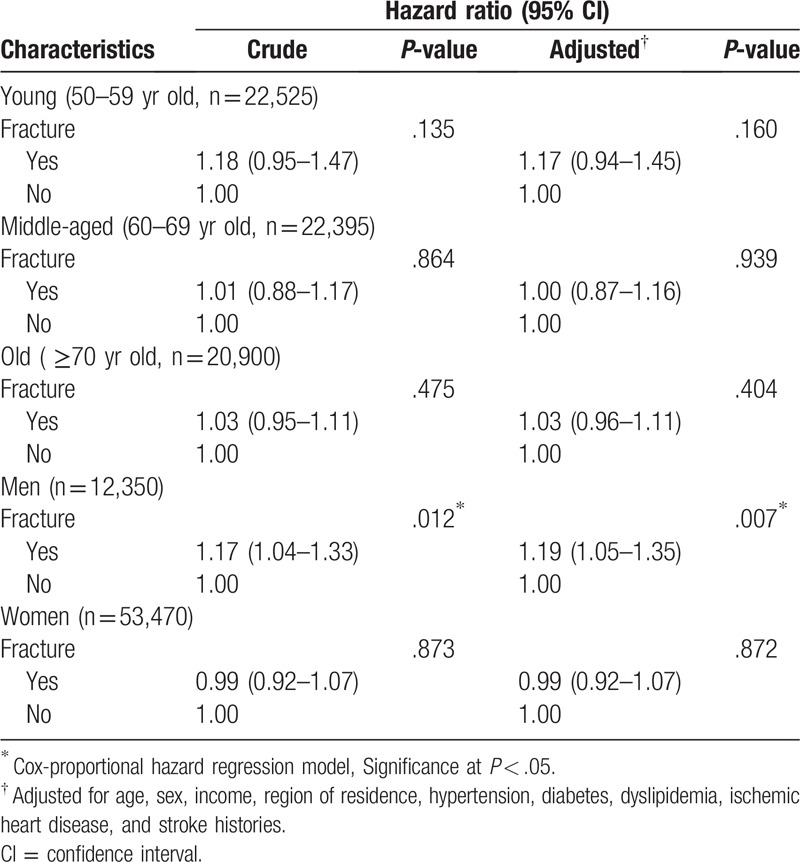
The mortality rate was not significantly higher in the DRF group than in the control group. The crude and adjusted HRs of mortality in the DRF group were 1.03 (95% CI = 0.97–1.10, P = .298) and 1.04 (95% CI = 0.98–1.11, P = .237), respectively (Table 2). In the subgroup analyses according to age and sex, the adjusted HR of mortality was not different for age. The DRF group showed an increased HR and adjusted HR of mortality in the only men subgroup (adjusted HR = 1.19, 95% CI = 1.05–1.35, P < .007) (Table 3).
Table 2.
Crude and adjusted hazard ratios (95% confidence interval) of distal radius fracture for mortality.
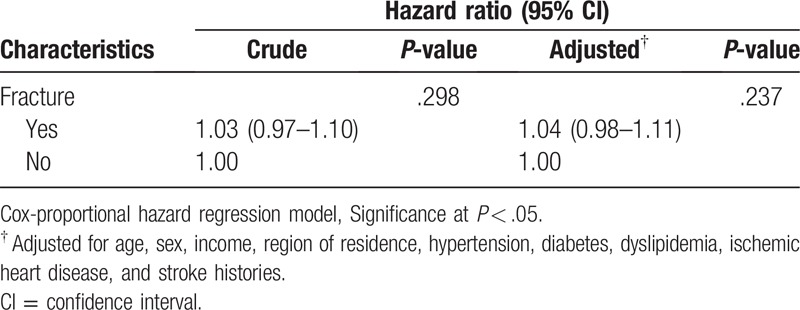
Table 3.
Subgroup analysis of crude and adjusted hazard ratios (95% confidence intervals) of distal radius fracture for mortality according to age and sex.
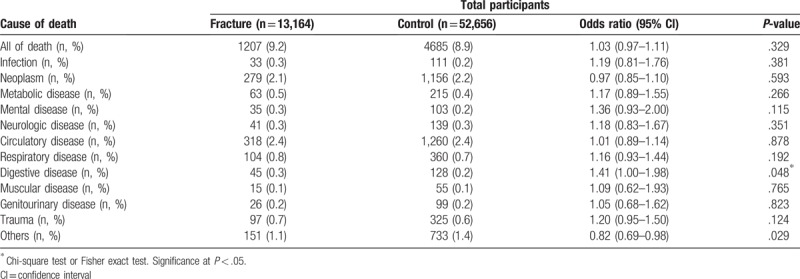
The mortality ratios according to the cause of death were analyzed (Table 4). In the DRF group, the odds ratio (OR) of overall mortality was 1.03 (95% CI = 0.97–1.11, P = .329). Mortality by digestive disease was higher in the DRF group than in the control group (OR = 1.41, 95% CI = 1.00–1.98, P = .048). Mortality caused by infection, neoplasm, metabolic disease, mental disease, neurologic disease, circulatory disease, respiratory disease, and muscular disease were not significantly different between the DRF and control groups.
Table 4.
The difference in mortality between the distal radius fracture and control groups according to the cause of death.
4. Discussion
Our findings (adjusted HR 1.04) were consistent with those of previous studies, which found similar or reduced mortality rates in patients with DRFs, with HRs ranging from 0.58 to 1.94[9,17,18] and risk ratios (RRs) ranging from 0.8 to 3.5.[8,10,11,13,15] However, most previous studies were performed in Western countries, and data from Asian patients have rarely been reported. A recent study in Korea found that the mean of SMRs of DRFs at 1-year postfracture was 1.45 in men and 1.17 in women in the over 50 years age group.[21] However, we cannot compare their results to ours given their different study design; the previous study used only the cumulative mortality rate within the first 12 months, not the overall mortality. To our knowledge, ours is the first study to demonstrate that DRFs were not associated with increased mortality in a national cohort even after adjusting for age, sex, income, region of residence, and past medical histories (hypertension, diabetes, dyslipidemia, ischemic heart disease, and stroke histories); this is also the largest study of its kind to date. The present study extended observations made by prior studies by analyzing the impact of DRFs on the specific disease-related risk of mortality. The adjusted HR of our study (1.04) was relatively lower than those in previous studies; however, this is of little relevance owing to the major heterogeneity among the study methodologies and designs. Among previous publications, 5 did not include patients younger than sixty[11] or 65 years old,[8,14,18,19] 5 did not classify patients by age group,[9,13,15,16,19] 8 did not define the outcome event unequivocally as overall mortality,[8,9,11,17,18,19,20,21] and 4 did not include sufficient data to estimate the RR or HR with a 95% CI.[8,11,16,18] Only patients with an exact fracture of the lower end of radius were selected in the present study. In addition, the inclusion of a young population, that is, not confining the population to an elderly population, increased the accuracy of mortality in this study.
DRFs were not associated with increased overall mortality in this study, but an increased HR and adjusted HR of mortality was observed in the only men group. Morin et al reported an increased risk of mortality in men after wrist fracture (RR 1.5; 95% CI 1.2–1.9) within the first year postfracture.[10] Kwon et al also reported that the mean year mortality over 5 years in men with DRF (2.6%) within the first 12 months was 1.7 times higher than that in women (1.5%).[21] The present report is the third report of increased mortality in men with wrist fractures.
The OR of overall mortality in men with DRFs was 1.16 (95% CI = 1.02–1.33, P = .026), and this group showed a higher OR than the control group for mortality by trauma (Supplementary Table 1). Patients who sustained wrist fractures were nearly 5 times more likely to experience another wrist fracture[4] or a vertebral fracture.[5] A previous study reported that in elderly women, prior wrist fracture was a risk factor for radiographic vertebral fracture and was associated with an 43% age-adjusted excess rate of incident hip fracture.[6] Cuddihy et al reported that among women, vertebral fractures were associated with a 5.2-fold increase in risk and among men, a 10.7-fold increase in risk following a first distal forearm fracture.[5] Men with DRFs may be exposed to complex, multiple traumas or have weaker bones than healthy men.
There were several strengths in the present study. This study was based on an extremely large national population and was verified by a previous study. Because the NHIS data include all citizens without exception, there were no missing participants. While many previous studies focused on elderly adult patients or women of advanced age, the present study enrolled patients who were 50 years or older. The control participants were randomly selected and matched for age, sex, income, and region of residence to avoid confounding effects. Because income and region of residence determine the availability of medical care, the matching of these variables was important, and income was accurately collected based on the Korean NHIS data. Furthermore, an adjusted hazard model was used to minimize confounding by age; sex; income; region of residence; and hypertension, diabetes, dyslipidemia, ischemic heart disease, and stroke histories. To evaluate the specific effects of DRFs on each cause of death, the ORs for all the causes of death were separately analyzed. Finally, all wrist fracture patients were defined as presenting fracture of the lower end of the radius (ICD-10 codes: S525).
However, there were some limitations in the present study. Some potential confounders for mortality, including smoking, obesity, and body mass index, could not be considered in this study due to the lack of information in the NHIS-NSC database. It would be desirable to add a description of factors such as Irisin hormone[22] or platelet volume[23] that could affect the union after fracture. Second, the bone mineral density of DRF patients was not available in the NHIS-NSC data. Third, the heterogeneity of injuries and treatment modalities of DRFs could affect the association with the risk of mortality. Fourth, participants who received physical therapy or rehabilitation could not be determined. Fifth, the severity of each of the fractures related to death could not be assessed. Sixth, the control participants could have had other forearm fractures, and their effects on the risk of mortality remain to be elucidated. Seventh, there is no data on the timing of individual deaths; it would be better if we could reveal the relationship between mortality and the time of death. Finally, our data did not provide information on whether surgery was performed and the type of surgery. It would be more helpful if the relationship between surgery and mortality could be identified. As the purpose of the present study was to estimate the risk of mortality in DRF patients according to the cause of death, our data did not provide information on whether surgery was performed. If DRF patients were associated with increased overall mortality, evaluation of the method of treatment was beneficial. As with tibia,[24] the use of percutaneous plating has been reported in patients with DRF.[25]
5. Conclusion
DRFs were not associated with increased overall mortality in this study, but there was an increased HR and adjusted HR of mortality in the only men subgroup. The DRF group showed a significantly higher ratio of stroke than the control group. The mortality ratio related to digestive disease was high in both men and women, and trauma as the cause of death was more likely in men with DRFs.
Author contributions
Conceptualization: Jung Woo Lee.
Methodology: Jung Woo Lee.
Resources: Jung Woo Lee.
Software: Jung Woo Lee.
Supervision: Yong-Beom Lee, Bong Cheol Kwon, Je-Hyun Yoo.
Validation: Jung Woo Lee.
Writing – original draft: Jung Woo Lee.
Writing – review and editing: Jung Woo Lee, Yong-Beom Lee, Bong Cheol Kwon, Je-Hyun Yoo.
Hyo Geun Choi orcid: 0000-0003-1655-9549.
Jung Woo Lee orcid: 0000-0003-2989-4952.
Yong-Beom Lee orcid: 0000-0002-1500-3402.
Bong Cheol Kwon orcid: 0000-0001-9777-9409.
Je-Hyun Yoo orcid: 0000-0002-0777-1575.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, DRFs = distal radius fractures, HR = hazard ratio, ICD = international statistical classification of diseases and related health problems, NHIS-NSC = National Health Insurance Service-National Sample Cohort, OR = odds ratio, RRs = risk ratios, SD = standard deviation, SMR = standard mortality ratios.
How to cite this article: Lee JW, Lee YB, Kwon BC, Yoo JH, Choi HG. Mortality and cause of death in distal radius fracture patients: A longitudinal follow-up study using a national sample cohort. Medicine. 2019;98:52(e18604).
The manuscript was edited for proper English language, grammar, punctuation, spelling, and overall style by the highly qualified native English-speaking editors at American Journal Experts (631D-C3A5-E0B5–7FA0-A3D6).
This work was supported in part by a research grant (NRF-2015-R1D1A1A01060860).
from the NRF of Korea.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1]. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin 2012;28:113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Kim HY, Ha YC, Kim TY, et al. Healthcare costs of osteoporotic fracture in Korea: information from the national health insurance claims database, 2008-2011. J Bone Metab 2017;24:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Edwards BJ, Song J, Dunlop DD, et al. Functional decline after incident wrist fractures-study of osteoporotic fractures: prospective cohort study. BMJ 2010;341:c3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Robinson CM, Royds M, Abraham A, et al. Refractures in patients at least forty-five years old. A prospective analysis of twenty-two thousand and sixty patients. J Bone Joint Surg Am 2002;84:1528–33. [DOI] [PubMed] [Google Scholar]
- [5]. Cuddihy MT, Gabriel SE, Crowson CS, et al. Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int 1999;9:469–75. [DOI] [PubMed] [Google Scholar]
- [6]. Schousboe JT, Fink HA, Taylor BC, et al. Association between self-reported prior wrist fractures and risk of subsequent hip and radiographic vertebral fractures in older women: a prospective study. J Bone Miner Res 2005;20:100–6. [DOI] [PubMed] [Google Scholar]
- [7]. Chen CW, Huang TL, Su LT, et al. Incidence of subsequent hip fractures is significantly increased within the first month after distal radius fracture in patients older than 60 years. J Trauma Acute Care Surg 2013;74:317–21. [DOI] [PubMed] [Google Scholar]
- [8]. Barrett JA, Baron JA, Beach ML. Mortality and pulmonary embolism after fracture in the elderly. Osteoporos Int 2003;14:889–94. [DOI] [PubMed] [Google Scholar]
- [9]. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ 2009;181:265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Morin S, Lix LM, Azimaee M, et al. Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos Int 2011;22:2439–48. [DOI] [PubMed] [Google Scholar]
- [11]. Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int 2004;15:38–42. [DOI] [PubMed] [Google Scholar]
- [12]. Cooper C, Atkinson EJ, Jacobsen SJ, et al. Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993;137:1001–5. [DOI] [PubMed] [Google Scholar]
- [13]. Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int 2000;11:556–61. [DOI] [PubMed] [Google Scholar]
- [14]. Browner WS, Pressman AR, Nevitt MC, et al. Mortality following fractures in older women. The study of osteoporotic fractures. Arch Intern Med 1996;156:1521–5. [PubMed] [Google Scholar]
- [15]. Melton LJ, 3rd, Achenbach SJ, Atkinson EJ, et al. Long-term mortality following fractures at different skeletal sites: a population-based cohort study. Osteoporos Int 2013;24:1689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Endres HG, Dasch B, Lungenhausen M, et al. Patients with femoral or distal forearm fracture in Germany: a prospective observational study on health care situation and outcome. BMC Public Health 2006;6:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Shortt NL, Robinson CM. Mortality after low-energy fractures in patients aged at least 45 years old. J Orthop Trauma 2005;19:396–400. [DOI] [PubMed] [Google Scholar]
- [18]. Shauver MJ, Zhong L, Chung KC. Mortality after distal radial fractures in the medicare population. J Hand Surg Eur Vol 2015;40:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Rozental TD, Branas CC, Bozentka DJ, et al. Survival among elderly patients after fractures of the distal radius. J Hand Surg Am 2002;27:948–52. [DOI] [PubMed] [Google Scholar]
- [20]. Oyen J, Diamantopoulos AP, Haugeberg G. Mortality after distal radius fracture in men and women aged 50 years and older in southern Norway. PLoS One 2014;9:e112098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Kwon GD, Jang S, Lee A, et al. Incidence and mortality after distal radius fractures in adults aged 50 years and older in Korea. J Korean Med Sci 2016;31:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Serbest S, Tiftikci U, Tosun HB, et al. The Irisin hormone profile and expression in human bone tissue in the bone healing process in patients. Med Sci Monit 2017;23:4278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Serbest S, Tiftikci U, Tosun HB, et al. Is there a relationship between fracture healing and mean platelet volume? Ther Clin Risk Manag 2016;12:1095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Tosun HB, Agir I, Gumustas S, et al. Tibial lengthening using a fixator-assisted lengthening plate: a new technique. Trauma Mon 2016;21:e25340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Wei XM, Sun ZZ, Rui YJ, et al. Minimally invasive percutaneous plate osteosynthesis for distal radius fractures with long-segment metadiaphyseal comminution. Orthop Traumatol Surg Res 2016;102:333–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


