Abstract
Background:
Primary dysmenorrhea is common and troublesome. The comparative efficacy of over-the-counter analgesics (OTCAs) for dysmenorrhea is unclear. This study was aimed at conducting a network meta-analysis to assess the efficacy and safety of 5 OTCAs – naproxen, ibuprofen,diclofenac, aspirin, and ketoprofen – in patients with primary dysmenorrhea.
Methods:
The study was registered with PROSPERO (number: CRD42019133556). The search strategy involved a review of PubMed, Embase, Cochrane Library, Web of Science, and CINAHL for relative randomized controlled trials of the 5 analgesics from the date of database establishment to July 2019. The outputs are presented as odds ratios (ORs), their corresponding 95% confidence intervals (CIs), and the surface under the cumulative ranking area (SUCRA) probabilities.
Results:
Thirty-five trials with 4383 participants were included in our study. As for efficacy outcomes, all the included analgesics except aspirin were more effective than placebo in treating dysmenorrhea [naproxen (OR 3.99, 95% CI 2.18–7.30), ibuprofen (OR 10.08, 95% CI 3.29–30.85), diclofenac (OR 11.82, 95% CI 2.66–52.48), and ketoprofen (OR 5.12, 95% CI 1.57–16.69). The OTCAs were superior to the placebo in terms of pain relief in primary dysmenorrhea. Aspirin was less effective than ibuprofen (OR 0.17, 95% CI 0.04–0.73) and diclofenac (OR 1.17, 95% CI 0.02–0.85). The SUCRA curves showed that diclofenac and ibuprofen were the most and second most effective (85.1% and 83.8%, respectively), followed by ketoprofen, naproxen, and aspirin. Regarding safety, there was no significant difference between the 5 OTCAs included and the placebo. Diclofenac versus ibuprofen (OR 4.31, 95% CI 1.18–15.67), ketoprofen versus diclofenac (OR 0.18, 95% CI 0.04–0.78), and ketoprofen versus aspirin (OR 0.41, 95% CI 0.18–0.97) presented statistically significant differences. Ketoprofen and ibuprofen were ranked the best (SUCRA 90.6% and 79.6%), followed by naproxen, aspirin, and diclofenac.
Conclusion:
Considering the efficacy and safety, ibuprofen is recommended as the optimal OTCA for primary dysmenorrhea. Further well-designed studies that directly compare these analgesics are needed to support our conclusion.
Keywords: network meta-analysis, over-the-counter analgesic, primary dysmenorrhea
1. Introduction
Dysmenorrhea, also known as annoying monthly menstrual flow, is the most common gynecologic condition and the main reason for short-term absenteeism of teenagers and adolescents from school or work.[1,2] Studies have reported that the prevalence of dysmenorrhea in adolescents ranges from 60% to 93% and that of severe dysmenorrhea from 36% to 52.5%.[3–5] Dysmenorrhea is commonly categorized into primary dysmenorrhea and secondary dysmenorrhea. When menstrual pelvic pain is not associated with an identifiable pathological condition, it is called primary dysmenorrhea.[5] It usually occurs from the onset of menstruation or after 6 to 12 months. The pain usually lasts for 2 to 3 days.[6]
Effective solutions for primary dysmenorrhea include nonsteroidal anti-inflammatory drugs (NSAIDs),[7] oral contraceptives,[8,9] acupuncture,[10,11] and low levels of topical heat.[12] When choosing a treatment for dysmenorrhea, it is important to consider treatment availability. In most women with dysmenorrhea, the most severe pain occurs within 12 to 14 hours from the onset of menstruation,[13] and some women cannot determine the exact date of menstruation. Therefore, it is necessary to take painkillers at the beginning of menstruation. Since the 1980s, NSAIDs have become a routine treatment option for dysmenorrhea.[14] Currently, there are several types of NSAIDs, because the results of basic research have indicated that prostaglandins are involved in the pathogenesis of primary dysmenorrhea and that NSAIDs act by blocking the production of prostaglandin. As the most common pain relievers in the management of dysmenorrhea, NSAIDs are available as over-the-counter analgesics (OTCAs) in pharmacies in several countries. In China[15] and Italy,[16] naproxen, ibuprofen, aspirin, diclofenac (prescription required in the US), and ketoprofen are common OTCAs.
In several countries, the use of OTCAs for the treatment of dysmenorrhea is common. A survey of 1539 students in 6 Mexican universities showed that 65% of women with dysmenorrhea practiced self-medication, and the most commonly used medications were OTC drugs.[17] Sugumar et al reported that out of 641 respondents with primary dysmenorrhea, 42% self-medicated.[18] Young women are usually confused about various OTC drug choices, and they often do not know the best analgesic for them. Studies have shown that the majority of prevalent self-medication methods among women are inappropriate, and this is attributable to them being poorly informed about appropriate drug selection, the therapeutic dose, and the associated adverse effects.[4,18,19] In some countries, menstruating women are subjected to cultural restrictions. Adolescents are often reluctant to discuss about menstruation and seldom seek for optimal menstrual health.[20] A lack of knowledge about analgesics among women leads to their refusal to take drugs for fear of safety, but most women use non-drug treatment methods, some of which have no obvious effect.[21] Consequently, whether OTCAs should be used to treat dysmenorrhea and, if yes, which OTCA should be recommended for dysmenorrhea have become topics worth exploring for public health service personnel in pain management.
Indeed, the evidence for OTCA recommendations is notably insufficient. Moore et al[22] conducted a meta-analysis to compare ibuprofen and paracetamol at the standard doses for painful conditions, including dysmenorrhea. The results of a clinical trial of the efficacy of diclofenac potassium relative to a placebo for dysmenorrhea were also reported recently.[23] A Cochrane systematic review[24] compared 20 different NSAIDs versus placebo using the standard meta-analysis to elucidate whether NSAIDs are effective and safe in the treatment of primary dysmenorrhea, but the conclusions of this study are mostly about prescription NSAIDs, with limited evidence of pairwise comparison of different drugs. There are no recommendations pertaining to the use of OTCAs for dysmenorrhea. We therefore conducted a systematic review of the efficacy and safety of 5 OTCAs – naproxen, ibuprofen, diclofenac, and ketoprofen – used for primary dysmenorrhea, to provide a self-medication approach for patients with primary dysmenorrhea and provide evidence for clinical staff and pain specialists in health-care settings.
2. Methods
2.1. Protocol registration
This review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and was registered on June 6, 2019, with the International Prospective Register of Ongoing Systematic Reviews (CRD42019133556).
2.2. Eligibility criteria
Parallel-group or crossover randomized controlled trials of the 5 analgesics (naproxen, ibuprofen, diclofenac, aspirin, and ketoprofen) for the treatment of primary dysmenorrhea were eligible for inclusion, if they met the following criteria:
-
-
Types of participants: Women of reproductive age with primary dysmenorrhea, which was not formally diagnosed with a physical or gynecological examination, were included as long as there were no clinical indications of pelvic pathology.
-
-
Types of interventions: 1 of the 5 analgesics was compared with a placebo; comparison between either 2 of the 5 OTCAs.
The doses of NSAIDs varied, but were within commonly recommended dose range.[24] Average doses for the included OTCAs are shown in Table 1.
Table 1.
Dosing parameters of the five analgesics included in the study.

-
-
Types of outcomes: Pain relief was measured in terms of percentage of effectiveness as dichotomous data (at least moderate pain relief or pain score reduction of more than 50% were considered indicative of effectiveness). If pain scales were used, we converted these into dichotomous data according to the author's description of the scale. Adverse effects were measured in terms of their incidence.
2.3. Search strategy
A systematic literature search of PubMed, Embase, Cochrane Library, Web of Science, and CINAHL was performed from the date of inception of the databases to July 21, 2019. There was no restriction on language, date of trial, or setting. The databases were searched using the following medical subject headings or text keywords in 4 elements of PICOs: P (patient/population) –“primary dysmenorrheal” or “dysmenorrhea” or “dysmenorrhoea” or “pelvic pain” or “menstrual cramps” or “menstrual pain” or “pain-pelvic” or “painful menstruations”; I and C (intervention and comparison) – “non-steroidal” or “non steroidal” or “NSAID” or “NSAIDs” or “naproxen” or “naprosyn” or “ibuprofen” or “brufen” or “diclofenac” or “aspirin” or “acetylsalicylic acid” or “ketoprofen” or “profenid”; O (outcome) – “pain” or “adverse effect” or “adverse reaction” or “safety”; and S (study design) – “randomized controlled trial” or “controlled clinical trial” or “randomized” or “placebo” or “randomly” or “trial.” Attempts were also made to identify trials from the Website of Clinical Trials Register. In order to identify other potentially overlooked literature, an additional manual search of references in the selected trials included and systematic reviews was performed.
2.4. Study selection
The identified studies were selected by 2 authors independently. Titles and abstracts were scanned initially, and then the full articles were examined according to the inclusion criteria. The authors attempted to contact the authors of these studies, as required, to determine study eligibility. Disagreements were resolved by consensus and by consulting a third reviewer.
2.5. Data extraction
Two reviewers independently performed data extraction using standardized data extraction forms. For each study, data on the general characteristics of the study, research methods, participants, interventions, outcome measures, results, and other information were extracted. Any disagreements were resolved by consensus or discussion with a third reviewer.
2.6. Risk of bias assessment
Two authors independently assessed the risk of bias for each study. According to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0), 7 quality domains were considered, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Disagreements between the 2 authors were resolved by consensus and discussion with a third reviewer.
2.7. Data analysis
We used Review Manager 5.3 to evaluate literature quality. Stata 12.0 was used to perform the network meta-analysis. Inconsistency factors and 95% confidence interval (95% CI) were used to assess the consistency of each closed loop. The 95% CI lower limit was equal to 0, and it was regarded as consistent. Otherwise, the closed loop was regarded as obviously inconsistent.[28] Odds ratio (OR) was used to combine the effect sizes, and interval estimation was performed with 95% CIs, where the upper limit was less than 1 or the lower limit was greater than 1, which indicated a statistically significant difference; otherwise the difference was not statistically significant. The surface under the cumulative ranking area (SUCRA) was used to rank the performance of different interventions.[29] The highest SUCRA score was 100%, and a higher SUCRA score indicated better efficacy and safety. Publication bias was evaluated using a funnel plot.
3. Results
3.1. Study identification and selection
According to the retrieval strategy and data collection method, 1278 reports were identified. EndNote X7 document management software was used to eliminate duplicate documents and 692 articles were eligible. By reading the title and abstract, 626 reports were excluded owing to being duplicates or not conforming to the inclusion criteria for participants and intervention measures. Further screening of the full text of 66 reports showed that 9 were not actually non-randomized controlled trials, 4 were not associated with primary dysmenorrhea, 8 were not focused on the drug included, and 5 did not have relevant outcomes. Moreover, 3 studies involved OTCA treatment combined with the application of traditional Chinese medicine and 2 studies involved non-oral treatment administration. Ultimately, 35 articles were included in the meta-analysis (Fig. 1).
Figure 1.
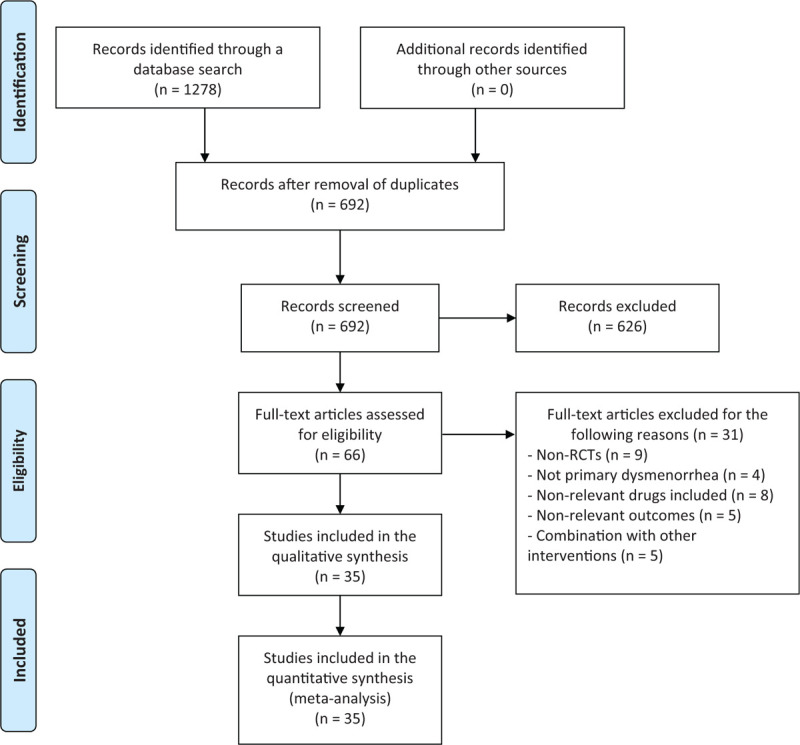
Flow chart of literature selection.
3.2. Characteristics and quality of the included studies
Thirty-five randomized controlled trials with 4383 patients were analyzed in our study. The percentage of effectiveness was reported in 27 studies and the incidence of adverse reactions was reported in 28 studies. The characteristics of the included studies are described in Table 2. The quality of these 35 studies is summarized in Figure 2. The 35 studies mentioned the use of random methods, but only 18 studies described the method of randomization. Among the studies included, only 7 described allocation concealment. In addition, only 22 studies reported the withdrawal of enrolled patients and the causes.
Table 2.
Characteristics of the included studies.
Figure 2.
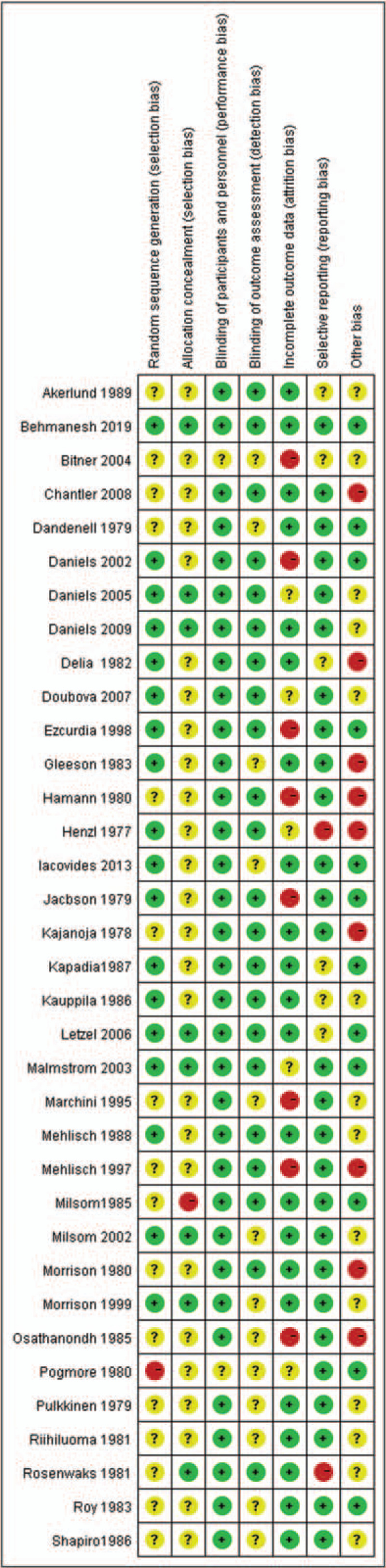
Summary of methodological quality.
Our study involved 6 interventions: naproxen, ibuprofen, diclofenac, aspirin, ketoprofen, and placebo. Figure 3A and 3B show the network plots of treatment comparisons for the efficacy and safety outcomes. Each vertex represents a drug intervention, and the diameter of the vertices represents the total sample size for drug intervention. Every 2 connecting vertices show a direct comparison between 2 interventions. The thickness of line between 2 drug points is indicative of the number of studies directly comparing the 2 drugs; the thicker the line, the higher the number of such studies. Interventions without a connecting line were analyzed through a network meta-analysis.
Figure 3.
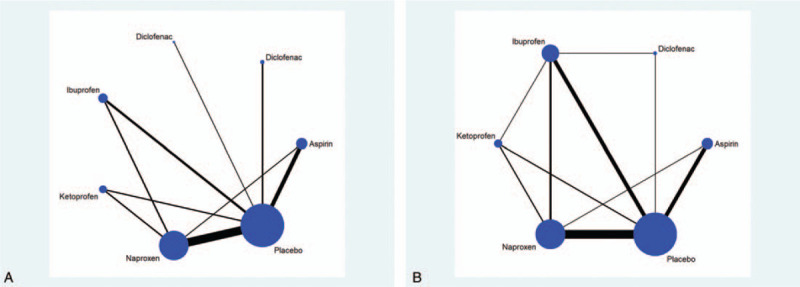
(A) Network connections of the included trials for pain relief. (B) Network connections of the included trials for adverse effects.
3.3. Network meta-analysis of efficacy outcome
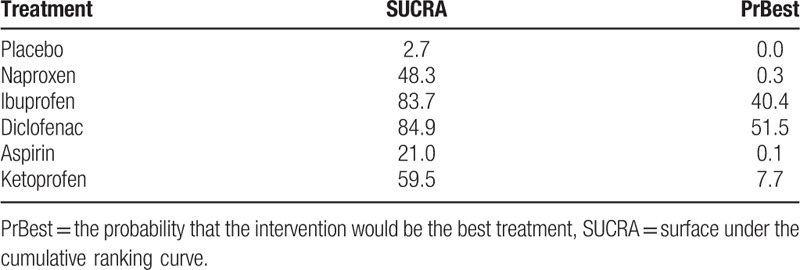
The results of the network meta-analysis revealed that naproxen (OR 3.99, 95% CI 2.18–7.30), ibuprofen (OR 10.08, 95% CI 3.29–30.85), diclofenac (OR 11.82, 95% CI 2.66–52.48), and ketoprofen (OR 5.12, 95% CI 1.57–16.69) were more effective than placebo in treating dysmenorrhea (Fig. 4A). Aspirin was less effective than ibuprofen (OR 0.17, 95% CI 0.04–0.73) and diclofenac (OR 1.17, 95% CI 0.02–0.85). In addition, ranking of efficacy of the various treatments is shown in Table 3. Diclofenac was ranked the best (SUCRA 84.9%), followed by ibuprofen (83.7%), ketoprofen (59.5%), naproxen (48.3%), aspirin (21.0%), and placebo (2.7%).
Figure 4.
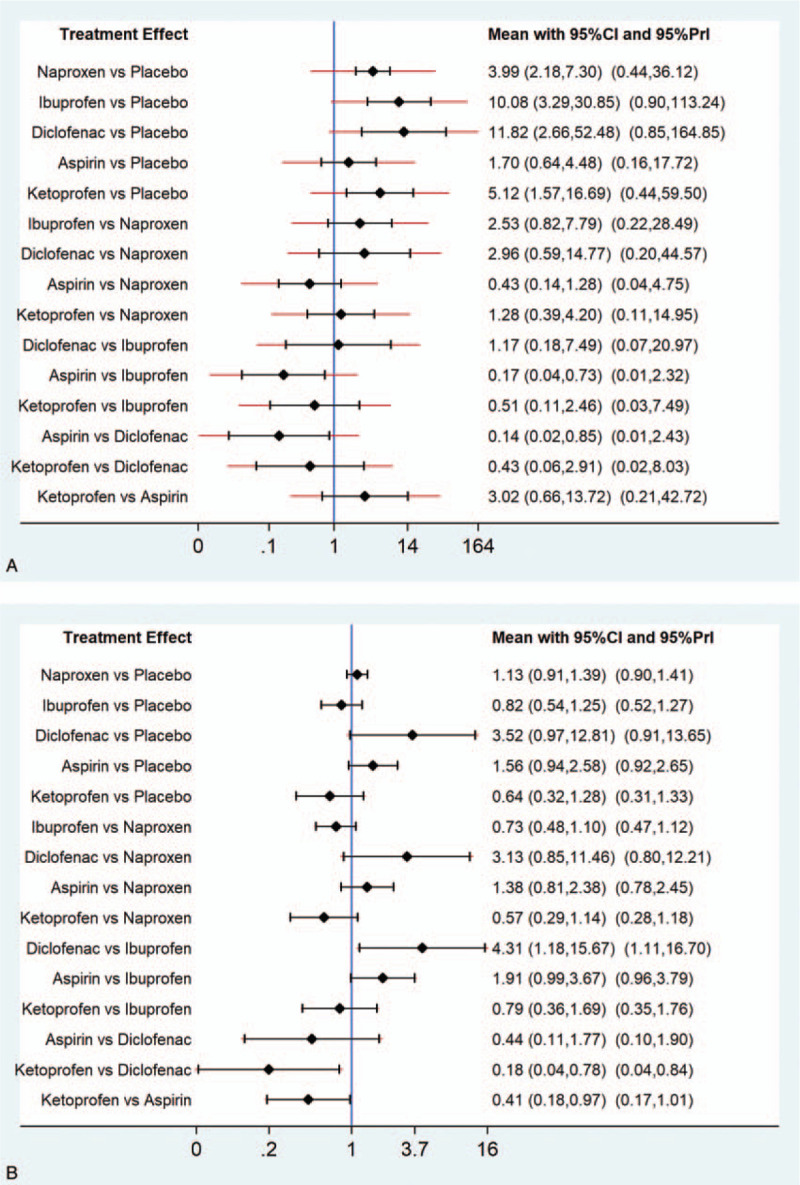
(A) Results of the network meta-analysis for efficacy parameters of the included analgesics. (B) Results of the network meta-analysis for safety parameters of the included analgesics.
Table 3.
Ranking according to efficacy outcomes of SUCRA curves.
3.4. Network meta-analysis of the safety outcomes
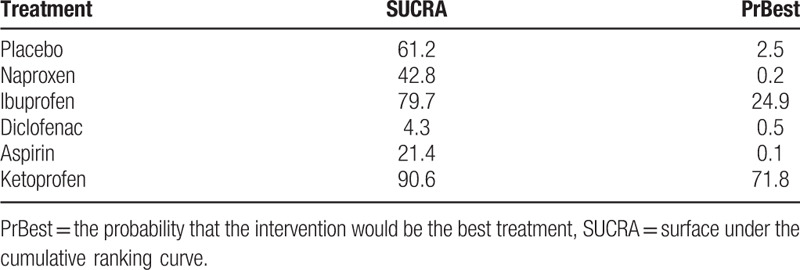
With respect to adverse effects, there was no significant difference among the 5 OTCAs included and placebo (Fig. 4B). Diclofenac versus ibuprofen (OR 4.31, 95% CI 1.18–15.67), ketoprofen versus diclofenac (OR 0.18, 95% CI 0.04–0.78), and ketoprofen versus aspirin (OR 0.41, 95% CI 0.18–0.97) presented statistically significant differences. Ranking according to safety outcomes of SUCRA curves is shown in Table 4. Ketoprofen was associated with the highest probability of being the safest drug (SUCRA, 90.6%), followed by ibuprofen (79.7%), placebo (61.2%), naproxen (42.8%), aspirin (21.4%), and diclofenac (4.3%).
Table 4.
Ranking according to safety outcomes of SUCRA curves.
3.5. Publication bias and data consistency
No obvious publication bias was detected in a visual inspection of funnel-plot symmetries (Fig. 5A, 5B). In terms of network connections regarding efficacy data, 3 closed triangular loops were formed (Fig. 3A). With respect to safety data, there were 6 closed loops (Fig. 3B). Therefore, the node-splitting analysis was conducted to detect if any significant data inconsistency exists. The results demonstrated that all P values were more than .05 (Fig. 6A and 6B), indicating that the closed loop consistency was good.
Figure 5.
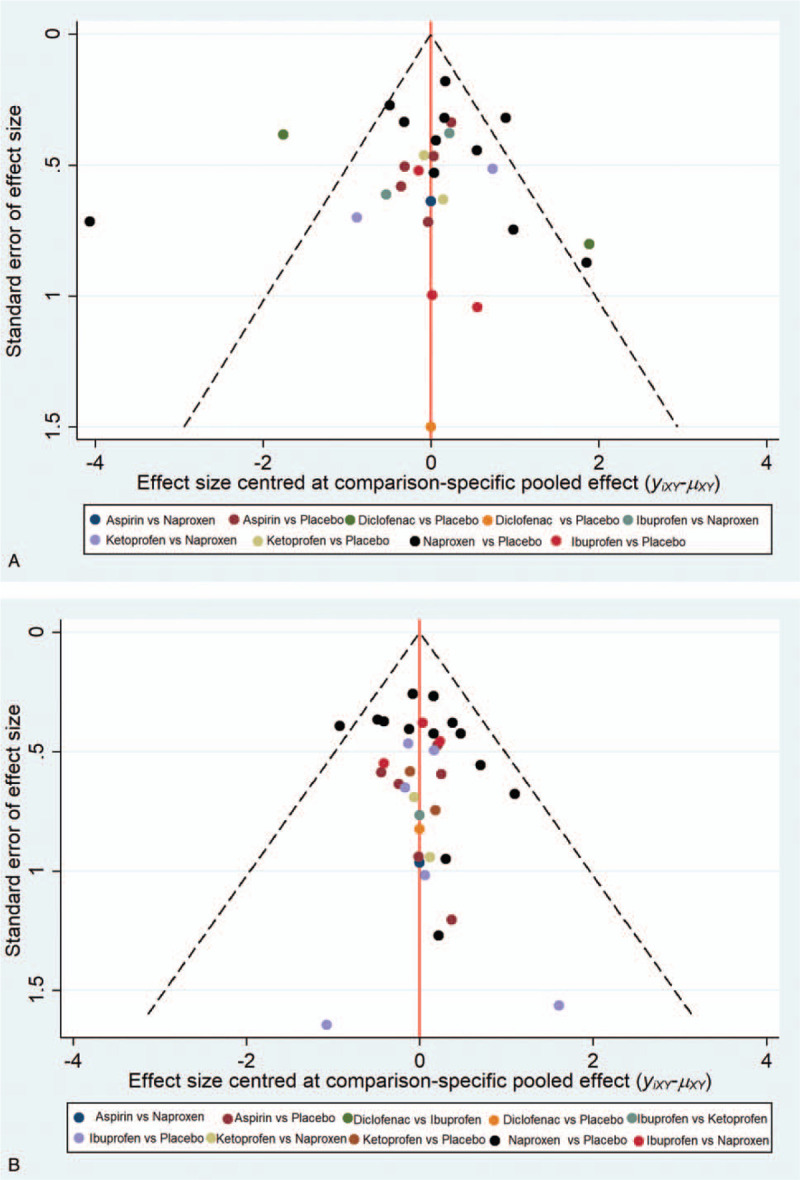
(A) Funnel plot of the efficacy of 5 over-the-counter analgesics and placebo. (B) Funnel plot of the safety of 5 over-the-counter analgesics and placebo.
Figure 6.
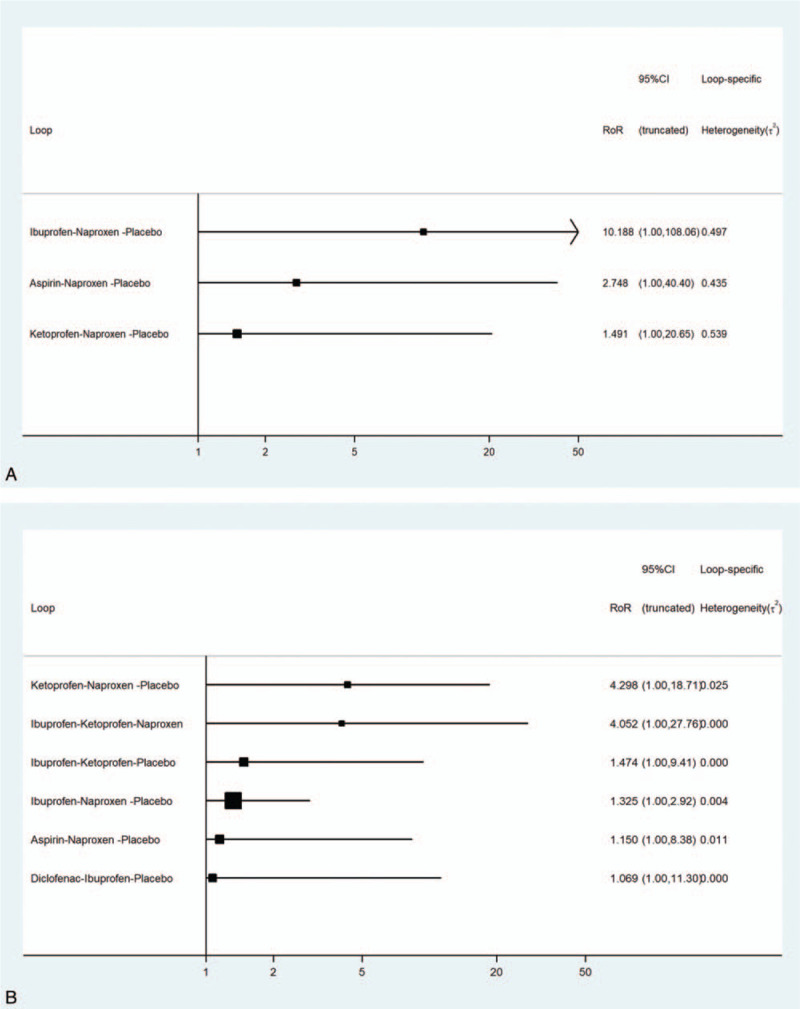
(A) Node-splitting analysis of the network meta-analysis for efficacy outcome. (B) Node-splitting analysis of the network meta-analysis for safety outcome.
4. Discussion
4.1. Overall analysis of the included studies
Primary dysmenorrhea, a high-frequency disease in women, affects their normal quality of life.[1] There are several types of prescribed NSAIDs, which are used as a first-line treatment,[64] and they act by inhibiting cyclooxygenase (COX) enzymes including COX-1 and COX-2. OTCAs, which are widely used, are certainly effective in relieving the pain of primary dysmenorrhea, but there is no clinical consensus on the best choice. Therefore, the purpose of this network meta-analysis was to develop an optimal treatment strategy with OTCAs through a systematic review and statistical analysis. Although a few studies have been conducted in recent years, the results of our study are valuable, as OTCAs have been widely used to relieve pain in primary dysmenorrhea in the past few decades. In this study, randomized controlled trials of the 5 OTCAs included (naproxen, ibuprofen, diclofenac, aspirin, and ketoprofen) were selected through careful reading of literature and employing an evaluation methodology without language restriction. In the studies that met the inclusion criteria, different statistics were used for the efficacy outcome indicators of dysmenorrhea and incidence of adverse events. Some studies used dichotomous variables, whereas others used continuous variables; therefore, some data could not be combined. Only binary variable data and the results that can be converted into binary variable data were integrated in our study. The overall quality of the included studies was not very high. This might be because some of the studies on the treatment of primary dysmenorrhea with OTCAs were published several years ago, and they did not adequately focus on the detailed description of research methodology.
4.2. Efficacy of the 5 OTCAs for primary dysmenorrhea
With respect to the effectiveness of the 5 OTCAs, the results of the present network meta-analysis showed that all the included analgesics except aspirin were superior to the placebo in terms of pain relief in primary dysmenorrhea. The results are consistent with those of a systematic review conducted by Zhang[65]; that is, the efficacy of the treatment of primary dysmenorrhea was significant with naproxen (OR = 0.38, 95% CI 0.32–0.44) and ibuprofen (OR = 0.23, 95% CI 0.13–0.41), but the effect of aspirin (OR = 0.79, 95% CI 0.58–1.08) was not obviously better than placebo. Marjoribanks et al[24] analyzed 11 kinds of NSAIDs in the treatment course of primary dysmenorrhea and reported that while NSAIDs are very effective in alleviating dysmenorrhea, studies directly comparing 2 kinds of drugs are limited. They also reported that the size of sample in such studies was small. Hence, it is difficult to decide whether a drug is more effective or safer than another. In our network meta-analysis, most of the results compensate for the limitations of the traditional meta-analysis, by ranking the drugs based on the SUCRA score providing information for more effective treatment. Diclofenac and ibuprofen showed the best efficacy among the 5 OTCAs. Naproxen, the most widely used drug with the highest number of studies, showed moderate efficacy in ranking.
4.3. Safety of the 5 OTCAs for primary dysmenorrhea
In terms of safety, Marjoribanks et al[24] reported that the use of NSAIDs in the treatment course of primary dysmenorrhea was highly effective when compared with placebo (OR = 4.50, 95% CI 3.85–527), but attention should be paid to their adverse reactions (OR = 1.37, 95% CI 1.12–1.66). However, in our study, the 5 OTCAs used for dysmenorrhea did not cause more adverse effects relative to those associated with placebo. This may be because analgesics for dysmenorrhea are usually needed for only a few days a month, and OTCAs, as drugs that can be purchased and taken by patients themselves, are relatively safe. In all the included studies, no serious adverse reaction was reported. This can be attributed to the fact that the duration of all these studies was no more than 6 months. Our network meta-analysis showed that the OTCAs were well tolerated as a pain-relief option for dysmenorrhea over a period of 6 months. Our safety outcomes obtained using SUCRA curves showed that ketoprofen and ibuprofen were the safest OTCAs, even better than placebo, whereas diclofenac was the worst one.
4.4. Overall completeness and applicability of evidence
Although our network meta-analysis results were relatively comprehensive, there were still some limitations, which may weaken the reliability of the results. First, we only evaluated the efficacy and safety differences among these OTCAs; however, the dosage and frequency of medication were not taken into consideration. Second, although various trials were included in the study, the sample size included was small. Among the 22 studies included, only 8 had a total sample size of more than 100 cases and seldom mentioned the estimation of sample size; therefore, the strength of the data was slightly weakened.
It would be useful to know whether the benefits of OTCAs can be maintained with reduced adverse effects by combining lower doses of OTCAs with codeine, paracetamol, acupuncture, or transcutaneous electrical nerve stimulation.[24] It would also be useful to know whether dysmenorrhea in oral contraceptives or intrauterine contraceptive device users can be treated in a similar way to primary dysmenorrhea. However, these questions are beyond the scope of the present study.
4.5. Relevance for practice
As previously mentioned, dysmenorrhea has been shown to cause repeated short-term pain, which is very serious at some instances. OTCAs have been suggested for pain relief in the treatment of primary dysmenorrhea[64,66]. However, some adolescents use overdoses of OTCAs without guidance, while others who experience intense pain do not seek treatment with painkillers owing to potential serious adverse reactions. Thus, it is important for health-care providers to review pertinent literature and discern available pain practice data[66] and to recommend which type of OTCAs is more effective and safe. The results of our study may serve as a reasonable medication recommendation for the health-care staff in evidence-based pain management interventions and education programs.
5. Conclusions
The results of both effectiveness and safety network meta-analyses showed that diclofenac, as the OTCA with the best effectiveness and worst safety, is similar to a double-edged sword in its application. Therefore, we recommend ibuprofen, which was ranked second in terms of effectiveness and safety, to patients with primary dysmenorrhea. Naproxen, one of the most widely used rugs for dysmenorrhea, did not show higher efficacy or safety in our study. However, the findings are applicable only to choosing a certain type of OTCA for dysmenorrhea. A previous study has shown that pharmacokinetics and pharmacodynamics parameters are significantly affected by drug-manufacturing technique and dosage forms (conventional and chewable tablets, enteric-coated capsule, oral suspensions, and liquid capsules).[67] For instance, the median times to reach the maximum blood concentration after a single oral dose of ibuprofen tablet and enteric-coated ibuprofen capsule (200 mg) were 60 and 240 minutes, respectively.[68] The oral bioavailability of a drug also depends on the dosing vehicle or formulation, physicochemical properties of the compounds, pathway of drug absorption, and physiological conditions.[69] Therefore, our results cannot be used to recommend specific doses of drugs with a specific trade name for the treatment of dysmenorrhea. In future studies, objective outcome parameters reflecting the severity of dysmenorrhea should be included in the evaluations. In addition, it is suggested that clinical nurses and researchers carry out more randomized controlled trials with large samples for direct comparison between OTCAs, in order to more accurately compare the differences in effectiveness, safety, and economy among various non-prescription analgesics.
Acknowledgments
We are grateful to Jing Tang and Lijing Zhao, who assisted in literature screening. We thank Zhifang Jia for her instructions on statistical analyses. We also acknowledge the authors who supplied additional data for clarifying the results reported in their study publications.
Author contributions
All authors provided final approval and agree to be accountable for the work.
Conceptualization: Lisheng Wang.
Data curation: Wenbo Nie, Ping Xu, Chunyan Hao, Yingying Chen, Yanling Yin, Lisheng Wang.
Formal analysis: Ping Xu, Yingying Chen.
Investigation: Yingying Chen, Yanling Yin.
Methodology: Wenbo Nie, Lisheng Wang.
Resources: Chunyan Hao, Yanling Yin.
Software: Wenbo Nie, Ping Xu.
Writing – Original Draft: Wenbo Nie.
Writing – Review & Editing: Lisheng Wang.
Footnotes
Abbreviations: CI = confidence interval, COX = cyclooxygenase, NSAID = nonsteroidal anti-inflammatory drug, OR = odds ratio, OTCA = over-the-counter analgesic, SUCRA = surface under the cumulative ranking area.
How to cite this article: Nie W, Xu P, Hao C, Chen Y, Yin Y, Wang L. Efficacy and safety of over-the-counter analgesics for primary dysmenorrhea: a network meta-analysis. Medicine. 2020;99:19(e19881).
This work was funded by the Interdisciplinary Research Funding Program for PhD Students of Jilin University (grant number 10183201849) and by the Jilin Provincial Department of Finance (grant number 3D518V103429).
The authors acknowledge that all authors meet the authorship criteria according to the latest guidelines of the International Committee of Medical Journal Editors and approve the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Jiang ZQ, Wang J, Guo XN, et al. Menstrual disorders and occupational exposures among female nurses: a nationwide cross-sectional study. Int J Nurs Stud 2019;95:49–55. [DOI] [PubMed] [Google Scholar]
- [2].Schoep ME, Nieboer TE, van der Zanden M, et al. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol 2019;220:569.e1–7. [DOI] [PubMed] [Google Scholar]
- [3].Till SR, As-Sanie S, Schrepf A. Psychology of chronic pelvic pain: prevalence, neurobiological vulnerabilities, and treatment. Clin Obstet Gynecol 2019;62:22–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Soderman L, Edlund M, Marions L. Prevalence and impact of dysmenorrhea in Swedish adolescents. Acta Obstet Gynecol Scand 2019;98:215–21. [DOI] [PubMed] [Google Scholar]
- [5].Rafique N, Al-Sheikh MH. Prevalence of primary dysmenorrhea and its relationship with body mass index. J Obstet Gynaecol Res 2018;44:1773–8. [DOI] [PubMed] [Google Scholar]
- [6].Fernandez-Martinez E, Onieva-Zafra MD, Parra-Fernandez ML. Lifestyle and prevalence of dysmenorrhea among Spanish female university students. PLoS One 2018;13:e0201894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sharghi M, Mansurkhani SM, Larky DA, et al. An update and systematic review on the treatment of primary dysmenorrhea. JBRA Assist Reprod 2019;23:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Davis AR, Westhoff CL. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J Pediatr Adolesc Gynecol 2001;14:3–8. [DOI] [PubMed] [Google Scholar]
- [9].Jaisamrarn U, Santibenchakul S. A comparison of combined oral contraceptives containing chlormadinone acetate versus drospirenone for the treatment of acne and dysmenorrhea: a randomized trial. Contracept Reprod Med 2018;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang F, Sun M, Han S, et al. Acupuncture for primary dysmenorrhea: an overview of systematic reviews. Evid Based Complement Alternat Med 2018;2018:8791538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shetty GB, Shetty B, Mooventhan A. Efficacy of acupuncture in the management of primary dysmenorrhea: a randomized controlled trial. J Acupunct Meridian Stud 2018;11:153–8. [DOI] [PubMed] [Google Scholar]
- [12].Jo J, Lee SH. Heat therapy for primary dysmenorrhea: a systematic review and meta-analysis of its effects on pain relief and quality of life. Sci Rep 2018;8:16252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Deligeoroglou E. Dysmenorrhea. Ann N Y Acad Sci 2000;900:237–44. [DOI] [PubMed] [Google Scholar]
- [14].Milsom I, Minic M, Dawood MY, et al. Comparison of the efficacy and safety of nonprescription doses of naproxen and naproxen sodium with ibuprofen, acetaminophen, and placebo in the treatment of primary dysmenorrhea: a pooled analysis of five studies. Clin Ther 2002;24:1384–400. [DOI] [PubMed] [Google Scholar]
- [15].Chen H, Ung COL, Chi P, et al. Consumers’ perceptions about pharmaceutical care provided by community pharmacists in China in relation to over-the-counter drugs: a qualitative study. Inquiry 2018;55:46958018793292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Motola G, Russo F, Mazzeo F, et al. Over-the-counter oral nonsteroidal anti-inflammatory drugs: a pharmacoepidemiologic study in southern Italy. Adv Ther 2001;18:216–22. [DOI] [PubMed] [Google Scholar]
- [17].Ortiz MI. Primary dysmenorrhea among Mexican university students: prevalence, impact and treatment. Eur J Obstet Gyn R B 2010;152:73–7. [DOI] [PubMed] [Google Scholar]
- [18].Sugumar R, Krishnaiah V, Channaveera GS, et al. Comparison of the pattern, efficacy, and tolerability of self-medicated drugs in primary dysmenorrhea: a questionnaire based survey. Indian J Pharmacol 2013;45:180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mehuys E, Crombez G, Paemeleire K, et al. Self-medication with over-the-counter analgesics: a survey of patient characteristics and concerns about pain medication. J Pain 2019;20:215–23. [DOI] [PubMed] [Google Scholar]
- [20].Chang YT, Lin ML. Menarche and menstruation through the eyes of pubescent students in eastern Taiwan: implications in sociocultural influence and gender differences issues. J Nurs Res 2013;21:10–8. [DOI] [PubMed] [Google Scholar]
- [21].Su JJ, Lindell D. Promoting the menstrual health of adolescent girls in China. Nurs Health Sci 2016;18:481–7. [DOI] [PubMed] [Google Scholar]
- [22].Moore RA, Derry S, Wiffen PJ, et al. Overview review: comparative efficacy of oral ibuprofen and paracetamol (acetaminophen) across acute and chronic pain conditions. Eur J Pain 2015;19:1213–23. [DOI] [PubMed] [Google Scholar]
- [23].Moore RA, Derry S. Diclofenac potassium in acute postoperative pain and dysmenorrhoea: results from comprehensive clinical trial reports. Pain Res Manag 2018;2018:9493413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Marjoribanks J, Ayeleke RO, Farquhar C, et al. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst Rev 2015;7:CD001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ezzy A. Recommending OTC medicines for period pain. Aust Pharm 2014;33:44–6, 48. [Google Scholar]
- [26].Meeves S, Leyva R, Richardson C, et al. Actual use of and adherence to ibuprofen 400 mg tablet dosing instructions in a simulated OTC environment. Int J Clin Pharmacol Ther 2017;55:547–57. [DOI] [PubMed] [Google Scholar]
- [27].Lavonas EJ, Fries JF, Furst DE, et al. Comparative risks of non-prescription analgesics: a structured topic review and research priorities. Expert Opin Drug Saf 2012;11:33–44. [DOI] [PubMed] [Google Scholar]
- [28].Del Giovane C, Vacchi L, Mavridis D, et al. Network meta-analysis models to account for variability in treatment definitions: application to dose effects. Stat Med 2013;32:25–39. [DOI] [PubMed] [Google Scholar]
- [29].Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Akerlund M, Stromberg P. Comparison of ketoprofen and naproxen in the treatment of dysmenorrhoea, with special regard to the time of onset of pain relief. Curr Med Res Opin 1989;11:485–90. [DOI] [PubMed] [Google Scholar]
- [31].Behmanesh E, Delavar MA, Kamalinejad M, et al. Effect of eryngo (Eryngium caucasicum Trautv) on primary dysmenorrhea: a randomized, double-blind, placebo-controlled study. Taiwan J Obstet Gynecol 2019;58:227–33. [DOI] [PubMed] [Google Scholar]
- [32].Bitner M, Kattenhorn J, Hatfield C, et al. Efficacy and tolerability of lumiracoxib in the treatment of primary dysmenorrhoea. Int J Clin Pract 2004;58:340–5. [DOI] [PubMed] [Google Scholar]
- [33].Chantler I, Mitchell D, Fuller A. The effect of three cyclo-oxygenase inhibitors on intensity of primary dysmenorrheic pain. Clin J Pain 2008;24:39–44. [DOI] [PubMed] [Google Scholar]
- [34].Dandenell LO, Lalos O, Lisciak J, et al. Clinical experience of naproxen in the treatment of primary dysmenorrhea. Acta Obstet Gynecol Scand Suppl 1979;87:95–100. [DOI] [PubMed] [Google Scholar]
- [35].Daniels SE, Talwalker S, Torri S, et al. Valdecoxib, a cyclooxygenase-2-specific inhibitor, is effective in treating primary dysmenorrhea. Obstet Gynecol 2002;100:350–8. [DOI] [PubMed] [Google Scholar]
- [36].Daniels SE, Torri S, Desjardins PJ. Valdecoxib for treatment of primary dysmenorrhea. A randomized, double-blind comparison with placebo and naproxen. J Gen Intern Med 2005;20:62–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Daniels S, Robbins J, West CR, et al. Celecoxib in the treatment of primary dysmenorrhea: results from two randomized, double-blind, active- and placebo-controlled, crossover studies. Clin Ther 2009;31:1192–208. [DOI] [PubMed] [Google Scholar]
- [38].DeLia JE, Emery MG, Taylor RH, et al. Flurbiprofen in dysmenorrhea. Clin Pharmacol Ther 1982;32:76–80. [DOI] [PubMed] [Google Scholar]
- [39].Doubova SV, Morales HR, Hernandez SF, et al. Effect of a Psidii guajavae folium extract in the treatment of primary dysmenorrhea: a randomized clinical trial. J Ethnopharmacol 2007;110:305–10. [DOI] [PubMed] [Google Scholar]
- [40].Ezcurdia M, Cortejoso FJ, Lanzón R, et al. Comparison of the efficacy and tolerability of dexketoprofen and ketoprofen in the treatment of primary dysmenorrhea. J Clin Pharmacol 1998;38: Suppl 12: 65S–73S. [PubMed] [Google Scholar]
- [41].Gleeson S, Sorbie J. Efficacy of ketoprofen in treating primary dysmenorrhea. Can Med Assoc J 1983;129:842–4. [PMC free article] [PubMed] [Google Scholar]
- [42].Hamann GO. Severe, primary dysmenorrhea treated with naproxen. A prospective, double-blind, crossover investigation. Prostaglandins 1980;19:651–7. [DOI] [PubMed] [Google Scholar]
- [43].Henzl MR, Buttram V, Segre EJ, et al. The treatment of dysmenorrhea with naproxen sodium: a report on two independent double-blind trials. Am J Obstet Gynecol 1977;127:818–23. [DOI] [PubMed] [Google Scholar]
- [44].Iacovides S, Baker FC, Avidon I. The 24-h progression of menstrual pain in women with primary dysmenorrhea when given diclofenac potassium: a randomized, double-blinded, placebo-controlled crossover study. Arch Gynecol Obstet 2014;289:993–1002. [DOI] [PubMed] [Google Scholar]
- [45].Jacobson J, Cavalli-Björkman K, Lundström V, et al. Prostaglandin synthetase inhibitors and dysmenorrhea. A survey and personal clinical experience. Acta Obstet Gynecol Scand Suppl 1979;87:73–9. [DOI] [PubMed] [Google Scholar]
- [46].Kajanoja P. Indomethacin in the treatment of primary dysmenorrhoea. Archiv fur Gynakologie 1978;225:1–5. [DOI] [PubMed] [Google Scholar]
- [47].Kapadia L. A study of naproxen sodium and ibuprofen in primary dysmenorrhoea. J Soc Occup Med 1987;37:77–80. [DOI] [PubMed] [Google Scholar]
- [48].Kauppila A, Makila UM, Makarainen L, et al. Tiaprofenic acid in the treatment of primary dysmenorrhoea. Eur J Obstet Gynecol Reprod Biol 1986;22:359–63. [DOI] [PubMed] [Google Scholar]
- [49].Letzel H, Megard Y, Lamarca R, et al. The efficacy and safety of aceclofenac versus placebo and naproxen in women with primary dysmenorrhoea. Eur J Obstet Gynecol Reprod Biol 2006;129:162–8. [DOI] [PubMed] [Google Scholar]
- [50].Malmstrom K, Kotey P, Cichanowitz N, et al. Analgesic efficacy of etoricoxib in primary dysmenorrhea: results of a randomized, controlled trial. Gynecol Obstet Invest 2003;56:65–9. [DOI] [PubMed] [Google Scholar]
- [51].Marchini M, Tozzi L, Bakshi R, et al. Comparative efficacy of diclofenac dispersible 50 mg and ibuprofen 400 mg in patients with primary dysmenorrhea. A randomized, double-blind, within-patient, placebo-controlled study. Int J Clin Pharmacol Ther 1995;33:491–7. [PubMed] [Google Scholar]
- [52].Mehlisch DR. Ketoprofen, ibuprofen, and placebo in the treatment of primary dysmenorrhea: a double-blind crossover comparison. J Clin Pharmacol 1988;28: Suppl 1: S29–33. [DOI] [PubMed] [Google Scholar]
- [53].Mehlisch DR, Fulmer RI. A crossover comparison of bromfenac sodium, naproxen sodium, and placebo for relief of pain from primary dysmenorrhea. J Womens Health 1997;6:83–92. [DOI] [PubMed] [Google Scholar]
- [54].Milsom I, Andersch B. Ibuprofen and naproxen-sodium in the treatment of primary dysmenorrhea: a double-blind cross-over study. Int J Gynaecol Obstet 1985;23:305–10. [DOI] [PubMed] [Google Scholar]
- [55].Morrison JC, Ling FW, Forman EK, et al. Analgesic efficacy of ibuprofen for treatment of primary dysmenorrhea. South Med J 1980;73:999–1002. [DOI] [PubMed] [Google Scholar]
- [56].Morrison BW, Daniels SE, Kotey P, et al. Rofecoxib, a specific cyclooxygenase-2 inhibitor, in primary dysmenorrhea: a randomized controlled trial. Obstet Gynecol 1999;94:504–8. [DOI] [PubMed] [Google Scholar]
- [57].Osathanondh R, Caldwell BV, Kaul AF, et al. Efficacy of fenoprofen in the treatment of primary dysmenorrhea. J Reprod Med 1985;30:915–9. [PubMed] [Google Scholar]
- [58].Pogmore JR, Filshie GM. Flurbiprofen in the management of dysmenorrhoea. Br J Obstet Gynaecol 1980;87:326–9. [DOI] [PubMed] [Google Scholar]
- [59].Pulkkinen MO, Csapo AI. Effect of ibuprofen on menstrual blood prostaglandin levels in dysmenorrheic women. Prostaglandins 1979;18:137–42. [DOI] [PubMed] [Google Scholar]
- [60].Riihiluoma P, Wuolijoki E, Pulkkinen MO. Treatment of primary dysmenorrhea with diclofenac sodium. Eur J Obstet Gynecol Reprod Biol 1981;12:189–94. [DOI] [PubMed] [Google Scholar]
- [61].Rosenwaks Z, Jones GS, Henzl MR, et al. Naproxen sodium, aspirin, and placebo in primary dysmenorrhea. Reduction of pain and blood levels of prostaglandin F2-alpha metabolite. Am J Obstet Gynecol 1981;140:592–8. [DOI] [PubMed] [Google Scholar]
- [62].Roy S. A double-blind comparison of a propionic acid derivative (ibuprofen) and a fenamate (mefenamic acid) in the treatment of dysmenorrhea. Obstet Gynecol 1983;61:628–32. [PubMed] [Google Scholar]
- [63].Shapiro SS. Flurbiprofen for the treatment of primary dysmenorrhea. Am J Med 1986;80:71–5. [DOI] [PubMed] [Google Scholar]
- [64].Oladosu FA, Tu FF, Hellman KM. Nonsteroidal antiinflammatory drug resistance in dysmenorrhea: epidemiology, causes, and treatment. Am J Obstet Gynecol 2018;218:390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].WY Z, A LWP. Efficacy of minor analgesics in primary dysmenorrhoea: a systematic review. British journal of obstetrics and gynaecology. 1998;105:780–789. [DOI] [PubMed] [Google Scholar]
- [66].Klaess CC, Urton M, Whitehead P, et al. Pain management pillars for the clinical nurse specialist: summary of National Association of Clinical Nurse Specialists Opioid Pain Management Task Force. Clin Nurse Spec 2019;33:136–45. [DOI] [PubMed] [Google Scholar]
- [67].Irvine J, Afrose A, Islam N. Formulation and delivery strategies of ibuprofen: challenges and opportunities. Drug Dev Ind Pharm 2018;44:173–83. [DOI] [PubMed] [Google Scholar]
- [68].Moghadamnia Y, Kazemi S, Rezaee B, et al. New formulation of ibuprofen on absorption-rate: a comparative bioavailability study in healthy volunteers. Caspian J Intern Med 2019;10:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Aungst BJ. Optimizing oral bioavailability in drug discovery: an overview of design and testing strategies and formulation options. J Pharm Sci 2017;106:921–9. [DOI] [PubMed] [Google Scholar]


