We assess the efficacy of an array of transgenic restriction factors in suppressing Plasmodium infection in the mosquito vector.
Abstract
The malaria parasite’s complex journey through the Anopheles mosquito vector provides multiple opportunities for targeting Plasmodium with recombinant effectors at different developmental stages and different host tissues. We have designed and expressed transgenes that efficiently suppress Plasmodium infection by targeting the parasite with multiple independent endogenous and exogenous effectors at multiple infection stages to potentiate suppression and minimize the probability for development of resistance to develop. We have also addressed the fitness impact of transgene expression on the mosquito. We show that highly potent suppression can be achieved by targeting both pre-oocyst stages by transgenically overexpressing either the endogenous immune deficiency immune pathway transcription factor Rel2 or a polycistronic mRNA encoding multiple antiparasitic effectors and simultaneously targeting the sporozoite stages with an anti-sporozoite single-chain antibody fused to the antiparasitic protein Scorpine. Expression of the selected endogenous effector systems appears to pose a lower fitness cost than does the use of foreign genes.
INTRODUCTION
The malaria parasite Plasmodium falciparum is transmitted by Anopheles mosquitoes and causes malaria, which remains one of the most devastating diseases of humankind. The lack of effective vaccines and rapid development of drug-resistant parasites and insecticide-resistant mosquitoes have underscored the need to develop novel alternative strategies for disease control. Multiple approaches based on the development of engineered refractory mosquitoes incapable of transmitting the parasite have been explored and have gained leverage through the ongoing development of mosquito gene-drive technologies that can enable the replacement of malaria-susceptible mosquito populations with populations refractory to malaria (1–4).
Malaria parasites have to complete a complex journey in the mosquito vector to accomplish successful transmission to the human host. The insect’s innate immune system and physical barriers play important roles in this process, with a variety of host and restriction factors influencing Plasmodium’s success in finally moving through the mosquito into the human host (5). Restriction factors can be used for Plasmodium blocking by being transgenically overexpressed in an appropriate tissue and at an appropriate time point to effectively target the parasites.
Most known Plasmodium restriction factors are components of the mosquito’s innate immune system, and the extensive study of this field over the past 20 years has generated a plethora of promising candidates for transgenesis [reviewed in (5–9)]. Blood meal–inducible expression of the mosquito’s nuclear factor κB (NF-κB) transcription factor Rel2, the positive regulator of the anti–P. falciparum immune deficiency (IMD) immune pathway, in the midgut or fatbody tissue results in potent suppression of multiple P. falciparum isolates without measurable fitness cost under laboratory conditions (10, 11). One can also use exogenous restriction factors to suppress Plasmodium infection, either by directly killing the parasites or by interfering with their interaction with the midgut or salivary glands (12–18). A variety of antimicrobial peptides (AMPs) such as Melittin, Scorpine, and Shiva toxin have been shown to exert in vitro and in vivo anti-Plasmodium activity. Expression of a modified P. falciparum circumsporozoite protein (CSP)–targeting single-chain antibody (m2A10) has been shown to greatly decrease the ability of the parasites to reach and invade the Anopheles salivary glands (19). An example of small peptides that can block the parasite’s interaction with the mosquito midgut is the Plasmodium enolase-plasminogen interaction peptide (EPIP), which prevents the binding of plasminogen to the ookinete surface during the parasite’s invasion of the midgut epithelium (16, 20). One can also combine parasite-binding single-chain antibodies with antiparasitic peptides to potentiate Plasmodium targeting and killing (19, 21). Transgenic mosquitoes expressing a parasite-binding ScFv fused to the anti-Plasmodium peptide CecA have been able to achieve almost complete parasite suppression by targeting the sporozoites alone (19).
The malaria parasite has a notorious ability to develop resistance to antimalarial drugs, and this feature may also enable it to develop resistance to mosquito-encoded blocking mechanisms. For this reason, it is beneficial to engineer mosquitoes in ways that would suppress Plasmodium infection at multiple sporogonic stages through independent mechanisms, akin to using combinatorial drug therapy for pathogen suppression. Here, we have explored the Plasmodium-blocking efficacy of single or combinations of versatile endogenous and exogenous transgenic anti-Plasmodium restriction factors that can target multiple parasite stages in different mosquito body compartments. For targeting early stages of Plasmodium infection in the midgut tissue, we explored the carboxypeptidase promoter (AgCp)–driven expression of the endogenous IMD pathway transcription factor Rel2, which controls multiple anti–P. falciparum effectors (10), and the simultaneous expression of multiple anti-Plasmodium effectors (Melittin, TP10, Shiva1, EPIP, and Scorpine) from a single transgene array. For inhibition of post-ookinete infection stages, we used the blood meal–inducible fatbody-specific vitellogenin promoter (AsVg) to express either an anti-sporozoite stage single-chain antibody (ScFv of m2A10 targeting CSP) alone or fused with the antiparasitic protein Cecropin C (or Cec3, AGAP000694), Apis mellifera phospholipase PLA2 (XM_391951), or Scorpine toxin (16, 22, 23). We also explored the combination of both midgut- and fatbody-expressed anti-Plasmodium effector systems to optimize parasite suppression. Last, we have assessed the fitness of the various transgenic mosquito lines, as measured by longevity, size, and fecundity.
In summary, we have addressed the following key requirements for transgenic Anopheles mosquitoes that would render them suitable for a population suppression–based malaria control strategy: (i) effector transgenes that efficiently suppress Plasmodium infection; (ii) targeting of the malaria parasite with multiple independent effectors to potentiate suppression and minimize the probability for the development of resistance; (iii) spatiotemporal specificity in expressing these transgenes for effective targeting of different developmental stages of the parasite; and (iv) transgene selection to allow minimal fitness cost to the mosquitoes.
RESULTS
Targeting the malaria parasite in the midgut with multiple antiparasitic effectors
Several endogenous and exogenous transgenes have been shown to effectively suppress P. falciparum infection in the midgut tissue. We have previously shown that blood meal–inducible midgut expression of the IMD immune pathway transcription factor Rel2 results in an up-regulation of multiple anti–P. falciparum effectors and a potent suppression of infection (10). The up-regulation of multiple effectors likely potentiates suppression and renders it more difficult for the parasite to develop resistance to the blocking mechanisms. Here, we have explored an alternative approach to unleash a multifactorial attack against the malaria parasite in the midgut tissue at the ookinete to pre-oocyst stage without the use of multiple independent transgenes (Fig. 1A). Previous studies have identified several small anti-Plasmodium peptides that suppress the parasites either through directly killing or by interfering with parasitic invasion of the midgut. We explored the blood meal–inducible midgut expression of a polycistronic mRNA encoding a polypeptide containing an array of five different anti–P. falciparum effectors separated by self-cleaving viral P2A sequences (Fig. 1B) (24). The five effectors are listed in Table 1: Melittin, TP10 dimer (TP10)2, Shiva1, four repeats of the Plasmodium EPIP4, and Scorpine (16, 20, 22, 23, 25, 26). The bee venom AMP Melittin has previously been shown to have in vitro anti-Plasmodium killing activity at 50 μM (27). The self-cleaving viral P2A sequences may also have enhanced the anti-Plasmodium activity of Melittin through the additional C-terminal modification (EENPG) derived from P2A (28). The cell-penetrating peptide TP10 has broad-spectrum antiparasitic activity against the blood and mosquito stages of P. falciparum and blood-stage Trypanosoma brucei (25, 26). Cell-penetrating peptides are molecules that can translocate into cells without causing membrane damage, and TP10 is one of the best characterized peptides of this class that can successfully translocate into various cell types (25, 26). EPIP prevents the binding of plasminogen to the ookinete surface during parasite invasion, thereby also inhibiting the parasite-vector interaction (16, 20). The scorpion venom protein Scorpine has antibacterial activity and can inhibit Plasmodium gametogenesis and ookinete formation at low concentrations both in vitro and in vivo (16, 22, 23). These five anti–P. falciparum effectors have previously been tested for anti-Plasmodium activity through in vitro or in vivo studies using synthetic peptides or para-transgenic approaches. Rather than developing individual transgenic lines for each effector, here, we explored the utility of expressing multiple antiparasitic effectors, through a single transgene cassette, for blocking the human malaria parasite.
Fig. 1. Generation of transgenic mosquitoes (MultiEff) using an AgCp-driven transgene array with five anti-Plasmodium effectors targeting the malaria parasite in the midgut, and P. falciparum infection phenotypes at both oocyst and sporozoite stages.
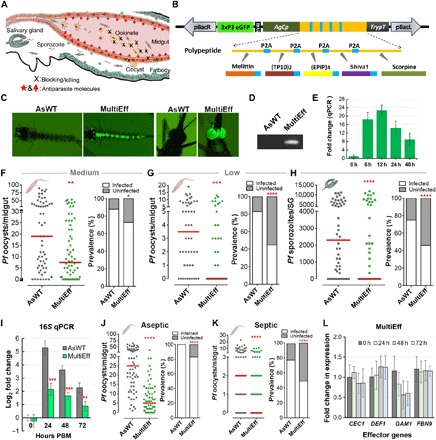
(A) Schematic illustration of transgenic targeting of parasite midgut infection stages and the design of the transgenes to target the parasites at this stage. (B) Five known anti-Plasmodium effectors (Melittin, TP10, EPIP, Shiva1, and Scorpine) were selected. The gene array with these five anti-Plasmodium effector genes was synthesized through GenScript Inc. and cloned under the AgCp promoter, followed by the trypsin terminator (TrypT) in the pBac[3xP3-EGFPafm] vector. These five antiparasitic effector genes were transcribed on one cassette separated by viral P2A sequences, translated into one polypeptide, and self-spliced into five individual peptides with an extra viral P2A amino acid tag on the first four peptides. (C) Fluorescent images of a positive larva and an adult transgenic mosquito. (D) Polymerase chain reaction (PCR) validation of the partial [500 base pairs (bp)] transgene cassette of MultiEff in the transgenic mosquitoes. (E) Transcript abundance of the transgene in the gut of MultiEff transgenic mosquitoes at various time points post-blood meal (PBM). Each bar represents the relative fold change in the transgene as compared to the control at time 0 hour. The S7 ribosomal gene was used to normalize the complementary DNA (cDNA) templates. Error bars indicate SEM. (F to H) P. falciparum (NF54) oocyst and sporozoite infection intensities and prevalence at 8 days post-infection (dpi) in the gut or 14 dpi in the salivary glands (SG) when fed on blood with a medium (0.05%) (F) or low (0.01%) (G and H) gametocytemia. At least three biological replicates were pooled for the dot plots. Each dot represents the number of parasites in an individual gut or a pair of salivary glands, with the median values indicated by red bars. P values were calculated by a Mann-Whitney test. Detailed statistical analysis is presented in table S2. (I) Midgut microbial flora of female transgenic MultiEff and WT control (AsWT) mosquitoes at 0-, 24-, 48-, and 72-hour PBM (mean ±SEM). (J and K) P. falciparum oocyst infection intensities and prevalence in the aseptic (antibiotic-treated) and septic (non–antibiotic-treated) transgenic and AsWT mosquitoes at 8 dpi. (L) Expression of AMP and anti-Plasmodium effector as fold change in expression through quantitative reverse transcription PCR (qRT-PCR). Error bars indicate SEM. CEC1, Cecropin 1; DEF1, Defensin 1; GAM1, Gambicin 1; FBN9, Fibrinogen-related protein 9.
Table 1. Selected AMPs and anti-Plasmodium peptides for transgenic expression in the genetically modified mosquitoes.
| Effectors | Characterization | Parasite stage(s) |
Function or mechanism |
Expression method |
Inhibition (%) | Reference |
| Melittin | Bee antimicrobial peptides |
Ookinete | Lysis of parasites | Synthesized | 100.0% | (27) |
| TP10 dimer | Wasp antimicrobial peptides |
Ookinete | Lysis of parasites | Synthesized | 100.0% | (25–27) |
| Shiva1 | Cecropin-like synthetic peptide |
Gametes Ookinete | Lysis of parasites | Para-transgenesis | 94.3% | (27) |
| EPIP four repeats | Enolase- plasminogen interaction peptide |
Ookinete | Block midgut invasion |
Para-transgenesis | 97.7% | (20) |
| Scorpine | Scorpion venom | Gametogenesis Ookinete |
Cecropin and defensin-like lytic peptide |
Para-transgenesis | 97.8% | (23) |
The coding sequences for these five effectors were codon-optimized and synthesized through GenScript Inc. (Supplementary Materials). The transgene cassette (hereafter referred to as MultiEff) was fused to the Anopheles gambiae Cp (AgCp) promoter including its signal peptide sequences and terminated with the A. gambiae trypsin gene terminator (TrypT), and then cloned into the piggyBac-based plasmid pBac[3xP3-EGFPafm] containing the eye-specific 3xP3 promoter–driven eGFP as an eye marker for screening of the positive transgenic mosquitoes at both the larval and adult stages, according to our previously published method (10). Through microinjection of the transformation construct plasmid, together with the phsp-pBac helper plasmid, into approximately 620 Anopheles stephensi embryos, we generated five pools of mosquitoes that were crossed with the wild type (AsWT), resulting in three AgCp-driven MultiEff transgenic mosquito lines (#2, #3, and #4) that showed stable expression of the GFP eye fluorescence in both larval and adult stages (Fig. 1C). Chromosomal integration of transgenes was confirmed by polymerase chain reaction (PCR) (Fig. 1D and table S1). The heterozygous G2 generation of the three transgenic lines was screened for P. falciparum suppression, allowing the selection of the MultiEff-2 line demonstrating the most potent anti–P. falciparum activity. The temporal expression specificity of the transgene was monitored through quantitative reverse transcription PCR (qRT-PCR), showing a >22-fold induction within a 6- to 12-hour time period after blood feeding (Fig. 1E).
To assess the resistance of homozygous transgenic MultiEff mosquitoes to P. falciparum, we fed them on both medium- and low-concentration gametocyte cultures (gametocytemia at 0.05 and 0.01%, respectively) and then investigated the infection phenotype. MultiEff transgenic mosquitoes displayed significant lower permissiveness to pre-oocyst stage P. falciparum when fed on blood with a medium gametocytemia (0.05%), resulting in an infection intensity unnaturally higher than that observed for field-caught mosquitoes. The midguts of the WT (AsWT) mosquitoes had a median of 19 oocysts and an infection prevalence of 88% (Fig. 1F and table S2). The prevalence at the oocyst infection stage decreased by 17.1% (Fisher’s exact test, P < 0.05; Fig. 1F and table S2), and the median infection intensity was reduced to 39.5% of AsWT control mosquitoes (Mann-Whitney test, P = 0.0034; Fig. 1F and table S2). When the mosquito cohorts were fed on a fivefold lower gametocytemia (0.01%) to mimic a natural infection system, the MultiEff transgenic mosquitoes displayed strong parasite suppression, with a median oocyst count of 0, whereas the AsWT control mosquitoes had a median of 3.5 oocysts per midgut (Fig. 1G). The MultiEff transgenic mosquitoes displayed an infection prevalence of 45%, in contrast to 83% for the AsWT (Fig. 1G and table S2). Under natural infection intensity conditions, the MultiEff transgenic mosquitoes displayed a profound suppression that resulted in a median sporozoite count of zero in the salivary glands. The sporozoite infection prevalence showed a 1.6-fold decrease in the MultiEff transgenic mosquitoes (46% in the MultiEff versus 75% in the control mosquitoes) (Fig. 1H and table S2).
Mosquitoes harbor a microbiota, including an intestinal flora comprising a variety of bacteria and fungi that can influence vector competence for Plasmodium by stimulating the mosquito’s innate immune system or through direct effects on the parasite (29). We have previously shown that transgenically immune enhanced mosquitoes have an altered midgut flora (10). Considering that some of our five selected anti-Plasmodium effectors are also AMPs and thereby might influence the midgut microbiota, we assayed the bacterial loads of transgenic and WT mosquitoes at 24- to 72-hour post-blood meal (PBM) as described in (11). As expected, the MultiEff transgenic line harbored a lower microbiota load (2 to 3 log2 decrease in 16S ribosomal RNA abundance) than the AsWT mosquitoes (Fig. 1I). To investigate whether the MultiEff-mediated suppression of the microbiota could counteract the antiparasitic activity of the recombinant effectors (30), we performed identical infection assays with MultiEff transgenic and AsWT mosquitoes under aseptic (antibiotic-treated) and septic (without antibiotic treatment) conditions. In the absence of midgut microbiota, both mosquito groups are more susceptible to P. falciparum infection, but the MultiEff-mediated suppression of the parasite, in terms of infection prevalence and intensity, remained at the same significance levels as that of the septic mosquitoes (P < 0.0001; Fig. 1, J and K, and table S2). The difference in infection prevalence between the septic transgenic and WT mosquito cohorts was greater than that between aseptic transgenic and WT cohorts, suggesting that the anti-Plasmodium activity exerted by the recombinant effectors is greater than that exerted by the bacteria. The mosquito microbiota has been shown to influence immune gene expression, including AMPs and anti-Plasmodium effectors (30). We therefore investigated whether the expression of the MultiEff transgene could influence the expression of the endogenous AMPs and anti–P. falciparum effector genes Cecropin 1 (CEC1), Defensin 1 (DEF1), Gambicin 1 (GAM1), and Fibrinogen-related protein 9 (FBN9) (7, 31). This assay only showed an insignificant reduction of endogenous GAM1 gene expression at 24- to 72-hour PBM (Fig. 1L; qRT-PCR primers are presented in table S1), suggesting that the modulation of Plasmodium infection is attributed to the expression of the recombinant effectors rather than the regulation of the mosquito immune system.
Targeting the sporozoite-stage malaria parasite in the hemolymph with single-chain antibodies fused to antiparasitic effectors
Moving forward, we hypothesized that incorporating another mechanism that would target the sporozoite stage would result in a greater resistance to P. falciparum (Fig. 2A). Previous studies have shown that transgenic expression of Cecropin A (CecA or CEC1) fused to a CSP-binding single-chain antibody (m2A10 ScFv) effectively blocks P. falciparum sporozoite-stage infection (19). We explored the three A. stephensi vitellogenin (AsVg) promoter–driven transgenes comprising the CSP-ScFv fused to either Cecropin C (CecScFv), PLA2 (PLAScFv), or Scorpine (ScorpScFv) for efficacy in blocking Plasmodium at the sporozoite stage (Fig. 2B). The transgene cassette, including the AsVg promoter, CecScFv, and AsVg 3′-UTR and terminator sequences, was codon-optimized (for ScFv-CSP), synthesized through GenScript Inc. (Supplementary Materials), and cloned into the pUC57-Kan cloning vector. The CecC signal peptide sequences and the coding sequences were flanked with Pme I and Spe I restriction sites for ease in cloning other effector genes. The PLA2 and Scorpine sequences were synthesized through GenScript Inc. (Supplementary Materials) with the same restriction sites for CecC, and PLA2 and Scorpine sequences were separately cloned to replace CecC on the pUC57-Kan vector. The three constructs were separately incorporated into the piggyBac-based plasmid pBac[3xP3-dsRed] containing the eye-specific 3xP3 promoter–driven dsRed as an eye marker to identify transgenic larvae and mosquitoes (Fig. 2, B and C). About 800 embryos were injected together with the helper plasmid phsp-pBac for each construct, and from the 20% of larvae that hatched, about 90% survived to adulthood and were outcrossed to control mosquitoes as described by Dong et al. (10). This outcrossing resulted in four, two, and two lines for CecScFv, ScorpScFv, and PLAScFv, respectively. All the insertions of the transgenes into the chromosomes of transgenic G2 larval offspring were confirmed through PCR on larval genomic DNA (gDNA) and the fluorescence markers in both larvae and adult mosquito eyes (Fig. 2, C and D, and table S1). The heterozygotes of the G3 generation of all the transgenic lines were screened for P. falciparum suppression. The CecScFv-4 and ScorpScFv-2 lines, but none of the PLAScFv lines, showed strong anti–P. falciparum activity (fig. S1) and were therefore selected for further study. Because the ScFv targets the sporozoite stage, whereas the transgenes are driven by the AsVg promoter that is induced several days (12- to 24-hour PBM) before sporozoite formation (reaching a >20-fold increase in recombinant transcript abundance) (Fig. 2E), we blood-fed the mosquitoes 5 and 9 days after the P. falciparum–infected blood meal to ensure an enrichment of recombinant protein at the time of sporozoite release from the oocysts (fig. S1). Positive transgenic mosquitoes were outcrossed with WT (AsWT) control mosquitoes for the first four generations, and homozygotes were obtained for the most potent Plasmodium-resistant lines.
Fig. 2. Generation of transgenic mosquitoes targeting the sporozoite-stage malaria parasite in the hemolymph with single-chain antibodies fused to antiparasitic effectors and P. falciparum infection phenotypes at both the oocyst and sporozoite stages.
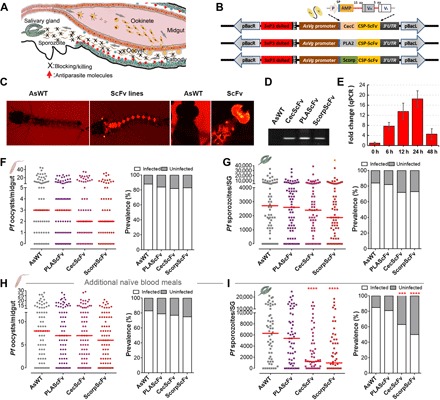
(A) Schematic illustration of transgenic targeting of parasite sporozoite infection stages and the design of AsVg-driven transgenes to be expressed in the fatbody after the blood meal, to specifically target the parasites at this stage. (B) Schematic representation of single-chain antibody (ScFv) targeting the CSP protein fused to AMP. The single-chain antibodies consist of variable regions of the VH heavy and VL light chains. Each transgene encodes a short 5–amino acid polypeptide linker between VH and VL and a long 15–amino acid (aa) sequence linking the VH to the AMP peptides (CecC, PLA2, and Scorpine), including the CecA signal peptide sequence (SP). Three individual transformation plasmids, pBAC-AsVg-CecC (or PLA2, Scorpine)–ScFv (with the red fluorescent eye reporter gene 3xP3 dsRed), were used for the germline transformation. AsVg promoter with the same AsVg endogenous terminator (AsVg 3′-UTR) was used. (C) Fluorescent images of a positive larva and an adult transgenic ScorpScFv mosquito. (D) PCR validation of the partial transgene cassette (~500 bp) of CecScFv, PLAScFv, and ScorpScFv in the transgenic mosquitoes. (E) Transcript abundance of the transgene in the fatbody of ScorpScFv transgenic line at various time points PBM. Each bar represents the relative fold change in the transgene as compared to the control at time 0 hour. The S7 ribosomal gene was used to normalize the cDNA templates. Error bars indicate SEM. (F to I) P. falciparum (NF54) oocyst and sporozoite infection intensities and prevalence of the three transgenic ScFv lines (PLAScFv, CecScFv, and ScorpScFv) at 8 dpi in the gut or 14 dpi in the salivary glands (SG) without (F and G) or with (H and I) additional naïve blood meals at days 5 and 9 post-infectious blood meal (PIBM). Each dot represents the number of parasites in an individual midgut or salivary glands, and the horizontal lines (red) indicate the median values. Detailed statistical analysis is presented in table S2.
Homozygous transgenic CecScFv (line 4), PLAScFv (line 2), and ScorpScFv (line 2) mosquitoes were fed on an NF54 P. falciparum gametocyte culture, and infection prevalence and intensity at both the oocyst and sporozoite stages were assayed (Fig. 2, F to I). As in earlier observations (19), the oocyst loads in the midguts of CecScFv and ScorpScFv mosquitoes showed a nonsignificant decrease either without or with additional naïve blood meals at 5 and 9 days after feeding on the infectious blood meal (PIBM) (P > 0.05; Fig. 2, F and H, and table S2). Without additional naïve blood meals, the salivary gland infection intensity of ScorpScFv decreased significantly (P < 0.05; Fig. 2G and table S2), whereas when the mosquitoes had been given additional blood meals, both the sporozoite load and infection prevalence of CecScFv and ScorpScFv, but not PLAScFv, mosquitoes were significantly reduced (Fig. 2I). The salivary gland infection prevalence decreased by 25.9 and 41.2% (χ2 test, P < 0.001, P < 0.0001; Fig. 2I and table S2), and the median infection intensity was reduced by 80.5 and 84.6% for CecScFv and ScorpScFv, respectively, when compared to the AsWT control mosquitoes (Mann-Whitney test, P < 0.0001; Fig. 2I and table S2).
Targeting the malaria parasite with multiple transgenes at multiple infection stages results in near-complete refractoriness
We then investigated whether expression of multiple anti-Plasmodium transgenes in both the midgut and fatbody compartments would potentiate resistance to P. falciparum infection. Dual transgene-expressing lines were generated by crossing virgin ScorpScFv homozygote females with the CpRel2 homozygote males (10, 11). The larval offspring were screened for both red and green fluorescent eye markers. These dual-transgene mosquitoes (referred to as hybrids, or Rel2ScFv) were fed on gametocyte cultures, along with the AsWT controls and the single-transgene mosquito lines ScorpScFv and CpRel2 (Fig. 3, A and B). The oocyst infection intensity and prevalence at 8 days post-infection (dpi) for the single-transgenic CpRel2 and hybrid line Rel2ScFv were significantly reduced (Fig. 3A and table S3). At 14 dpi, the sporozoite infection intensity and prevalence of the ScorpScFv, CpRel2, and hybrid (Rel2ScFv) lines were significantly suppressed, with the median sporozoite number in the Rel2ScFv salivary glands being zero, indicating near-complete refractoriness (Fig. 3, A and B). We also generated and tested a dual-transgene mosquito line expressing MultiEff in the midgut and ScorpScFv in the fatbody by crossing virgin ScorpScFv homozygote females with MultiEff homozygote males to obtain the hybrid line denoted MultiEffScFv. Feeding mosquitoes on a low-concentration gametocyte culture resulted in a significantly reduced oocyst infection intensity and prevalence in both the MultiEff and hybrid MultiEffScFv transgenic mosquitoes (Fig. 3C and table S3). The sporozoite infection intensity and prevalence in the salivary glands were also significantly suppressed when compared to AsWT, with the median infection intensity being zero for all three lines (ScorpScFv, MultiEff, and MultiEffScFv) (Fig. 3D and table S3). The sporozoite loads were significantly lower in both hybrid lines (Rel2ScFv and MultiEffScFv) compared to the corresponding individual transgenic lines CpRel2/ScorpScFv and MultiEff/ScorpScFv, respectively (Mann-Whitney test, P < 0.05; Fig. 3, B and D, and table S3). These data suggest that the spatiotemporal expression of multiple antiparasitic effectors targeting different stages of the parasite produces an elevated resistance level in the transgenic mosquitoes, as illustrated in Fig. 3E.
Fig. 3. The hybrid lines ScorpScFv with CpRel2 or with MultiEff targeting the malaria parasite at multiple infection stages result in near-complete refractoriness.
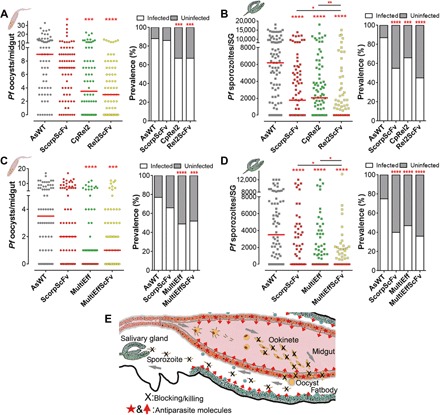
(A and B) P. falciparum (NF54) oocyst and sporozoite infection intensities and prevalence of two transgenic lines (ScorpScFv and CpRel2) and the hybrid line of the two transgene cassettes (Rel2ScFv) at 8 dpi in the gut (A) or 14 dpi in the salivary glands (SG) (B) with additional blood meals at days 5 and 9 PIBM. (C and D) P. falciparum (NF54) oocyst and sporozoite infection intensities of two transgenic lines (ScorpScFv and MultiEff) and the MultiEffScFv hybrid transgenic mosquitoes at 8 dpi in the gut (C) or 14 dpi in the salivary glands (SG) (D), with additional blood meals on days 5 and 9 PIBM. Assays were performed with at least four biological replicates, and the horizontal lines (red) indicate the median values. Mann-Whitney test was used to calculate P values and determine the significance of parasite numbers. A χ2 test was used to compare infection prevalence values. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Detailed statistical analysis is presented in table S3. (E) Schematic illustration of transgenic targeting of parasite midgut and sporozoite infection stages using carboxypeptidase and vitellogenin promoter-driven transgenes.
Fitness impact of CecScFv, ScorpScFv, and MultiEff effector gene expression
Because trade-offs between the expression of foreign genes and fitness parameters are likely to occur, we compared the longevity, mosquito body size, egg production, and hatch rate of the transgenic and AsWT control mosquitoes (Fig. 4). We measured the longevity of the transgenic lines when maintained on 10% sugar alone and when provided one naïve blood meal. The life span of the transgenic mosquitoes did not differ from that of the AsWT mosquitoes when maintained on a 10% sugar solution (Fig. 4A). Provision of a single naïve blood meal resulted in a significantly decreased longevity in the ScorpScFv and MultiEff mosquitoes but not in the CecScFv transgenic mosquitoes (Fig. 4B).
Fig. 4. Fitness effects of three transgenic lines with anti-Plasmodium activity.
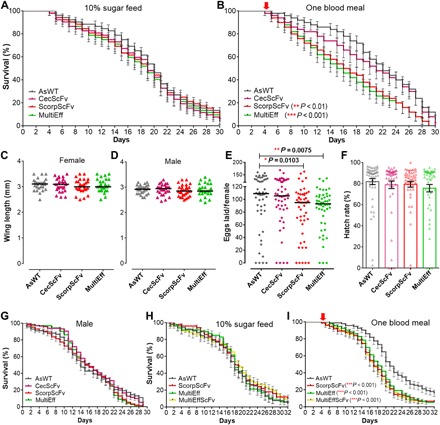
(A and B) Life spans of the female mosquitoes of the three transgenic A. stephensi lines maintained on 10% sucrose solution (A) or after one blood meal on mice (B) with WT control (AsWT). The life spans of ScorpScFv and MultiEff mosquitoes were significantly shorter than those of the controls when the mosquitoes were fed once on the naïve mice (P < 0.01 and P < 0.001, respectively). The pooled values from three replicates are shown, with SE. Survival rates were analyzed by Kaplan-Meier survival analysis with Wilcoxon test to determine the significance. (C and D) The wing lengths of the three anti-Plasmodium transgenic mosquito (CecScFv, ScorpScFv, and MultiEff) females or males did not differ from those of the control mosquitoes (AsWT). (E) Numbers of eggs laid by female homozygous transgenic ScorpScFv and MultiEff were significantly lower than those of the control AsWT mosquitoes. Each dot represents the eggs laid by an individual female after a single blood meal on mice. The median values (black horizontal bars) are shown. The P values were calculated with a Mann-Whitney test. (F) Hatch rates indicate the average percentage of eggs giving rise to first- and second-instar larvae, as shown with the bars indicating the mean values and SE. Each dot represents the hatch rate of the eggs laid by an individual female after blood meal. (G) Life spans of male mosquitoes of the three transgenic lines and AsWT controls maintained on 10% sucrose solution. (H and I) Comparison of life spans of the female hybrid transgenic line mosquitoes (MultiEffScFv) to the parental single transgenic lines (MultiEff and ScorpScFv) and the AsWT controls maintained on 10% sucrose solution (H) or after one naïve blood meal on mice (I).
None of the three transgenic mosquito lines had a body size different from that of the AsWT mosquitoes, as measured by the wing lengths of female and male mosquitoes (Fig. 4, C and D). Fecundity, measured as the number of eggs laid after a blood meal, was significantly lower for ScorpScFv and MultiEff transgenic mosquitoes than for the controls (Mann-Whitney test, P < 0.05 and P < 0.01, respectively; Fig. 4E). The egg hatch rate did not differ from the AsWT mosquitoes to any of the transgenics (Fig. 4F).
None of the transgenic lines showed any change in male longevity compared to the AsWT controls when reared at insectary conditions with a 10% sugar solution (Fig. 4G). The fitness of males is important to ensure spread of the transgene to offspring.
We also investigated the fitness of the hybrid transgenic line that targets multiple infection stages through expression of MultiEff and ScorpScFv in the midgut and fatbody tissues, respectively. We selected this hybrid line because both MultiEff and ScorpScFv transgenic lines showed significant decreased life spans after one blood meal (Fig. 4B). As shown in Fig. 4 (G and H), when the transgenic mosquitoes were maintained on the regular 10% sugar solution, neither the hybrid line nor the individual transgenic lines showed impaired life spans, and the hybrid transgenic line (MultiEffScFv) did not show a shorter life span after one naïve blood meal compared to the corresponding individual transgenic lines (MultiEff and ScorpScFv).
DISCUSSION
We have explored the use of versatile anti-Plasmodium transgenes, expressed in the mosquito midgut and fatbody compartments after blood feeding, to achieve refractoriness to the human malaria parasite P. falciparum in the Asian malaria vector A. stephensi.
The selection of effector molecules for parasite blocking has been facilitated by the existence of a variety of both mosquito-encoded endogenous and nonmosquito exogenous candidates. We have previously shown that transgenic midgut-specific, blood meal–inducible expression of the IMD pathway transcription factor gene Rel2 results in a multi-effector attack on the malaria parasite in the midgut lumen and epithelium, thereby achieving near-complete refractoriness (10). Transgenic midgut expression of Rel2 also provides a reproductive fitness advantage in a cage population because of a modification in the mosquito’s microbiota that, in turn, influences mate choice (11). We have now transgenically expressed multiple antiparasitic factors in the midgut of Anopheles mosquitoes from a single transgene through a construct that produces polycistronic mRNAs after blood feeding (28). While it is possible that this strategy results in a lower quantity of each of the effectors than would have been achieved through single-effector constructs (24), targeting the parasite with five different effectors surely impedes the development of resistance and may also potentiate blocking through synergistic effects (28).
Most studies on mosquito anti-Plasmodium immunity have focused on the pre-oocyst stages, and only relatively little is known about mosquito-encoded defense molecules that target the sporozoite-stage parasite. Inspired by earlier studies on transgenic mosquitoes expressing a CSP protein–targeting m2A10 single-chain antibody fused with Cecropin A, we developed transgenic mosquitoes expressing similar single-chain antibodies but fused to one of the three anti-Plasmodium effectors PLA2, CecC, or Scorpine. Both ScorpScFv and CecScFv transgenic mosquitoes showed increased resistance to Plasmodium. As predicted, the most effective suppression of sporozoites was achieved when the mosquitoes were provided additional naïve blood meals at 5 and 9 days after the infectious blood meal; this supplemental feeding resulted in the enrichment in recombinant antiparasitic effectors when the oocysts ruptured and released sporozoites (19). While the use of an adult-specific constitutive promoter would ensure the presence of antiparasitic recombinant proteins for Plasmodium targeting regardless of infection stage, such an approach would pose a high risk of adverse fitness effects. We therefore used two well-characterized and widely used blood meal–inducible promoters for driving our transgenes in the midgut or fatbody compartments after ingestion of the parasites. Provision of multiple blood meals also turns out to mimic a real field scenario because Anopheles mosquitoes acquire blood meals every 3 to 4 days throughout their life span (32).
Transgenic mosquitoes expressing the midgut-specific Rel2 or MultiEff in combination with the fatbody-specific ScorpScFv showed the most potent P. falciparum suppression. While expression of the midgut-specific effector transgenes Rel2 or MultiEff resulted in a sporozoite infection intensity and prevalence that were similar to those of the dual-transgenic mosquitoes, the expression of ScorpScFv or CecScFv alone in the fatbody tissue did not, suggesting that the most effective targeting of the Plasmodium parasite occurs in the midgut tissue, which is also considered the most important bottleneck in the malaria infection cycle (33). Nevertheless, coexpression of multiple effector transgenes that can target the parasite at multiple stages limits the probability of the emergence of parasite resistance and the loss of refractoriness should one of the transgenes become inactive. The emergence of parasite resistance to the transgenic blocking mechanisms is possible, but is very difficult to address experimentally, because no efficient animal models exists for studying transmission of the human malaria parasites. Using strategies such as those explored in our study can mitigate and delay the emergence of resistance, thereby providing a deployment time window that would have an epidemiologically significant impact on disease transmission.
While several of our tested transgenes and their combinations resulted in high-level resistance, total refractoriness was not attained in our assays. This may be a result of the high efficiency of our laboratory infection model or may indicate that the parasite targeting was not spatiotemporally perfect. It is also possible that some ookinete-stage parasites circumvented cells in which the effector transgenes were expressed or that some early or late events in midgut invasion or sporozoite translocation to the salivary glands occurred outside the peak concentration periods of the transgenic effectors. Nevertheless, several modeling studies have shown that total transmission blocking, or refractoriness, is not necessary to achieve an epidemiologically significant impact on disease prevalence and even a 35% transmission-blocking effect would result in malaria elimination from a hypoendemic area (34, 35). Here, we have shown that anti-Plasmodium effector-gene combinations targeting multiple infection stages can exceed a 35% reduction in P. falciparum infection prevalence assuming an effective population replacement of WT with transgenic mosquitoes.
While our Rel2-expressing mosquitoes did not display any measurable fitness cost under laboratory conditions as measured by longevity, size, and fecundity, the expression of the exogenous effector transgenes ScorpScFv and MultiEff was associated with a slightly decreased fecundity and longevity when mosquitoes were provided a blood meal. The transient upregulation of the transgenes is unlikely to pose an energetic cost that would affect these fitness parameters; also, we saw a lack of fitness costs when the endogenous Rel2 was transgenically expressed (11, 12). Hence, the decreased fecundity and longevity could be a result of the exogenous nature of the recombinant effectors that may adversely interfere with some of the mosquito’s biological processes. However, overexpression of an essential biological process could also adversely impact mosquito fitness as in the case when Akt, a single activated protein kinase that is essential to insulin signaling, was activated in the midguts (36). More realistic life-history studies must be performed under conditions that better mimic the natural environment of the mosquitoes. A slight transgene-mediated fitness cost may also not preclude its use in a transgenic mosquito–based malaria control strategy, because an effective gene-drive system could mitigate such effects (2, 4). Future modeling studies would have to consider multiple parameters, such as transgene spread and prevalence in the population, fitness costs, loss of transgene activity because of mutations or emergence of parasite resistance, and Plasmodium transmission-blocking efficacy.
MATERIALS AND METHODS
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH). The Johns Hopkins University Animal Care and Use Committee has approved this protocol, with permit number MO18H82. Commercial anonymous human blood was used for parasite cultures and mosquito feeding, and informed consent was therefore not applicable. The Johns Hopkins School of Public Health Ethics Committee has approved this protocol.
Mosquito rearing
A. stephensi Liston strain mosquitoes were maintained on sugar solution at 27°C and 80% humidity with a 12-hour light/dark cycle according to standard procedures (10). Larvae were reared on cat food pellets and ground Tetramin fish food powder. Adult mosquitoes were maintained on 10% sucrose and fed on mouse blood (from mice anesthetized with ketamine) for egg production.
Generation of aseptic mosquitoes through antibiotic treatment
On the basis of modifications of previously established methodology, adult female mosquitoes were provided a 10% filter-sterilized sugar solution containing 50 μg/ml of gentamicin sulfate (Sigma) and penicillin (100 U/ml)–streptomycin (100 μg/ml) (Thermo Fisher Scientific) immediately after eclosion (10, 30). The antibiotic treatment efficacy was confirmed by real-time qPCR using the bacterial ribosomal gene 16S primers as previously described in (11). To minimize possible effects of the antibiotics on Plasmodium, the antibiotic-supplemented sugar was replaced with regular filter-sterilized sugar 1 day before the P. falciparum–infected blood meal.
Generation of mosquito transformation constructs: pAsVg-CecScFv, pAsVg-ScorpScFv, pAsVg-PLAScFv, and pAgCp-MultiEff
A transgene cassette with AsVg-CecC-ScFv-3′UTR (hereafter denoted CecScFv) was paper-cloned and synthesized through GenScript Inc., including the AsVg promoter and its native terminator AsVg 3′-UTR. The transgene sequences are listed in the Supplementary Materials. The gene cassettes were first cloned into the cloning vector pUC57-Kan, followed by Asc I digestion and insertion of the transgene into the same restriction site on pBac[3xP3-DsRed]. For the AsVg-Scorp-ScFv-3′UTR (denoted ScorpScFv) and AsVg-PLA2-ScFv-3′UTR (denoted PLAScFv) constructs, the Scorpine or Phospholipase (PLA2) sequences were synthesized (IDTDNA gBlock) and followed by cloning into the pUC57Kan-AsVg-CecScFv plasmid by replacing the CecC with the Scorpine or PLA2 gene sequences at Pme I and Spe I RE sites. The Scorpine and PLA2 sequences are described in the Supplementary Materials. To generate the construct with an array of genes encoding five anti-Plasmodium effectors, the codons were optimized, and the whole transgene cassette, including the P2A viral sequences, was synthesized through GenScript Inc. The sequence information is listed in the Supplementary Materials. The gene cassette was first cloned into the pUC57-Kan plasmid and confirmed by sequencing. The pUC57-Kan-MultiEff plasmid was then digested with Bam HI and Xho I, and this gene fragment was inserted into the pENTR-carboxypeptidase P-antryp1T (provided by S. Yoshida) on the same RE sites (12). The cassette with the AgCp promoter, MultiEff transgene, and trypsin terminator (AgCp-MultiEff-TrypT) was then amplified through PCR using primers (AgCp-Pro-F and Tryp-Ter-R) in table S1 and cloned into the Fse I site of pBac[3xP3-eGFPafm].
Generation of transgenic mosquitoes
For the germline transformation, the mosquito transformation constructs generated above and the helper plasmid phsp-pBac were prepared using the Qiagen EndoFree Maxi Prep Kit and resuspended in 1× microinjection buffer according to an established protocol (10, 37). A mixture of 0.25 μg/μl of the plasmid containing the respective gene with helper plasmid DNA (0.20 μg/μl) was injected into A. stephensi embryos using the Eppendorf FemtoJet Microinjector and quartz needles as described in (37). In general, to generate transgenic mosquitoes, about 700 to 800 A. stephensi eggs were injected, and the larval survivors that hatched (~20%) were screened for transient expression of the 3xP3-GFP marker (green eyes) or 3xP3-dsRed marker (red eyes). Transient expression of GFP or dsRed was screened, and all the surviving adult mosquitoes were crossed to WT mosquitoes, giving rise to several independent transgenic lines (described in each section). Among all these lines, the most potent anti-Plasmodium lines were selected for further study. The AsWT control colony mosquitoes were reared in parallel to the transgenic mosquitoes for inclusion in all the infection assays.
gDNA and RNA isolation, PCR, and qRT-PCR
gDNA of the transgenic larvae or adult mosquitoes was prepared by using a Qiagen mini DNA kit; a pool of five larvae and five adult mosquitoes was used in each sample preparation. The PCR primers used to validate transgene insertion on the chromosome are listed in table S1.
To measure the activation of the transgene after a blood meal, midgut samples were collected from six midguts from AgCp transgenic mosquitoes at 3 hours after a blood meal and compared with unfed G8 homozygote mosquitoes. For AsVg transgenic mosquitoes, fatbody samples were collected 12 hours after blood feeding. RNA was extracted using TRIzol reagent (Thermo Fisher Scientific). Reverse transcription was carried out at 37°C for 2 hours using an MMLV kit (Promega), with 20 μl of reaction mixtures containing oligo(dT) primers and 1 μg of total RNA. The qRT-PCR assays were performed according to a standard protocol (38) by using SYBR Green PCR Master Mix (Thermo Fisher Scientific) and an ABI StepOne Plus real-time PCR system. The relative fold induction or repression of gene expression in the experimental samples was determined by comparing these values to their respective controls after normalizing the transcript levels with the A. stephensi ribosomal S7 gene. The primers used for qRT-PCR are listed in table S1.
To measure the possible influence of transgene expression on the expression of endogenous AMP and anti-Plasmodium effector genes, and the microbiota in the transgenic mosquitoes midguts, we measured mRNA abundance in midgut samples at 0-, 24-, 48-, and 72-hour PBM. Midgut total RNA was used for complementary DNA (cDNA) synthesis and subjected to ABI StepOne Plus real-time PCR using the primers listed in table S1.
P. falciparum infection assays and statistical analysis
To determine anti-Plasmodium activity, the transgenic and WT mosquitoes were fed on NF54 P. falciparum gametocyte cultures (provided by the Johns Hopkins Malaria Research Institute Core Facility) (39) through membranes at 37°C (38, 40). The adult mosquitoes were starved for 3 to 6 hours before feeding to ensure engorgement. Unfed mosquitoes were sorted, and the fed mosquitoes were further incubated for 7 days at 27°C for the counting of P. falciparum oocyst loads. Midguts were dissected out in phosphate-buffered saline (PBS), followed by staining in 0.1% PBS–buffered mercurochrome (MilliporeSigma) and examination under a light-contrast microscope (Olympus). At least three biological replicates were performed for each experiment, and equal numbers of samples from different replicates were pooled to produce dot plots using GraphPad Prism 8 software.
To determine the sporozoite loads in the salivary glands of the infected mosquitoes, salivary glands were dissected, and individual glands were placed in Eppendorf tubes with 30 μl of PBS and then homogenized on ice. The homogenate was centrifuged at 2000 rpm for 10 min to pellet tissue debris. Then, 10 μl of the suspension was placed in a Neubauer counting chamber and at least 5 min were allowed to elapse (to ensure that the sporozoites had sedimented at the bottom of the chamber) for accurately counting the sporozoites. The sporozoites were counted with a Leica phase-contrast microscope at ×400 magnification.
The dot plots of the oocyst and sporozoite numbers in the gut epithelium and salivary glands, respectively, for each treatment were generated using GraphPad Prism8 software, along with the median value. P values of infection intensities were calculated through the Mann-Whitney test and used to determine the significance of parasite numbers as described in (30). The significance of the infection prevalence was determined through the χ2 test or the Fisher’s exact test using GraphPad Prism 8.
Wing length, life span, fecundity, and egg hatching rate measurements
Adult wing length (in both males and females) was used as a surrogate measurement for mosquito size. Mosquitoes were anesthetized on ice and kept on a cold plate for wing length measurement. Wing length was measured manually from the distal end of the alula to the tip of the wing (without the hairy fringe), through a microscope objective containing a scale bar calibrated to a 1-mm stage micrometer, without taking pictures or using software (11).
To measure the life span of the various mosquito lines, adult mosquitoes were sexed into male and female cohorts and placed into cups within 12 hours of emergence, with a cotton pad constantly impregnated with a 10% sucrose solution. They were held there until all the mosquitoes in that cup had died, and the number of dead mosquitoes in the cup was recorded and the dead mosquitoes were removed daily (10, 11). For the life span assays of female mosquitoes receiving a single blood meal (blood-fed on mice), the mosquitoes were offered a blood meal 5 days after emergence, and only mosquitoes taking a blood meal on day 5 were retained for the rest of the study. The survival percentage represents the mean survival percentage for all three biological replicates of 35 mosquitoes each. Statistical significance was determined by Kaplan-Meier survival analysis with pooled data from three replicates by using GraphPad Prism8 software, and P values were determined by Wilcoxon test as described in (10, 36).
For the fecundity assay, approximately fifty 7-day-old adult female AsWT, CecScFv, ScorpScFv, and MultiEff mosquitoes were allowed to feed on mice. Mosquitoes were anesthetized on ice immediately following the blood meal, and all non-engorged mosquitoes were discarded. At 3 days after blood feeding, female mosquitoes were separated into individual vials (50-ml Falcon tubes) containing moist filter paper with 1 ml of water on the bottom and allowed to oviposit, and the number of eggs laid by each female was recorded 4 to 5 days after the blood feeding using light microscopy. The females that died before laying eggs were excluded from the assays. After each count, the eggs were submerged in larval rearing water in the same individual tubes to allow the eggs to hatch. First- and second-instar larvae were counted under a light microscope. Statistical significance was determined using the Mann-Whitney test with GraphPad Prism6 software. The control mosquitoes were reared under the same conditions as the transgenic mosquitoes.
Supplementary Material
Acknowledgments
We thank the Johns Hopkins Malaria Research Institute Insectary and Parasitology core facilities. We also thank D. McClellan for providing editing service. Funding: This work was supported by NIH/National Institute of Allergy and Infectious Disease grants R01AI061576, R01AI061576, R01AI122743, and R01AI122743; the Bloomberg Philanthropies; and a Johns Hopkins Malaria Research Institute postdoctoral fellowship to M.L.S. Author contributions: Conceived and designed the experiments: Y.D. and G.D.; performed the experiments and maintained the transgenic mosquito lines: Y.D. and M.L.S.; analyzed the data: Y.D. and G.D.; wrote the paper: Y.D. and G.D. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/20/eaay5898/DC1
REFERENCES AND NOTES
- 1.Gantz V. M., Jasinskiene N., Tatarenkova O., Fazekas A., Macias V. M., Bier E., James A. A., Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. U.S.A. 112, E6736–E6743 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammond A., Galizi R., Kyrou K., Simoni A., Siniscalchi C., Katsanos D., Gribble M., Baker D., Marois E., Russell S., Burt A., Windbichler N., Crisanti A., Nolan T., A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 34, 78–83 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond A. M., Kyrou K., Bruttini M., North A., Galizi R., Karlsson X., Kranjc N., Carpi F. M., D’Aurizio R., Crisanti A., Nolan T., The creation and selection of mutations resistant to a gene drive over multiple generations in the malaria mosquito. PLOS Genet. 13, e1007039 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyrou K., Hammond A. M., Galizi R., Kranjc N., Burt A., Beaghton A. K., Nolan T., Crisanti A., A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 36, 1062–1066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simōes M. L., Caragata E. P., Dimopoulos G., Diverse host and restriction factors regulate mosquito–pathogen interactions. Trends Parasitol. 34, 603–616 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Cirimotich C. M., Dong Y., Garver L. S., Sim S., Dimopoulos G., Mosquito immune defenses against Plasmodium infection. Dev. Comp. Immunol. 34, 387–395 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton A. M., Dong Y., Dimopoulos G., The Anopheles innate immune system in the defense against malaria infection. J. Innate Immun. 6, 169–181 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frolet C., Thoma M., Blandin S., Hoffmann J. A., Levashina E. A., Boosting NF-κB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 25, 677–685 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Blandin S., Shiao S.-H., Moita L. F., Janse C. J., Waters A. P., Kafatos F. C., Levashina E. A., Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116, 661–670 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Dong Y., Das S., Cirimotich C., Souza-Neto J. A., McLean K. J., Dimopoulos G., Engineered anopheles immunity to Plasmodium infection. PLOS Pathog. 7, e1002458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pike A., Dong Y., Dizaji N. B., Gacita A., Mongodin E. F., Dimopoulos G., Changes in the microbiota cause genetically modified Anopheles to spread in a population. Science 357, 1396–1399 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Yoshida S., Shimada Y., Kondoh D., Kouzuma Y., Ghosh A. K., Jacobs-Lorena M., Sinden R. E., Hemolytic C-type lectin CEL-III from sea cucumber expressed in transgenic mosquitoes impairs malaria parasite development. PLOS Pathog. 3, e192 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshida S., Matsuoka H., Luo E., Iwai K., Arai M., Sinden R. E., Ishii A., A single-chain antibody fragment specific for the Plasmodium berghei ookinete protein Pbs21 confers transmission blockade in the mosquito midgut. Mol. Biochem. Parasitol. 104, 195–204 (1999). [DOI] [PubMed] [Google Scholar]
- 14.de Lara Capurro M., Coleman J., Beerntsen B. T., Myles K. M., Olson K. E., Rocha E., Krettli A. U., James A. A., Virus-expressed, recombinant single-chain antibody blocks sporozoite infection of salivary glands in Plasmodium gallinaceum-infected Aedes aegypti. Am. J. Trop. Med. Hyg. 62, 427–433 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Moreira L. A., Ito J., Ghosh A., Devenport M., Zieler H., Abraham E. G., Crisanti A., Nolan T., Catteruccia F., Jacobs-Lorena M., Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. J. Biol. Chem. 277, 40839–40843 (2002). [DOI] [PubMed] [Google Scholar]
- 16.Wang S., Ghosh A. K., Bongio N., Stebbings K. A., Lampe D. J., Jacobs-Lorena M., Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 109, 12734–12739 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito J., Ghosh A., Moreira L. A., Wimmer E. A., Jacobs-Lorena M., Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417, 452–455 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Abraham E. G., Donnelly-Doman M., Fujioka H., Ghosh A., Moreira L., Jacobs-Lorena M., Driving midgut-specific expression and secretion of a foreign protein in transgenic mosquitoes with AgAper1 regulatory elements. Insect Mol. Biol. 14, 271–279 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Isaacs A. T., Li F., Jasinskiene N., Chen X., Nirmala X., Marinotti O., Vinetz J. M., James A. A., Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. PLOS Pathog. 7, e1002017 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh A. K., Coppens I., Gårdsvoll H., Ploug M., Jacobs-Lorena M., Plasmodium ookinetes coopt mammalian plasminogen to invade the mosquito midgut. Proc. Natl. Acad. Sci. U.S.A. 108, 17153–17158 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshida S., Ioka D., Matsuoka H., Endo H., Ishii A., Bacteria expressing single-chain immunotoxin inhibit malaria parasite development in mosquitoes. Mol. Biochem. Parasitol. 113, 89–96 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Conde R., Zamudio F. Z., Rodríguez M. H., Possani L. D., Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 471, 165–168 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Fang W., Vega-Rodríguez J., Ghosh A. K., Jacobs-Lorena M., Kang A., Leger R. J. S., Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 331, 1074–1077 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daniels R. W., Rossano A. J., Macleod G. T., Ganetzky B., Expression of multiple transgenes from a single construct using viral 2A peptides in Drosophila. PLOS ONE 9, e100637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrighi R. B., Ebikeme C., Jiang Y., Ranford-Cartwright L., Barrett M. P., Langel Ü., Faye I., Cell-penetrating peptide TP10 shows broad-spectrum activity against both Plasmodium falciparum and Trypanosoma brucei brucei. Antimicrob. Agents Chemother. 52, 3414–3417 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arrighi R. B., Nakamura C., Miyake J., Hurd H., Burgess J. G., Design and activity of antimicrobial peptides against sporogonic-stage parasites causing murine malarias. Antimicrob. Agents Chemother. 46, 2104–2110 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter V., Underhill A., Baber I., Sylla L., Baby M., Larget-Thiery I., Zettor A., Bourgouin C., Langel Ü., Faye I., Otvos L., Wade J. D., Coulibaly M. B., Traore S. F., Tripet F., Eggleston P., Hurd H., Killer bee molecules: Antimicrobial peptides as effector molecules to target sporogonic stages of Plasmodium. PLOS Pathog. 9, e1003790 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Habtewold T., Tapanelli S., Masters E. K. G., Hoermann A., Windbichler N., Christophides G. K., Streamlined SMFA and mosquito dark-feeding regime significantly improve malaria transmission-blocking assay robustness and sensitivity. Malar. J. 18, 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dennison N. J., Jupatanakul N., Dimopoulos G., The mosquito microbiota influences vector competence for human pathogens. Curr. Opin. Insect Sci. 3, 6–13 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong Y., Manfredini F., Dimopoulos G., Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLOS Pathog. 5, e1000423 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei G., Lai Y., Wang G., Chen H., Li F., Wang S., Insect pathogenic fungus interacts with the gut microbiota to accelerate mosquito mortality. Proc. Natl. Acad. Sci. U.S.A. 114, 5994–5999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muriu S. M., Muturi E. J., Shililu J. I., Mbogo C. M., Mwangangi J. M., Jacob B. G., Irungu L. W., Mukabana R. W., Githure J. I., Novak R. J., Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in Mwea rice scheme, Kenya. Malar. J. 7, 43 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinden R. E., Alavi Y., Raine J. D., Mosquito–malaria interactions: A reappraisal of the concepts of susceptibility and refractoriness. Insect Biochem. Mol. Biol. 34, 625–629 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Blagborough A. M., Churcher T. S., Upton L. M., Ghani A. C., Gething P. W., Sinden R. E., Transmission-blocking interventions eliminate malaria from laboratory populations. Nat. Commun. 4, 1812 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blagborough A. M., Delves M. J., Ramakrishnan C., Lal K., Butcher G., Sinden R. E., Assessing transmission blockade in Plasmodium spp. Methods Mol. Biol. 923, 577–600 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Corby-Harris V., Drexler A., Watkins de Jong L., Antonova Y., Pakpour N., Ziegler R., Ramberg F., Lewis E. E., Brown J. M., Luckhart S., Riehle M. A., Activation of Akt signaling reduces the prevalence and intensity of malaria parasite infection and lifespan in Anopheles stephensi mosquitoes. PLOS Pathog. 6, e1001003 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobo N. F., Clayton J. R., Fraser M. J., Kafatos F. C., Collins F. H., High efficiency germ-line transformation of mosquitoes. Nat. Protoc. 1, 1312–1317 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Dong Y., Aguilar R., Xi Z., Warr E., Mongin E., Dimopoulos G., Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLOS Pathog. 2, e52 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talman A. M., Blagborough A. M., Sinden R. E., A Plasmodium falciparum strain expressing GFP throughout the parasite’s life-cycle. PLOS ONE 5, e9156 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franke-Fayard B., Trueman H., Ramesar J., Mendoza J., van der Keur M., van der Linden R., Sinden R. E., Waters A. P., Janse C. J., A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol. Biochem. Parasitol. 137, 23–33 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/20/eaay5898/DC1


