Supplemental Digital Content is available in the text
Keywords: APC, bioinformatics, gastric cancer, meta-analysis, methylation
Abstract
Background:
The methylation status of the adenomatous polyposis coli (APC) promoter has been shown to be associated with the occurrence of gastric cancer, but this finding remains controversial. The aim of this study was to investigate the relationship between methylation of the APC gene promoter and gastric cancer.
Methods:
We searched the Web of Science, EMBASE, Medline, and Cochrane Central Register of Controlled Trials (CENTRAL) databases from the date of creation until August 1, 2019. According to the inclusion criteria, the relationship between the methylation status of the APC gene promoter and gastric cancer was investigated. The incidence of APC promoter methylation in the tissues or blood of patients with and without gastric cancer was compared. The results are expressed as the odds ratio (OR) and 95% confidence interval (CI). The pooled OR of each study was estimated using a fixed effects model or a random effects model to generate forest plots. We further validated the results using the MethHC database.
Results:
Eight studies (985 samples) were included. Our meta-analysis showed that the incidence of APC promoter methylation in patients with gastric cancer was higher than that of patients without gastric cancer (OR = 3.86, 95% CI 1.71–8.74, P = .001). Methylation of the APC promoter is associated with the incidence of gastric cancer, and it increases the risk of gastric cancer.
Conclusion:
This study provides a new strategic direction for research on gastric cancer. Methylation of the APC promoter may be a potential biomarker for the diagnosis of gastric cancer, but the results of this work require further confirmation.
1. Introduction
Gastric cancer, the fifth most frequently diagnosed cancer, is the third leading cause of cancer-related deaths, with more than 10,000 new cases and more than 780,000 deaths reported in 2018.[1] As 80% of patients with gastric cancer have no symptoms in the early stage and have progressed to an advanced stage after diagnosis, the 5-year survival rate of gastric cancer patients (GCPs) is as low as 30%.[2] Therefore, early diagnosis and treatment are particularly important. The detection of serum tumor markers is a noninvasive diagnostic method that has been widely used in the clinic.[3,4] However, the traditional detection methods for carcinoembryonic antigen (CEA) and carbohydrate antigen 19–9 (CA19–9) are neither sensitive nor specific for the accurate diagnosis of gastric cancer.[5,6] Therefore, there is an urgent need for a new noninvasive molecular biomarker that not only improves the efficiency of early diagnosis but also guides treatment and improves prognosis. Epigenetic alterations in tumor-related genes are involved in the pathogenesis and development of gastric cancer, and they may be used as markers of cancer diagnosis and treatment.[7] As a manifestation of epigenetics, DNA methylation is an important mechanism of gene expression regulation. The abnormal hypermethylation of promoters leads to a loss of tumor gene function.[8] Adenomatous polyposis coli (APC) is an important tumor suppressor gene that participates in different molecular pathways and causes epigenetic inactivation in a variety of cancers.[9–11] In addition, methylation of the APC gene promoter is closely related to the occurrence and development of gastric cancer.[12] Although some studies have shown that APC promoter methylation is associated with the risk of gastric cancer, some studies have reported conflicting results.[13,14] Thus, it remains unclear whether methylation of the APC promoter is associated with the risk of gastric cancer. Therefore, we conducted a meta-analysis to assess the relationship between APC promoter methylation and the incidence of gastric cancer. This study aimed to provide a new research direction and biomarkers for the diagnosis of gastric cancer.
2. Materials and methods
The study protocol was registered through PROSPERO (http://www.crd.york.ac.uk/PROSPERO) under registration number CRD42019145955, and the study protocol can be found online at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=145955.
2.1. Search strategy
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria.[15] We searched the PubMed (www.ncbi.nlm.nih.gov/pubmed), EMBASE (www.embase.com), Cochrane Library (www.cochranelibrary.com), and Web of Science (http://apps.webofknowledge.com) databases. Articles published between the time the database was created until July 2, 2019, in English were included. The search strategy included a combination of medical subject heading (MESH) and EMTREE keyword searches. The following search terms were included: “Gastric Cancer”, “Gastric Carcinoma”, “Gastric Neoplasms”, “Stomach Cancer”, “Stomach Carcinoma”, “Stomach Neoplasms”, “Adenomatous Polyposis Coli”, “APC” and “Methylation”. The detailed search strategy is shown in Additional file: Table S1.
2.2. Inclusion criteria
This meta-analysis was implemented according to the following inclusion criteria. All original studies on the relationship between APC methylation and gastric cancer published in Chinese or English were included. All data included in the studies were complete, had similar purposes and used similar statistical methods. In addition, the GCPs selected in each study met the pathological diagnostic criteria of gastric cancer. Samples in the experimental group were derived from patients with gastric cancer, and samples in the control group were derived from patients with nongastric cancer. In each study, the PCR-based methylation assay was used to detect the methylation levels of the APC gene. Furthermore, data on the methylation levels of APC in the tissues or blood samples of patients with gastric cancer and normal or benign patients were complete.
2.3. Exclusion criteria
The exclusion criteria were as follows: studies performed only using animals or cells instead of patients; review articles; studies that did not provide relevant data on the relationship between methylation of the APC gene promoter and the occurrence of gastric cancer; defectively designed studies; and studies with incomplete data.
2.4. Study selection and data extraction
According to the inclusion and exclusion criteria, 2 researchers independently searched the literature and then evaluated whether the title and abstract met the inclusion and exclusion criteria. If it was difficult to make a judgment based on the title and abstract, the full text was consulted for verification, and any differences included in the study were resolved by discussion with a third investigator. Data extraction was also independently performed by 2 researchers, and any disagreements or problems were solved through group discussions. The extracted data included the first author, year of publication, country, methylation detection method, specimen type, control type, number of participants, and incidence of APC promoter methylation in the case and control groups.
2.5. Quality assessment
The quality of all included studies was evaluated by the Newcastle-Ottawa Scale (NOS) for case-control studies.[16] The assessment of quality was based on the following 3 parameters: selection, comparability, and outcomes. Studies could receive a maximum possible score of nine stars. The NOS scores were characterized as follows: scores of 7 to 9 represented high-quality reports, scores of 4 to 6 represented medium-quality reports, and scores of 0 to 3 represented low-quality reports.
2.6. Statistical analysis
The outcome was the incidence of gastric cancer. The odds ratios (ORs) and 95% confidence intervals (CIs) were used to determine the estimated effect size of the outcome. The fixed effects or random effects model was used to generate forest plots. In addition, heterogeneity was measured by the Higgins I2 statistic and Cochran Q test, and if I2 < 50% or P > .1, heterogeneity was not considered significant. If the heterogeneity was not significant, the fixed effects model was used; if it was significant, the random effects model was used. Stratified analysis was used to further investigate the potential sources of heterogeneity. Begg funnel plot[17] and Egger linear regression[18] were used to assess potential publication bias. Funnel plots were assessed visually for asymmetry. All statistical analyses were performed using STATA 14.0 (College Station, TX, 77845, Serial number: 401406267051).
2.7. Bioinformatics analysis
MethHC (http://methhc.mbc.nctu.edu.tw/php/index.php) is a database for human pan-cancer gene expression, methylation and microRNA expression. Based on this database, we further explored the incidence of APC promoter methylation in gastric cancer and normal gastric tissues.
3. Results
3.1. Study selection
Based on the search strategy described in the Materials and Methods, 192 studies were preliminarily screened, while 91 studies were retained after deleting duplicate articles. After screening the titles and abstracts, 38 articles were excluded, and the remaining 53 studies related to the results of this study were reviewed in full. Finally, 985 samples from 8 studies[13,14,19–24] were included in this meta-analysis. The flow diagram is shown in Figure 1.
Figure 1.
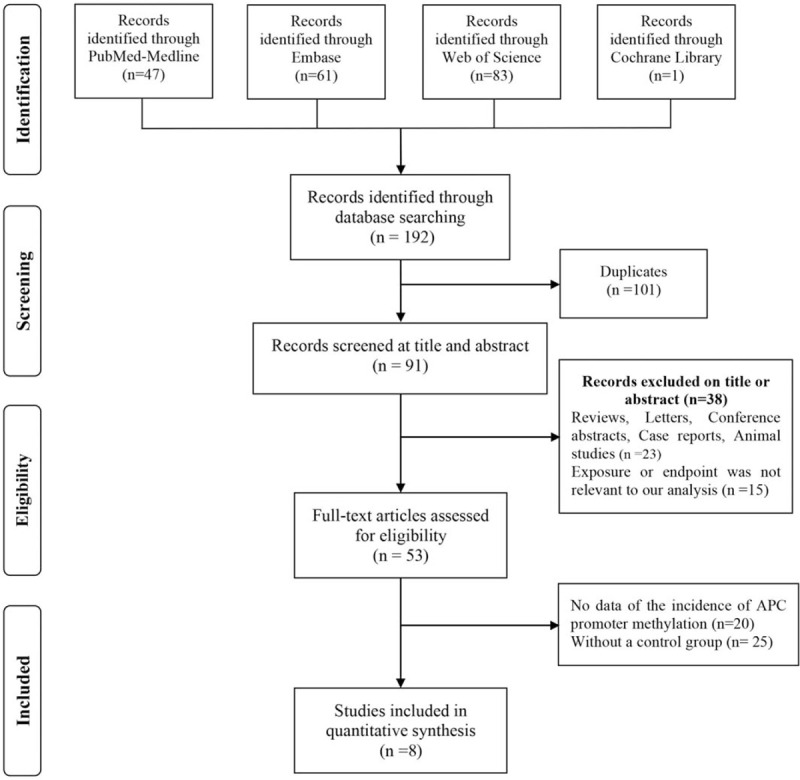
Flow diagram used to assess the evidence following Preferred Reporting Items for Systematic Review and Meta-analysis guidelines.
3.2. Study characteristics
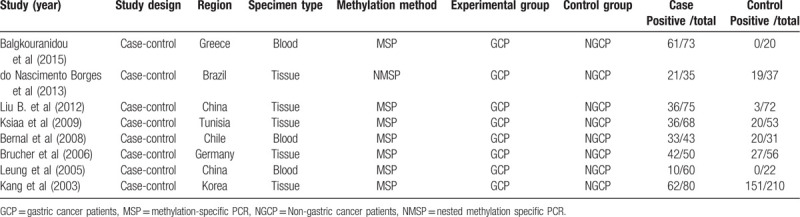
All 8 studies were case-control studies from Greece, Brazil, China, Tunisia, Chile, Germany, or South Korea. Five of the 8 studies utilized tissue specimens, and 3 studies utilized blood specimens. The methylation status was detected by methylation-specific polymerase chain reaction or nested methylation-specific polymerase chain reaction in all studies. The methylation frequencies of the APC promoter in the experimental and control groups are summarized in Table 1.
Table 1.
Characteristics of studies included in the meta-analysis.
3.3. Quality assessment
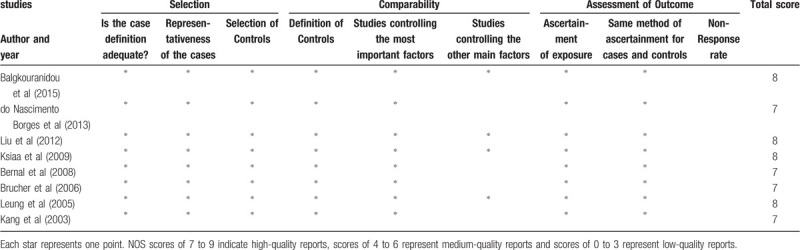
All 8 eligible studies were case-control studies, and the NOS scores were greater than or equal to 7 points. The details of each study are shown in Table 2.
Table 2.
The Newcastle–Ottawa Scale (NOS) to assess the quality of the case–control studies.
3.4. Meta-analysis
3.4.1. The relationship between APC promoter methylation and the incidence of gastric cancer
All 8 studies reported the relationship between APC promoter methylation and the incidence of gastric cancer. Because of the significant heterogeneity between studies (Q = 32.88, P = .000, I2 = 78.7%, Tau2 = 0.9534), we used the random effects model. The meta-analysis showed that the incidence of APC promoter methylation in GCPs was higher than that in nongastric cancer patients (NGCP) (OR = 3.86, 95% CI 1.71–8.74, P = .001) (Fig. 2). Thus, these findings demonstrate that APC promoter methylation is associated with the incidence of gastric cancer.
Figure 2.
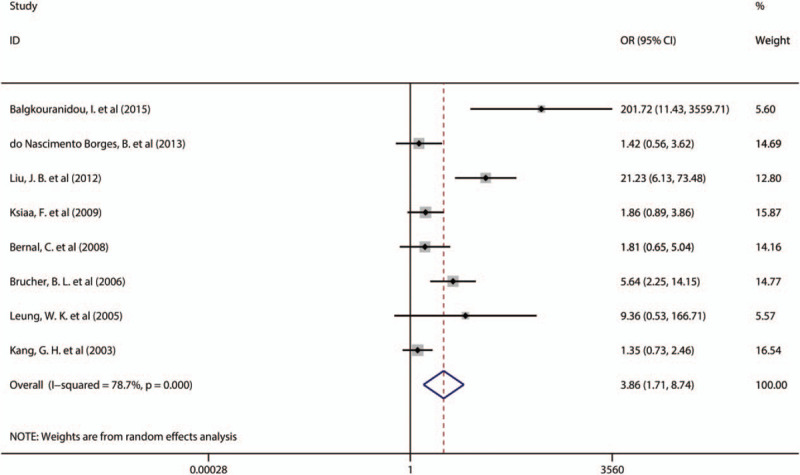
Forest plots of the association between methylation of the adenomatous polyposis coli promoter and gastric cancer risk. OR = odds ratio, CI = confidence interval.
3.4.2. Stratified analysis and meta-regression
To explore the sources of heterogeneity, we performed the meta-regression analysis and found that the methylation detection method (P = .719), the specimen type (P = .592) and the region (P = .372) were not sources of heterogeneity. When we used methylation detection methods or sample types as subgroups for analysis, we found no source of heterogeneity (Fig. 3A, B). However, when country was used as a subgroup, the heterogeneity between studies from China was low (Q = 0.27, P = .606, I2 = 0.0%, Tau2 = 0.000). The results of the subgroup analysis indicated that the heterogeneity of this meta-analysis may be due to the inclusion of studies from different countries. The results of the heterogeneity test are displayed in Additional file: Table S2.
Figure 3.
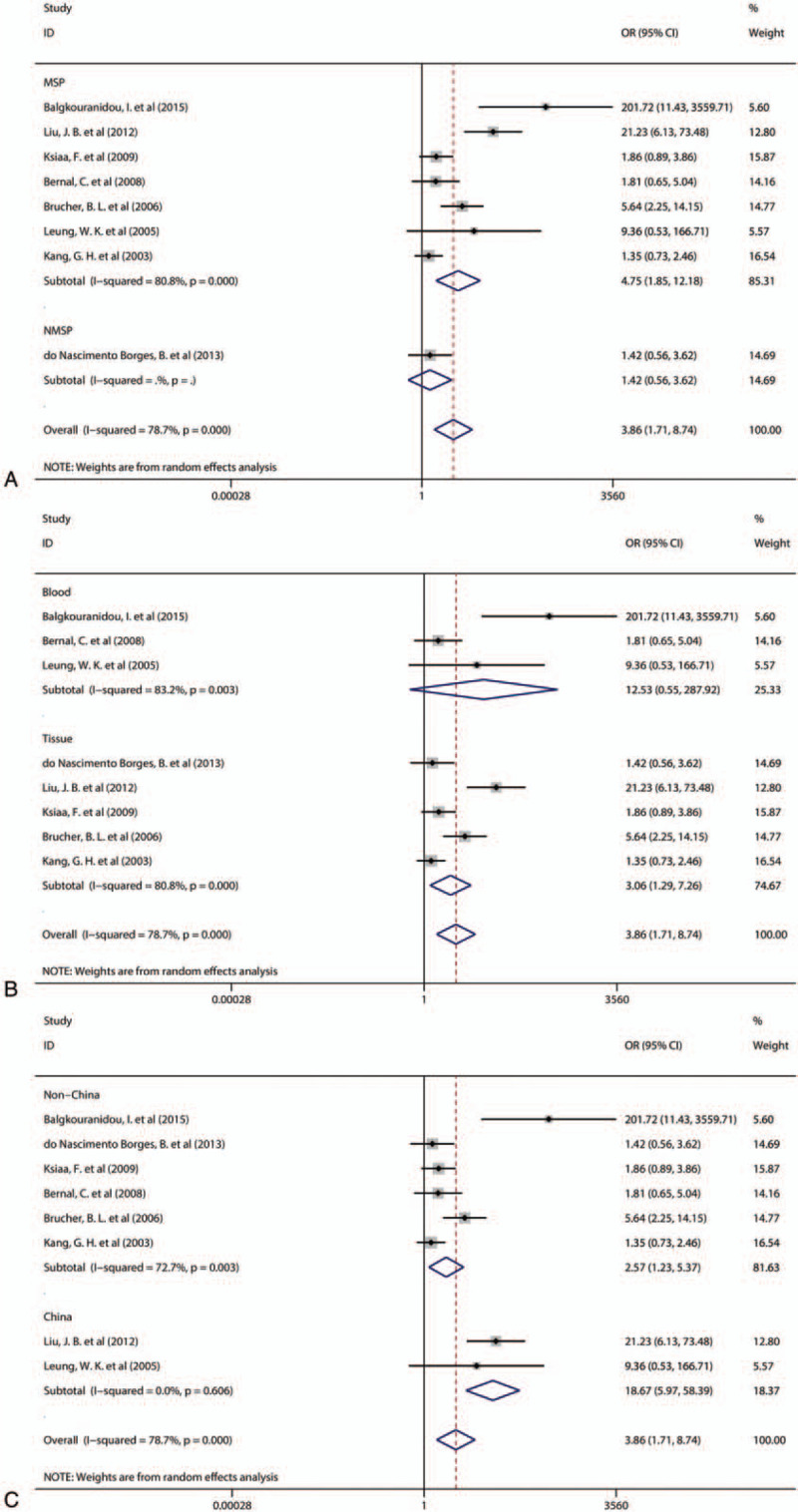
A: Forest plots of the subgroup analysis according to methylation detection methods. B: Forest plots of the subgroup analysis according to sample type. C: Forest plots of the subgroup analysis according to country. OR = odds ratio, CI = confidence interval.
3.4.3. Sensitivity analyses
To evaluate the stability of this meta-analysis, a sensitivity analysis was performed. The sensitivity analysis verified the impact of any study on the total estimate by omitting 1 study at a time. The results showed that the removal of any of the 8 studies had no significant effect on the results, indicating that this meta-analysis is robust and reliable (Fig. 4).
Figure 4.
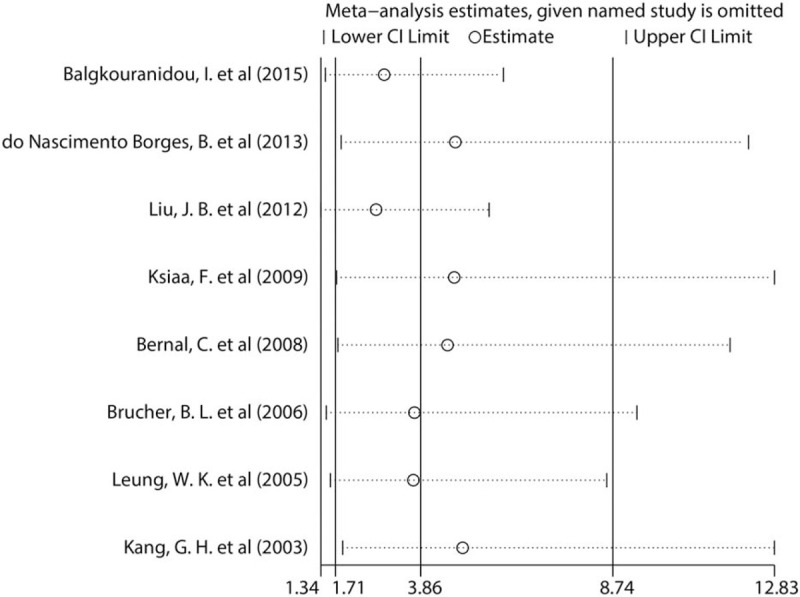
Sensitivity analysis of the association between methylation of the adenomatous polyposis coli promoter and the incidence of gastric cancer.
Due to the small number of studies included in this meta-analysis (<10), we did not construct funnel plots. Because the test efficiency is low when the number of studies is too few, this method cannot be used to test whether a funnel diagram is asymmetric.[25]
3.4.4. Validation of the results based on the MethHC database
To further verify the relationship between APC promoter methylation and the incidence of gastric cancer, we used a database of DNA methylation and gene expression in human cancer. The results showed that methylation of the APC promoter in transcripts NM_000038 and NM_001127510 was more likely to occur in gastric cancer samples than in normal gastric samples. However, methylation of the APC promoter in transcript NM_001127511 was less likely to occur in gastric cancer samples than in normal gastric samples (Fig. 5).
Figure 5.
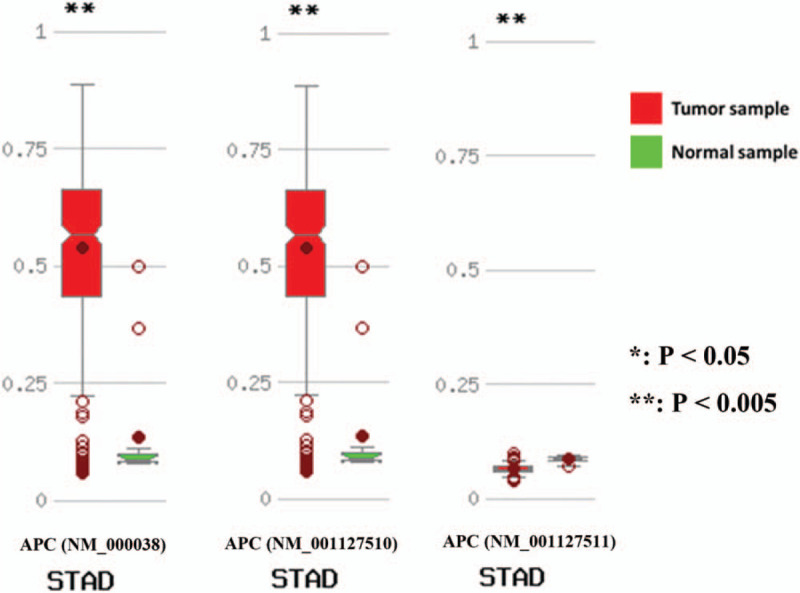
Bioinformatics analysis of adenomatous polyposis coli promoter methylation in gastric cancer.
4. Discussion
After a systematic review of the literature, this meta-analysis assessed the relationship between APC promoter methylation and the incidence of gastric cancer in 8 studies. Our results indicated that the incidence of APC promoter methylation was much higher in GCPs than in NGCPs.
There is increasing evidence that the promoter methylation of some tumor-associated genes may be a noninvasive biomarker.[26–28] Of course, as a tumor suppressor gene, aberrant methylation of the APC promoter usually occurs in different cancers, including breast cancer,[29] lung cancer,[30] prostate cancer,[31] colon cancer,[32] and gastric cancer.[13] APC is a gene that negatively regulates WNT signaling and is methylated in 34% to 83% of gastric cancers, but mutations in APC are rare.[33] Therefore, APC promoter methylation may be used as a diagnostic marker for gastric cancer. Balgkouranidou et al[13] detected the APC promoter methylation status in the blood of 73 patients with gastric cancer and 20 normal controls, and they reported APC methylation in 83.6% of GCPs, but APC promoter methylation was not observed in blood from the normal controls. However, other studies have shown no significant difference in the incidence of APC promoter methylation between gastric cancer and NGCPs.[14,21] Therefore, the topic of whether APC promoter methylation can increase the incidence of gastric cancer remains controversial.
Our meta-analysis showed that the methylation frequency of the APC promoter in GCPs was higher than that in NGCPs. These findings suggest that methylation of the APC promoter may play an important role in the pathogenesis of gastric cancer. The subgroup analysis showed that APC promoter methylation was associated with the incidence of gastric cancer in China (OR = 3.86, 95% CI, 1.71–8.704, P = .000) and countries other than China (OR = 2.57, 95% CI, 1.23–5.37, P = .012). However, China is more relevant than other countries. Finally, we further verified that the frequency of APC promoter methylation in gastric cancer samples was higher than that in normal gastric samples based on the bioinformatics analysis.
This meta-analysis has the following advantages. First, this meta-analysis confirmed that methylation of the APC promoter is associated with the incidence of gastric cancer, which may provide a new research direction for the diagnosis and treatment of gastric cancer. Second, the eight studies included in this meta-analysis were strictly in accordance with the inclusion and exclusion criteria, and they had high NOS scores, a wide range of samples, and good representativeness. Finally, the sensitivity analysis showed no significant change in the summary results after eliminating any of the 8 studies. Therefore, the results of this meta-analysis are robust and reliable.
However, this meta-analysis still had some limitations. First, we did not generate a funnel plot to detect publication bias because only 8 studies (<10) were included in this meta-analysis. Second, there were some confounding factors beyond our control that may have affected our results. Third, some of the original studies did not provide complete data on the age of patients, organization type and stage, preventing a comprehensive subgroup analysis. Fourth, when we searched the literature, we found that all the studies that met the inclusion criteria were case-control studies, and more randomized controlled trials might be needed to provide more reliable evidence.
Our results suggest that methylation of the APC promoter may increase the risk of gastric cancer. This study provides a new strategic direction for research on gastric cancer. Until these findings are confirmed, methylation of the APC promoter may be a potential biomarker for the diagnosis of gastric cancer. However, many randomized controlled clinical studies are needed to confirm this conclusion.
Acknowledgments
We would like to thank the Chinese Evidence Based Medicine Center at West China Hospital of Sichuan University for providing Stata 14.0 statistical software.
Author contributions
XZ and XH designed the research. XZ, DJ, MD, WZ and JC performed data acquisition and data analysis. ZL, HH and LL assisted with data acquisition and data analysis. HH, XZ, DJ and JC performed the statistical analysis. XZ, DJ and MD wrote the manuscript. All authors read and approved the final version of the manuscript.
Xinwei Han orcid: 0000-0003-4407-4864.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: APC = adenomatous polyposis coli, CI = confidence interval, GCP = gastric cancer patient, NGCP = Nongastric cancer patient, NOS = Newcastle-Ottawa Scale; OR = odds ratio.
How to cite this article: Zhou X, Jiao D, Dou M, Zhang W, Chen HH, Li Z, Li L, Han X. Association of APC gene promoter methylation and the risk of gastric cancer: a meta-analysis and bioinformatics study. Medicine. 2020;99:16(e19828).
XZ and DJ contributed equally to this work.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. The bioinformatics datasets generated and/or analyzed during the current study are available in the MethHC database (http://methhc.mbc.nctu.edu.tw/php/index.php).
The authors have no funding and conflicts of interests to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Lyons K, Le LC, Pham YT, et al. Gastric cancer: epidemiology, biology, and prevention: a mini review. Eur J Cancer Prev 2019;28:397–412. [DOI] [PubMed] [Google Scholar]
- [3].Park DJ, Seo AN, Yoon C, et al. Serum VEGF-A and tumor vessel VEGFR-2 levels predict survival in Caucasian but not Asian patients undergoing resection for gastric adenocarcinoma. Ann Surg Oncol 2015;22 Suppl 3:S1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kochi R, Yajima S, Nanami T, et al. Five-year postsurgical monitoring of serum p53 antibody for locally advanced esophageal squamous cell carcinoma. Clin J Gastroenterol 2018;11:278–81. [DOI] [PubMed] [Google Scholar]
- [5].Kanda M, Kodera Y. Recent advances in the molecular diagnostics of gastric cancer. World J Gastroenterol 2015;21:9838–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].He CZ, Zhang KH, Li Q, et al. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol May 2013;13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tahara E. Molecular mechanism of stomach carcinogenesis. J Cancer Res Clin Oncol 1993;119:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fukushige S, Horii A. DNA methylation in cancer: a gene silencing mechanism and the clinical potential of its biomarkers. Tohoku J Exp Med 2013;229:173–85. [DOI] [PubMed] [Google Scholar]
- [9].Zhao YF, Zhang YG, Tian XX, et al. Aberrant methylation of multiple genes in gastric carcinomas. Int J Surg Pathol 2007;15:242–51. [DOI] [PubMed] [Google Scholar]
- [10].Dulaimi E, Hillinck J, Ibanez de Caceres I, et al. Tumor suppressor gene promoter hypermethylation in serum of breast cancer patients. Clin Cancer Res 2004;10:6189–93. [DOI] [PubMed] [Google Scholar]
- [11].Dong SM, Kim HS, Rha SH, et al. Promoter hypermethylation of multiple genes in carcinoma of the uterine cervix. Clinical cancer research: an official journal of the American Association for Cancer Research 2001;7:1982–6. [PubMed] [Google Scholar]
- [12].Tamura G, Maesawa C, Suzuki Y, et al. Primary gastric carcinoma cells frequently lose heterozygosity at the APC and MCC genetic loci. Jpn J Cancer Res: Gann 1993;84:1015–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Balgkouranidou I, Matthaios D, Karayiannakis A, et al. Prognostic role of APC and RASSF1A promoter methylation status in cell free circulating DNA of operable gastric cancer patients. Mutat Res 2015;778:46–51. [DOI] [PubMed] [Google Scholar]
- [14].do Nascimento Borges B, Burbano RM, Harada ML. Analysis of the methylation patterns of the p16 INK4A, p15 INK4B, and APC genes in gastric adenocarcinoma patients from a Brazilian population. Tumour Biol 2013;34:2127–33. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269.w264. [DOI] [PubMed] [Google Scholar]
- [16].Wallis CJD, Saskin R, Choo R, et al. Surgery versus radiotherapy for clinically-localized prostate cancer: a systematic review and meta-analysis. Eur Urol 2016;70:21–30. [DOI] [PubMed] [Google Scholar]
- [17].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [18].Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ 1998;316:469.author reply 470-461. [PMC free article] [PubMed] [Google Scholar]
- [19].Liu JB, Wu XM, Cai J, et al. CpG island methylator phenotype and Helicobacter pylori infection associated with gastric cancer. World J Gastroenterol 2012;18:5129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ksiaa F, Ziadi S, Amara K, et al. Biological significance of promoter hypermethylation of tumor-related genes in patients with gastric carcinoma. Clin Chim Acta 2009;404:128–33. [DOI] [PubMed] [Google Scholar]
- [21].Bernal C, Aguayo F, Villarroel C, et al. Reprimo as a potential biomarker for early detection in gastric cancer. Clin Cancer Res 2008;14:6264–9. [DOI] [PubMed] [Google Scholar]
- [22].Brucher BL, Geddert H, Langner C, et al. Hypermethylation of hMLH1, HPP1, p14(ARF), p16(INK4A) and APC in primary adenocarcinomas of the small bowel. Int J Cancer 2006;119:1298–302. [DOI] [PubMed] [Google Scholar]
- [23].Leung WK, To KF, Chu ES, et al. Potential diagnostic and prognostic values of detecting promoter hypermethylation in the serum of patients with gastric cancer. Br J Cancer 2005;92:2190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kang GH, Lee S, Kim JS, et al. Profile of aberrant CpG island methylation along the multistep pathway of gastric carcinogenesis. Lab Invest 2003;83:635–41. [DOI] [PubMed] [Google Scholar]
- [25].Lau J, Ioannidis JP, Terrin N, et al. The case of the misleading funnel plot. BMJ 2006;333:597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhou X, Jiao D, Dou M, et al. Association of glutathione-S-transferase p1 gene promoter methylation and the incidence of prostate cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2019;145:1939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Potter NT, Hurban P, White MN, et al. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clin Chem 2014;60:1183–91. [DOI] [PubMed] [Google Scholar]
- [28].Hoque MO, Begum S, Topaloglu O, et al. Quantitative detection of promoter hypermethylation of multiple genes in the tumor, urine, and serum DNA of patients with renal cancer. Cancer Res 2004;64:5511–7. [DOI] [PubMed] [Google Scholar]
- [29].Qian X, Ruan L. APC gene promoter aberrant methylation in serum as a biomarker for breast cancer diagnosis: a meta-analysis. Thoracic Cancer 2018;9:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Feng H, Zhang Z, Qing X, et al. Promoter methylation of APC and RAR-beta genes as prognostic markers in non-small cell lung cancer (NSCLC). Exp Mol Pathol 2016;100:109–13. [DOI] [PubMed] [Google Scholar]
- [31].Wang Y, Fan C, Yu J, et al. APC methylation predicts biochemical recurrence of patients with prostate cancer: a meta-analysis. Int J Clin Exp Med 2015;8:15575–80. [PMC free article] [PubMed] [Google Scholar]
- [32].Ding Z, Jiang T, Piao Y, et al. Meta-analysis of the association between APC promoter methylation and colorectal cancer. OncoTargets Ther 2015;8:211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tamura G, Sato K, Akiyama S, et al. Molecular characterization of undifferentiated-type gastric carcinoma. Lab Invest 2001;81:593–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


