Abstract
Objective:
Apatinib mesylate is a novel vascular endothelial growth factor receptor 2 (VEGFR-2) inhibitor, which has exhibited good safety and efficacy in several types of solid tumors. The present study aimed to assess the clinical efficacy and safety of apatinib combined with chemotherapy and concurrent chemo-brachytherapy (CCBT) in patients with recurrent and advanced cervical cancer.
Methods:
A total of 52 patients with first diagnosed recurrent or untreated International Federation of Gynecology and Obstetrics stage IVB cervical cancer admitted at Shandong Cancer Hospital and Institute between July 2016 and May 2018 were analyzed in the current randomized controlled trial. The patients were randomly divided into 2 groups: the apatinib-treated group and the control group. Patients with recurrent cervical cancer in the apatinib-treated group were administered apatinib and carboplatin-paclitaxel as first-line chemotherapy. Patients with advanced cervical cancer were administered apatinib in combination with CCBT. In control group, patients with recurrent cervical cancer were treated with chemotherapy alone while patients with advanced cervical cancer received CCBT.
Results:
The progression-free survival was significantly prolonged in apatinib group compared with control group (10.1 months; 95% confidence interval (CI), 8.42–11.79 vs 6.4 months; 95% CI, 3.88–8.92; P < .01; hazard ratio (HR), 0.44; 95% CI, 0.25–0.78; P < .01). The objective response rate in apatinib group was obviously higher than that in control group (64.3% vs 33.3%, P < .05). Proteinuria, hand–foot syndrome, mucositis, and hypertension in all Grades were statistically more common in apatinib group than in control group. Apatinib did not obviously aggravate other radiotherapy or chemotherapy side effects.
Conclusion:
Apatinib exhibited promising clinical efficacy in cervical cancer patients, resulting in an improved response rate and prolonged progression-free survival compared with the control group, and had manageable side effects. Our study revealed that apatinib combination therapy, adenocarcinoma, and bone metastasis
Keywords: apatinib, chemotherapy, concurrent chemotherapy and brachytherapy, recurrent and advanced cervical cancer
Highlight
The efficacy of apatinib in recurrent and advanced cervical cancer was inspiring.
Apatinib addition, adenocarcinoma, and bone metastasis were independent prognostic factors.
Side effects of apatinib were manageable.
1. Introduction
Cervical cancer (CC) is one of the most common malignancies in females,[1] with 570,000 new cases and 311,000 mortalities reported in 2018 worldwide.[2] CC is the leading cause of cancer-associated mortalities in female malignant genital tumors and the fourth leading cause of cancer-associated mortalities in women.[2] This highlights the requirement for novel therapeutic strategies for the disease. Early stage disease (stage IA-IB1) is generally treated surgically. Locally advanced disease (IB2-IVA) may be treated with concurrent chemo-radiotherapy (CCRT),[3] and recurrent and advanced disease (stage IVB) is treated with chemotherapy and palliative radiotherapy including intra-cavity brachytherapy (IC-BT).[3,4] The early detection and prevention of CC through screening and vaccination has decreased the incidence of the disease in recent years; however, the overall prognosis for patients with recurrent and advanced disease remains poor.[5] Cisplatin-based chemotherapy was previously the standard of care for patients with recurrent and metastatic cervical cancer.[6] However, a recent systematic literature review revealed that a combination of carboplatin and paclitaxel is as equally effective and less toxic than cisplatin and paclitaxel.[7] Brachytherapy, an important treatment modality to achieve sufficient doses to the periphery and central part of the cervical carcinomas, can improve remission rate, recurrence rate, and survival rates of all types CC.[8] Therefore, brachytherapy should be considered in the treatment of stage IVB CC.[7–9] Targeted therapy is an area of ongoing research and may provide alternatives to traditional treatment modalities in cancer.[10]
The vascular endothelial growth factor (VEGF) inhibitor bevacizumab was approved by the Food and Drug Administration in 2014 for patients with recurrent or advanced CC.[11] A combination of platinum-based chemotherapy and bevacizumab improved the median overall survival (OS) of patients with recurrent or advanced CC by 3.5 months compared with chemotherapy alone.[12] However, the use of bevacizumab is limited by severe side effects, including gastrointestinal perforations and rectovaginal and vesicovaginal fistulas.[13] Apatinib mesylate (YN968D1) is a tyrosine kinase inhibitor that has a high selectivity for vascular endothelial growth factor receptor-2 (VEGFR-2). Apatinib inhibited endothelial cell migration and proliferation, decreased tumor microvascular density and prevented the formation of new blood vessels in tumor tissue[14] and exerted anticancer effects in several types of cancer, including gastric cancer, breast cancer, non-small-cell lung cancer, hepatocellular carcinoma, and ovarian cancer.[15–20] In addition, in a study of pancreatic cancer cells, apatinib inhibited the expression of hypoxia-inducible factor-1α (HIF-1α) and markers of the phosphoinositide 3-kinase (PI3K)/Akt/mTOR signaling pathway, which increased the levels of reactive oxygen species in vitro.[21] A recent study revealed that apatinib significantly increased the paclitaxel sensitivity of cervical cancer cells in vitro and in the mouse model.[22] Furthermore, the anti-tumor effects of apatinib in cervical cancer have been demonstrated in other studies.[22]
In order to further evaluate the efficacy and safety of apatinib, the current randomized controlled clinical trial investigated the potential synergistic anti-tumor activity of apatinib and chemotherapy or concurrent chemo-brachytherapy (CCBT) in recurrent or untreated stage IVB CC.
2. Methods
2.1. Patients
Patients with a first recurrence of CC or with stage IVB of the disease were analyzed in the current randomized controlled trial between July 2016 and May 2018 in Shandong Cancer Hospital and Institute (Jinan, China).
The criteria for inclusion were as follows:
-
i)
histologically confirmed cervical cancer;
-
ii)
age >18 year;
-
iii)
≥1 measurable lesion according to Response Evaluation Criteria in Solid Tumors[23] (RECIST; version 1.1);
-
iv)
Eastern Cooperative Oncology Group[24] (ECOG) performance status <3
-
v)
no serious heart, liver, or kidney insufficiency; and
-
vi)
patients with a first recurrence of CC or stage IVB.
The criteria for exclusion were as follows:
-
i)
patients allergic to apatinib;
-
ii)
patients with active hemorrhage;
-
iii)
patients with intestinal perforation or bowel ileus;
-
iv)
patients within 30 days of major surgery; and
-
v)
patients with uncontrolled hypertension.
The current study was approved by the Medical Ethical Committee of Shandong Cancer Hospital and Institute (SDTHEC201607004) and written informed consent was obtained from all patients. The current trial was registered with the Chinese Clinical Trials Registry (number ChiCTR1900024143; http://www.chictr.org.cn/showproj.aspx?proj=39833).
2.2. Grouping and treatment
All patients (n = 59) who met eligibility criteria were randomly divided into 2 groups (1:1 ratio): the apatinib group (n = 30) and the control group (n = 29). Patients with recurrent CC in the apatinib group were administered apatinib and carboplatin-paclitaxel as first-line chemotherapy. Patients with stage IVB CC in the apatinib group received apatinib and CCBT. In the control group, patients with recurrent CC were treated with carboplatin-paclitaxel chemotherapy and patients with advanced CC received CCBT. Patients in both groups received 135 to 175 mg/m2 paclitaxel (diluted in 500 ml of 0.9% saline and infused intravenously over 3 hours) on day 1 and carboplatin AUC 5 (diluted in 500 ml of 0.9% saline solution and infused intravenously over 30 minutes) on day 2 every 3 weeks, for 6 cycles. Patients in both groups with stage IVB CC received IC-BT in between chemotherapy cycles as a form of palliative radiotherapy. Patients with recurrent CC would not received BT. A high dose rate iridium-192 source based brachytherapy was used in the current clinical trial. IC-BT was performed according to a treatment planning system (Radionuclide After Loading Systems, Tian Jin, China). The dose delivered to point A was 5 Gy every fraction, once a week, for a total of 10 or 12 fractions (total dose range, 50–60 Gy). For smaller cervical tumors well covered by the brachytherapy dose, 10 fractions were given (totally 50 Gy). For massive cervical tumors, 2 fractions may be added in case tumour regression was not satisfactory after dose of 50 Gy (up to 60 Gy). Patients in the apatinib group received 500 mg apatinib mesylate (YN968D1, HengRui Medicine, Jiang Su, China) orally in between chemotherapy cycles. Apatinib was administered 30 minutes after a meal and the dose was reduced to 250 mg in the case of intolerable toxicity. One treatment cycle was based on regimens of 3 weeks. In each treatment cycle, a maximum of 2 apatinib withdrawals were accepted, and the total withdrawal time did not exceed 14 days. Patients were followed-up till disease progression, discontinuation of treatment due to intolerable toxicity, mortality or until the study cut-off date of May 1, 2018. Patients who had finished at least 2 cycles of therapy were analyzed in the current study.
2.3. Efficacy and safety assessments
Efficacy evaluation was assessed according to RECIST[23] 1.1 criteria. Tumor responses were divided into the following groups:
-
i)
complete response (CR);
-
ii)
partial response (PR);
-
iii)
stable disease (SD); and
-
iv)
progressive disease (PD).
The overall response rate (ORR) was calculated as the sum of CR and PR and the disease control rate (DCR) was calculated as the sum of CR, PR, and SD. Toxicities were reviewed and confirmed through medical history, laboratory and imaging examinations, and telephone follow-up, and were evaluated according to the National Cancer Institute Common Toxicity Criteria for Adverse Events[25] (version 4.0), ranging from 0 to 4.
The primary endpoint in the current study was progression-free survival (PFS). The secondary endpoints included ORR, DCR, and OS. PFS was defined as the interval between the first day of chemotherapy and disease progression or mortality, whichever occurred first. OS was defined as the time period between the first day of chemotherapy and mortality from any cause or the last follow-up visit. Two oncologists assessed tumor responses at the end of each treatment cycle or when significant signs of progression had occurred. All patients should accept pelvic examination from oncologists after each treatment cycle, especially for patients with stage IVB CC. Furthermore, serum Carcinoembryonic Antigen (CEA) and Squmaous Cell Carcinoma Antigen (SCCA) levels were measured and imaging assessments including abdominal and pelvic ultrasound or CT scan were performed at the end of each treatment cycle. Patient information was collected through telephone follow-ups as well as medical records.
2.4. Statistical analysis
All data were analyzed using SPSS software (version 24.0). The continuous data were analyzed using an unpaired t test. The categorical data, including adverse events, were analyzed using a χ2 test. The survival analyses were performed by the Kaplan–Meier method and log-rank test. The Cox risk regression model was used to perform univariate and multivariate analyses and to assess the hazard ratio (HR) and the 95% confidence interval (CI). P < .05 was considered to indicate a statistically significant difference.
3. Results
3.1. Patient characteristics
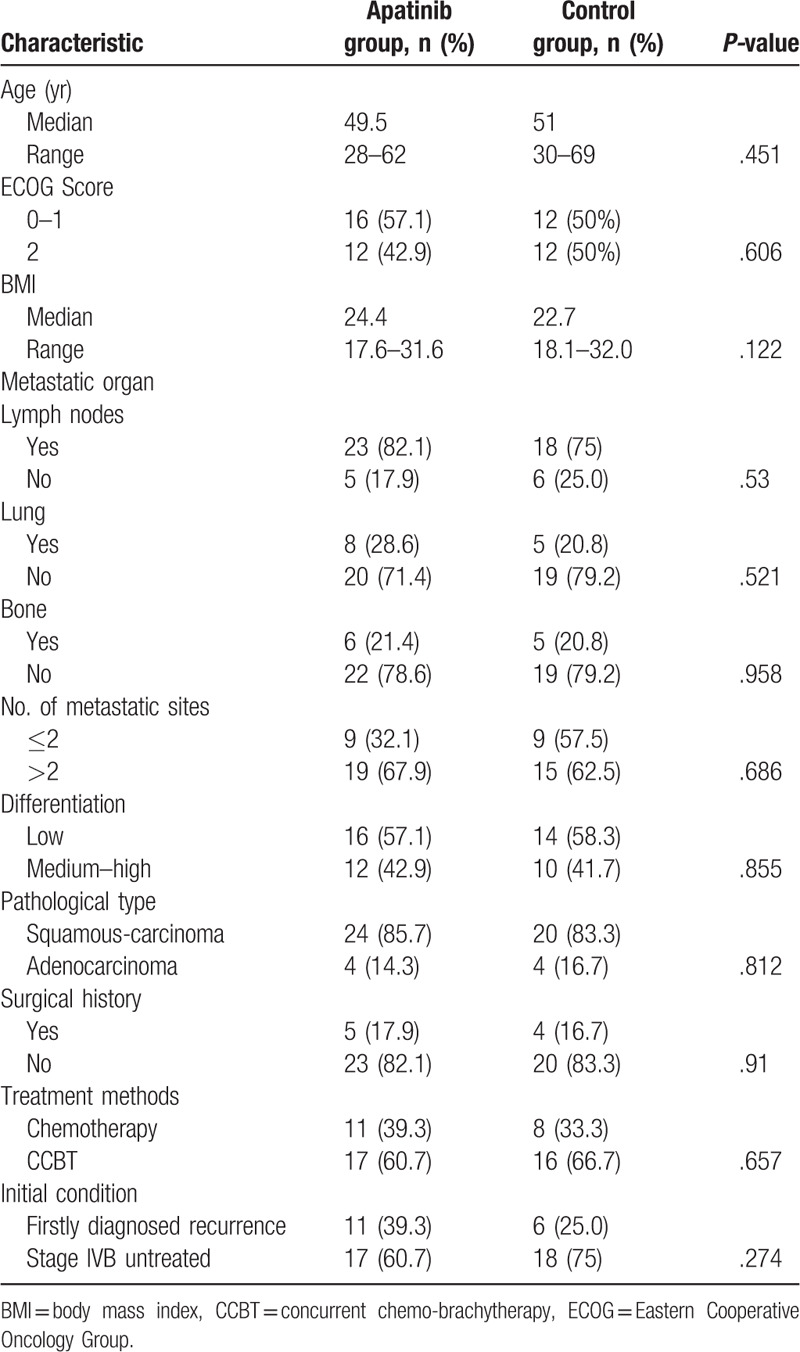
A total of 59 patients with recurrent and advanced CC were included in the current study randomly divided into the apatinib group (n = 30) and the control group (n = 29). One patient in apatinib group and 2 in control group separately discontinued the clinical trial before the first evaluation. A total of 4 patients were lost to follow-up (1 patient in apatinib group and 3 in control group). The main reasons were severe toxicities, disease progression, and personal factors. Eventually, 52 patients were analyzed (28 in apatinib group and 24 in control group). Baseline characteristics, including age, ECOG Score, body mass index, metastatic sites, number of metastatic sites, grade of differentiation, pathological type, surgical history, and treatment received, were similar in the 2 groups (Table 1). All patients were non-smokers and did not consume alcohol.
Table 1.
Patient characteristics.
3.2. Efficacy
The median follow-up time was 14.0 months (interquartile range, 9.6–19.3 months). By the end of April 2019, 27 patients (11 and 16 patients in the apatinib and control groups, respectively), accounting for 51.9% of the total number patients, had succumbed. The median PFS was significantly increased in the apatinib group compared with the control group (10.1 months, 95% CI 8.42–11.79 vs 6.4 months, 95% CI 3.88–8.92; P < .01; HR, 0.44, 95% CI, 0.25–0.78; P < .01; Fig. 1A). Stratified analyses revealed that the median PFS of patients who received chemotherapy was significantly increased in the apatinib group compared with the control group (10.1 months; 95% CI 8.16–12.04 vs 6.4 months; 95% CI 3.49–9.31; P < .01; Fig. 1B). The median PFS of patients who received CCBT was significantly increased in the apatinib group compared with the control group (10.3 months; 95% CI, 7.88–12.72 vs 6.1 months; 95% CI 2.77–9.43; P < .01; Fig. 1C).
Figure 1.
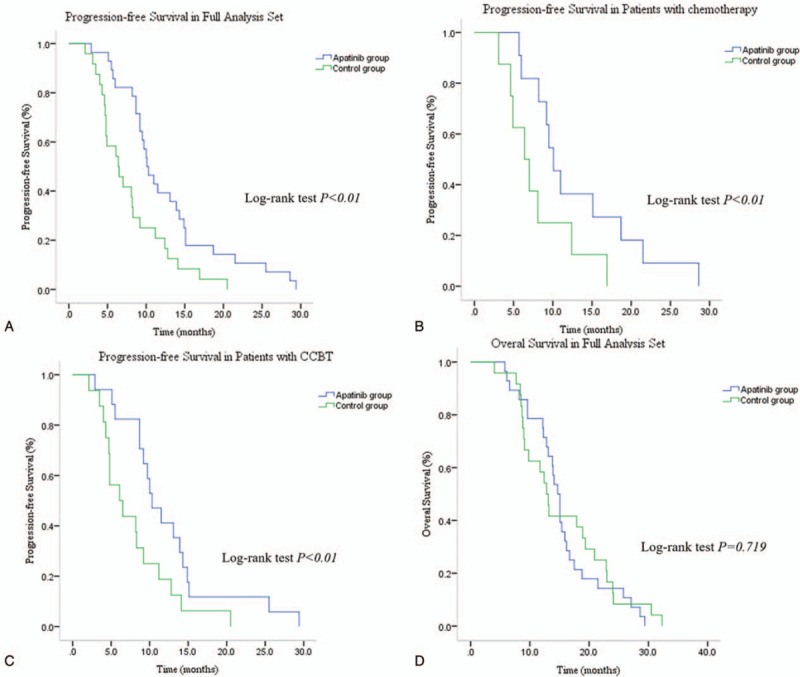
Kaplan–Meier estimates of PFS. (A) The median PFS for patients receiving chemotherapy or CCBT in the apatinib group was 10.1 mo compared with 6.4 mo for patients in the control group (log-rank test P < .01). (B) Stratified analyses revealed that the median PFS in patients who received chemotherapy was 10.1 mo in the apatinib group and 6.4 mo in the control group. (C) Stratified analyses demonstrated that the median PFS in patients receiving CCBT was 10.3 mo in the apatinib group and 6.1 mo in the control group (log-rank test P < .01). (D) The median overall survival in the full analysis set was 14.7 mo for patients in the apatinib group compared with 12.8 mo in the control group (log-rank test P = .719). CCBT = concurrent chemo-brachytherapy, PFS = progression-free survival.
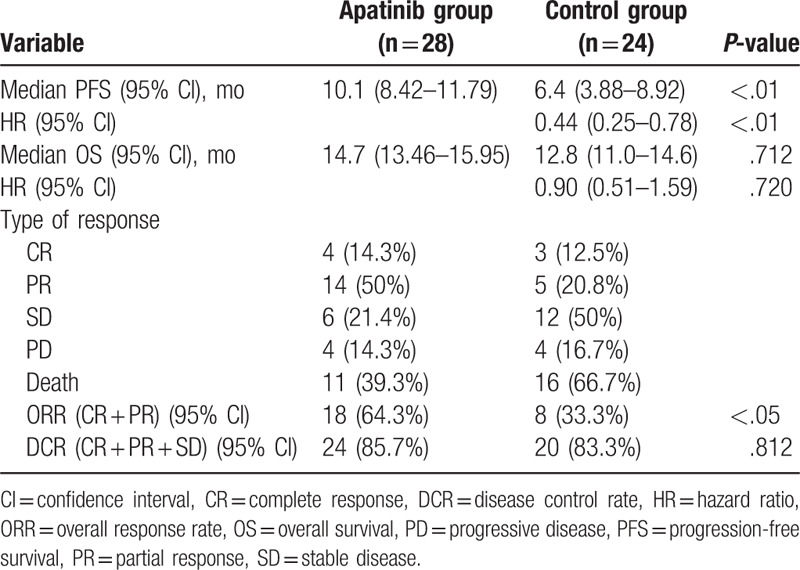
There was no significant difference in the median OS between the 2 groups (14.7 months; 95% CI 13.46–15.95 vs 12.8 months; 95% CI 11.0–14.6; P = .712; HR 0.90; 95% CI 0.51–1.59; P = .720; Fig. 1D and Table 2). The ORR in the apatinib and control groups were 64.3% and 33.3%, respectively (P < .05). The DCR in the apatinib group was slightly higher than in the control group (85.7% and 83%, respectively); however, the difference was not statistically significant (P = .812; Table 2).
Table 2.
Analysis of apatinib efficacy in the entire patient cohort.
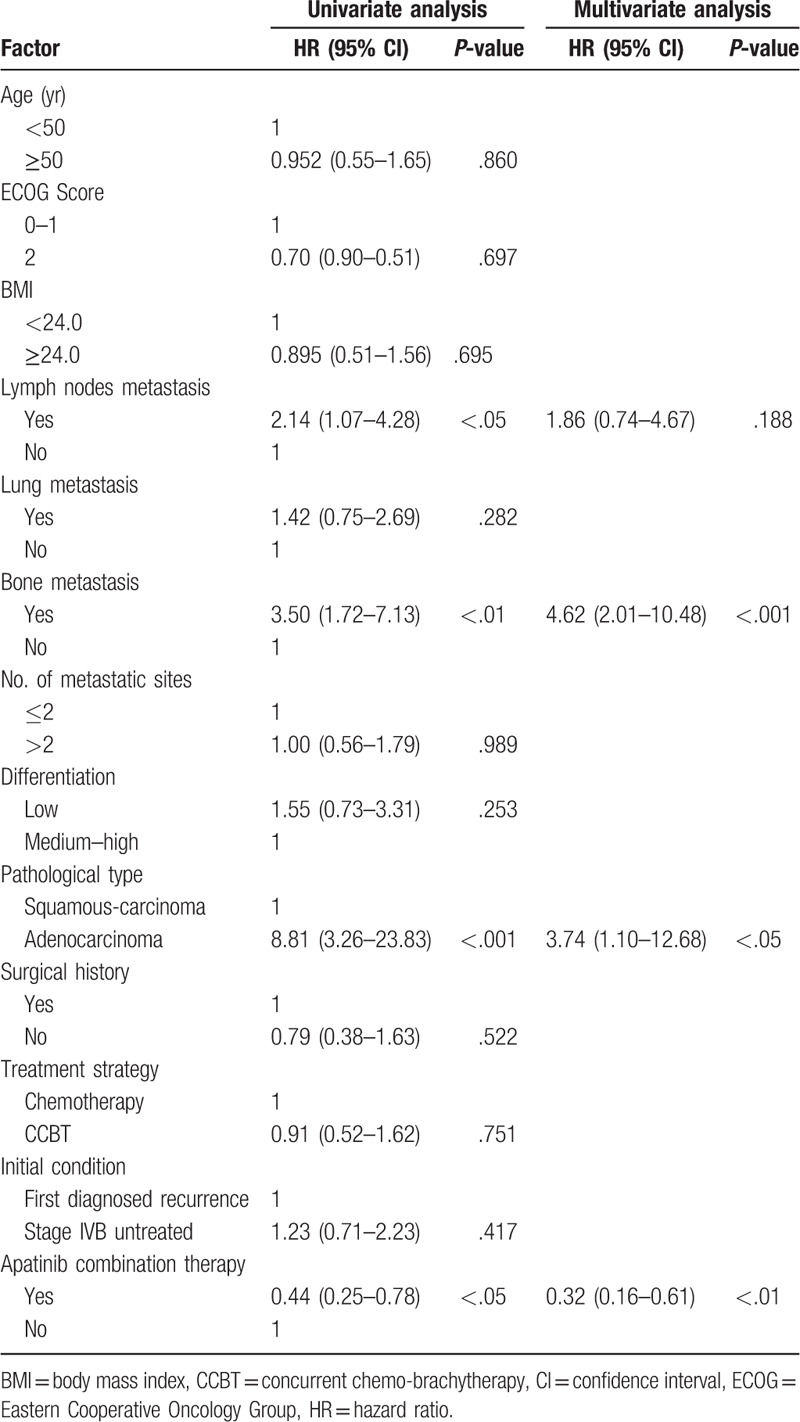
The associations between the clinical baseline variables and OS were analyzed by univariate analysis. Variables yielded P < .10 were subsequently included in multivariate analysis to predict the factors affecting survival outcomes. Variables drawn into multivariate analysis included lymph node metastasis (P < .05), bone metastasis (P < .01), pathological type (P < .001), and apatinib combination therapy (P < .05). Multivariate analysis with adjustment for confounding factors in the Cox regression model revealed that bone metastasis (P < .001), adenocarcinoma (P < .05), and apatinib combination therapy (P < .01) were independent prognostic factors, while lymph node metastasis was excluded (P = .188). Unlike bone metastasis and pathological type of adenocarcinoma, which led to poorer PFS, apatinib combination therapy served as a protective factor and increased the PFS (Table 3).
Table 3.
Multivariate analysis for overall survival and progression-free survival.
For the evaluation of PFS, data for patients who had not progressed or who had succumbed at the time of analysis were censored at the final assessment according to RECIST 1.1 criteria.[23] Overall survival was calculated from the date of recurrence or advanced cervical cancer diagnosis to the date of death from any cause or censored at the last recorded date which patients were known to have survived.
3.3. Adverse events
The apatinib dose was reduced to 250 mg/day for 3 patients due to severe treatment-associated toxicity, including hypertension and hand–foot syndrome. A total of 11 patients temporarily withdrew apatinib treatment in a short time primarily due to severe hematologic and non-hematologic toxicity following chemotherapy. Each patient withdrew therapy for a maximum of 2 times and the total withdrawal time was <14 days in each treatment cycle. Severe hypertension and hand–foot skin syndrome was adequately relieved when the drug dose was reduced or when patients were given symptomatic treatment to support medication adherence and apatinib tolerance.
In the apatinib group, the side effects with the highest incidence rates were neutropenia and anemia, followed by diarrhea, nausea and vomiting, proteinuria, hand–foot syndrome, fatigue, mucositis, hypertension, dysuria, urinary urgency and frequency, alopecia, thrombocytopenia, and liver toxicity (see Table 4). The incidence of adverse effects in the control group were as follows: neutropenia, anemia, diarrhea, nausea and vomiting, fatigue, dysuria, urinary urgency and frequency, alopecia, hand–foot skin syndrome, proteinuria, thrombocytopenia, mucositis, liver toxicity, and hypertension (see Table 4).
Table 4.
Analysis of adverse events.
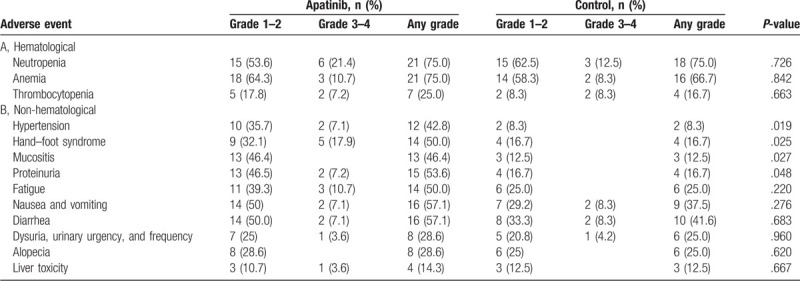
Apatinib did not significantly increase the incidence of neutropenia compared with the control group (75% vs 75%; P = .726), anemia (75% vs 66.7%; P = .842), diarrhea (57.1% vs 41.6%; P = .683), nausea and vomiting (57.1% vs 37.5%; P = .276), fatigue (50.0% vs 25.0%; P = .220), dysuria, urinary urgency, and frequency (28.6% vs 25%; P = .960), alopecia (28.6% vs 25%; P = .620), thrombocytopenia (25.0% vs 16.7%; P = .663), and liver toxicity (14.3% vs 12.5%; P = .667). However, proteinuria (53.6% vs 16.7%; P < .05), hand–foot syndrome (50% vs 16.7%; P < .05), mucositis (46.4% vs 12.5%; P < .05), and hypertension (42.8% vs 8.3%; P < .05) in all severities were significantly more common in the apatinib group compared with the control group.
4. Discussion
Patients presenting with recurrent and metastatic CC have traditionally received platinum-based doublet chemotherapy in combination with palliative radiotherapy, resulting in poor patient outcomes.[26] Clinical trials have demonstrated that the addition of anti-angiogenic agents improves the outcome of the aforementioned patients.[14,27] The Gynecologic Oncology Group (GOG) 240 trial revealed that the combination of bevacizumab and cisplatin-paclitaxel chemotherapy significantly improved the median PFS compared with chemotherapy alone (8.2 vs 6.0 months; HR 0.68; 95% CI, 0.56–84; P = .0002).[11] Apatinib mesylate is a novel type of VEGFR-2 inhibitor, which has exhibited good clinical efficacy in solid tumors, including hepatocellular carcinoma and gastric, breast, non-small-cell lung, and ovarian cancer, since its introduction in China in 2014.[14–20,28] Apatinib demonstrated good anti-tumor activity in cervical cancer cell lines and an in vivo mouse model.[22] However, evidence of efficacy and safety of apatinib in patients with recurrent or advanced CC is limited.[22]
The present randomized controlled study evaluated the efficacy of apatinib combination treatment with chemotherapy or CCBT in patients with firstly diagnosed recurrent or stage IVB CC. The results of the current study were promising compared with the outcome of the GOG 240 trial.[11]
The present study demonstrated that 64.3% of patients with recurrent or advanced CC treated with apatinib in combination with chemotherapy or CCBT achieved the ORR, compared with 33.3% of patients in the control group (P < .05). The median PFS was significantly increased in the apatinib group compared with the control group (10.1 months; 95% CI, 8.42–11.79 vs 6.4 months; 95% CI 3.88–8.92; P < .01; HR, 0.44; 95% CI, 0.25–0.78; P < .01). This result demonstrated that apatinib demonstrated antitumor effects in cervical cancer and suggested that apatinib may exhibit synergistic effects with radiotherapy and chemotherapy. The mechanisms underlying this potential synergism require further investigation. The median OS in the apatinib group was increased compared with the control group, although not significantly so (14.7 months; 95% CI 13.46–15.95 vs 12.8 months; 95% CI, 11.0–14.6; P = .712; HR 0.90; 95% CI 0.51–1.59; P = .720). The lack of significance may be due to the limited sample size, short-term follow-up of certain patients and patients lost to follow-up in the current study. Future studies with a larger sample size are therefore required to substantiate the results obtained in the current study.
The multivariate analysis performed in the current study revealed that patients with recurrent and advanced CC with bone metastasis and adenocarcinoma had a poorer PFS and a significantly increased risk of mortality, similar to results obtained in previously published studies.[29–32] Additionally, lymph node metastasis was associated with poor prognosis in the current study, but was not an independent prognosis predictor factor. Combination treatment with apatinib served as a protective factor that significantly prolonged the PFS and decreased the risk of mortality compared with controls.
Previous clinical trials have reported that hand–foot syndrome, hypertension, and proteinuria are the most common adverse events associated with apatinib.[19,20,22,33] In the present study, proteinuria, hand–foot syndrome, mucositis, and hypertension were significantly more common in the apatinib group compared with the control group, which was consistent with previous studies investigating gastric, breast, and ovarian cancer.[34–36] The addition of apatinib to chemotherapy or CCBT in the current study did not affect the incidence of neutropenia, anemia, diarrhea, nausea and vomiting, fatigue, dysuria, urinary urgency and frequency, alopecia, thrombocytopenia, and liver toxicity. Notably, apatinib did not aggravate radiotherapy and chemotherapy-associated side effects and caused manageable common adverse effects. Furthermore, apatinib is administered orally without the need for hospital admission, resulting in improved patient compliance and economic feasibility.
In conclusion, the present study revealed that apatinib in combination with chemotherapy or CCBT exhibited promising efficacy and manageable toxicities in patients with firstly diagnosed recurrent or stage IVB CC. Based on the results of the current study, a phase 3 trial to further investigate the effects of apatinib is warranted.
Author contributions
Conceptualization: Naifu Liu, Mingjiang Li.
Data curation: Qiufen Guo, Linli Rao.
Formal analysis: Qiufen Guo, Yawen Sun.
Investigation: Jinlong Chen, Qian Wu, Tingting Zhang.
Methodology: Qiufen Guo, Yawen Sun.
Resources: Li Sun.
Software: Qiufen Guo, Yawen Sun.
Validation: Qiufen Guo, Yawen Sun.
Writing – original draft: Qiufen Guo.
Writing – review & editing: Li Sun.
Footnotes
Abbreviations: BMI = body mass index, CCBT = concurrent chemo-brachytherapy, CCRT = concurrent chemo-radiotherapy, CI = confidence interval, CR = complete response, DCR = disease control rate, ECOG = Eastern Cooperative Oncology Group, FIGO = International Federation of Gynecology and Obstetrics, HIF-1α = hypoxia-inducible factor-1α, HR = hazard ratio, IC-BT = intra-cavity brachytherapy, ORR = overall response rate, OS = overall survival, PD = progression disease, PFS = progression-free survival, PI3K = phosphoinositide 3-kinase, PR = partial response, SD = stable disease, TR = tumor response, VEGFR-2 = vascular endothelial growth factor receptor 2.
How to cite this article: Guo Q, Sun Y, Kong E, Rao L, Chen J, Wu Q, Zhang T, Liu N, Li M, Sun L. Apatinib combined with chemotherapy or concurrent chemo-brachytherapy in patients with recurrent or advanced cervical cancer: A phase 2, randomized controlled, prospective study. Medicine. 2020;99:11(e19372).
The present study was supported by grants from The National Natural Science Foundation of China (grant no. 81671433), The Province Key Research and Development Project of Shandong (grant no. 2018GSF118238), The CSCO-HengRui Tumor Research Fund (grant no. Y-HR2018-200), The Medical Science and Technology Project of Shandong (grants no. 2016WS0562), and The Natural Science Foundation of Shandong (grant no. ZR2012HM010).
The authors have no conflicts of interest to disclose.
References
- [1].Small WJ, Bacon MA, Bajaj A, et al. Cervical cancer: a global health crisis. Cancer 2017;123:2404–12. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [3].Aghili M, Andalib B, Karimi MZ, et al. Concurrent chemo-radiobrachytherapy with cisplatin and medium dose rate intra-cavitary brachytherapy for locally advanced uterine cervical cancer. Asian Pac J Cancer Prev 2018;19:2745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bhatla N, Aoki D, Sharma DN, et al. Cancer of the cervix uteri. Int J Gynaecol Obstet 2018;143: Suppl 2: 22–36. [DOI] [PubMed] [Google Scholar]
- [5].Mountzios G, Soultati A, Pectasides D, et al. Developments in the systemic treatment of metastatic cervical cancer. Cancer Treat Rev 2013;39:430–43. [DOI] [PubMed] [Google Scholar]
- [6].Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel plus carboplatin versus paclitaxel plus cisplatin in metastatic or recurrent cervical cancer: the open-label randomized Phase III Trial JCOG0505. J Clin Oncol 2015;33:2129–35. [DOI] [PubMed] [Google Scholar]
- [7].Lorusso D, Petrelli F, Coinu A, et al. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol Oncol 2014;133:117–23. [DOI] [PubMed] [Google Scholar]
- [8].Karlsson J, Dreifaldt AC, Mordhorst LB, et al. Differences in outcome for cervical cancer patients treated with or without brachytherapy. Brachytherapy 2017;16:133–40. [DOI] [PubMed] [Google Scholar]
- [9].Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet 2019;393:169–82. [DOI] [PubMed] [Google Scholar]
- [10].Kunos CA. Novel biological radiochemotherapy approaches in locally advanced-stage cervical cancer management. Discov Med 2014;17:179–86. [PubMed] [Google Scholar]
- [11].Tewari KS, Sill MW, Penson RT, et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017;390:1654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tewari KS, Sill MW, Long HR, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med 2014;370:734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Minion LE, Tewari KS. The safety and efficacy of bevacizumab in the treatment of patients with recurrent or metastatic cervical cancer. Expert Rev Anticancer Ther 2017;17:191–8. [DOI] [PubMed] [Google Scholar]
- [14].Zhou JG, Zhou NJ, Zhang Q, et al. Apatinib for patients with advanced or recurrent cervical cancer: study protocol for an open-label randomized controlled trial. Trials 2018;19:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [17].Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kong Y, Sun L, Hou Z, et al. Apatinib is effective for treatment of advanced hepatocellular carcinoma. Oncotarget 2017;8:105596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu J, Liu X, Yang S, et al. Clinical response to apatinib monotherapy in advanced non-small cell lung cancer. Asia Pac J Clin Oncol 2018;14:264–9. [DOI] [PubMed] [Google Scholar]
- [20].Lan CY, Wang Y, Xiong Y, et al. Apatinib combined with oral etoposide in patients with platinum-resistant or platinum-refractory ovarian cancer (AEROC): a phase 2, single-arm, prospective study. Lancet Oncol 2018;19:1239–46. [DOI] [PubMed] [Google Scholar]
- [21].He K, Wu L, Ding Q, et al. Apatinib promotes apoptosis of pancreatic cancer cells through downregulation of hypoxia-inducible factor-1 alpha and increased levels of reactive oxygen species. Oxid Med Cell Longev 2019;5152072. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [22].Qiu H, Li J, Liu Q, et al. Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor growth in cervical cancer and synergizes with Paclitaxel. Cell Cycle 2018;17:1235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aras M, Erdil TY, Dane F, et al. Comparison of WHO, RECIST 1.1, EORTC, and PERCIST criteria in the evaluation of treatment response in malignant solid tumors. Nucl Med Commun 2016;37:9–15. [DOI] [PubMed] [Google Scholar]
- [24].Neeman E, Gresham G, Ovasapians N, et al. Comparing physician and Nurse Eastern Cooperative Oncology Group Performance Status (ECOG-PS) ratings as predictors of clinical outcomes in patients with cancer. Oncologist 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grover S, Bvochora-Nsingo M, Yeager A, et al. Impact of human immunodeficiency virus infection on survival and acute toxicities from chemoradiation therapy for cervical cancer patients in a limited-resource setting. Int J Radiat Oncol Biol Phys 2018;101:201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hirte H, Kennedy EB, Elit L, et al. Systemic therapy for recurrent, persistent, or metastatic cervical cancer: a clinical practice guideline. Curr Oncol 2015;22:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rosen VM, Guerra I, McCormack M, et al. Systematic review and network meta-analysis of bevacizumab plus first-line topotecan-paclitaxel or cisplatin-paclitaxel versus non-bevacizumab-containing therapies in persistent, recurrent, or metastatic cervical cancer. Int J Gynecol Cancer 2017;27:1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu WJ, Liu XJ, Xu J, et al. EGFR-targeting, beta-defensin-tailored fusion protein exhibits high therapeutic efficacy against EGFR-expressed human carcinoma via mitochondria-mediated apoptosis. Acta Pharmacol Sin 2018;39:1777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen JL, Huang CY, Huang YS, et al. Differential clinical characteristics, treatment response and prognosis of locally advanced adenocarcinoma/adenosquamous carcinoma and squamous cell carcinoma of cervix treated with definitive radiotherapy. Acta Obstet Gynecol Scand 2014;93:661–8. [DOI] [PubMed] [Google Scholar]
- [30].Lee JY, Kim YT, Kim S, et al. Prognosis of cervical cancer in the era of concurrent chemoradiation from National Database in Korea: a comparison between squamous cell carcinoma and adenocarcinoma. PLoS One 2015;10:e0144887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou J, Wu SG, Sun JY, et al. Comparison of clinical outcomes of squamous cell carcinoma, adenocarcinoma, and adenosquamous carcinoma of the uterine cervix after definitive radiotherapy: a population-based analysis. J Cancer Res Clin Oncol 2017;143:115–22. [DOI] [PubMed] [Google Scholar]
- [32].Manders DB, Sims TT, Albuquerque KV, et al. Emphasis on systemic therapy in women with pelvic bone metastasis at time of diagnosis of cervical cancer. Am J Clin Oncol 2018. [DOI] [PubMed] [Google Scholar]
- [33].Scott AJ, Messersmith WA, Jimeno A. Apatinib: a promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today (Barc) 2015;51:223–9. [DOI] [PubMed] [Google Scholar]
- [34].Roviello G, Ravelli A, Polom K, et al. Apatinib: a novel receptor tyrosine kinase inhibitor for the treatment of gastric cancer. Cancer Lett 2016;372:187–91. [DOI] [PubMed] [Google Scholar]
- [35].Liu X, Qin S, Wang Z, et al. Early presence of anti-angiogenesis-related adverse events as a potential biomarker of antitumor efficacy in metastatic gastric cancer patients treated with apatinib: a cohort study. J Hematol Oncol 2017;10:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fan M, Zhang J, Wang Z, et al. Phosphorylated VEGFR2 and hypertension: potential biomarkers to indicate VEGF-dependency of advanced breast cancer in anti-angiogenic therapy. Breast Cancer Res Treat 2014;143:141–51. [DOI] [PubMed] [Google Scholar]


