Abstract
Background:
There is considerable evidence that prolonged use of cervical collars potentially cause detrimental effects including increase in optic nerve sheath diameter (ONSD) among healthy volunteers. Different types of cervical collars immobilize cervical spine variably well and may presumably differently influence the venous compression and hence the intracranial pressure. We therefore aimed to evaluate the influence of cervical spine immobilization with 5 different types of cervical collars on ONSD measured noninvasively by ultrasound on healthy volunteers.
Methods:
We conducted a randomized crossover trial including 60 adult healthy volunteers. Control assessment of the optic nerve sheath thickness was performed in both sagittal and transverse planes. Patient was placed supine on a transport stretcher, cervical collar was placed, and ONSD measurement was performed after 5 and 20 minutes. During the next days, the procedure was repeated with random allocation of participants and random cervical collar.
Results:
Sixty healthy volunteers were included in our study. ONSD left diameter [mm] for the baseline was 3.8 [interquartile range (IQR): 3.65–3.93)] mm. Using AMBU after 5 min, ONSD was changed up to 4.505 (IQR 4.285–4.61; P < .001) mm. The largest change at 5 minutes and 20 minutes was using Philly 4.73 (IQR: 4.49–4.895; P < .001) and 4.925 (IQR: 4.65–5.06; P < .001), respectively. Necklite reported the lower change in ONSD: 3.92 (IQR: 3.795–4; P = 1.0) mm in 5 minutes and 3.995 (IQR: 3.875 – 4.1; P = 1.0) mm in 20 minutes. ONSD right diameter [mm] for the baseline was 3.8 (IQR 3.675–3.9) mm. Using AMBU after 5 minutes, ONSD was changed up to 4.5 (IQR 4.21–4.6) mm. The largest change at 5 minutes and 20 minutes was using Philly 4.705 (IQR 4.455–4.9) and 4.93 (IQR 4.645–5.075), respectively. Necklite reported the lower change in ONSD -33.9 (IQR 3.795–3.99) mm in 5 minutes and 3.995 (IQR 3.86–4.09) mm in 20 minutes.
Conclusion:
We report significant increase of ONSD from the baseline after cervical collar placement among healthy volunteers at 5 minutes and 20 minutes interval. In addition, no significant difference was noted between ONSD measurements at 5 and 20 minutes. Clinicians should take proactive steps to assess the actual need of cervical collar case by case basis. Nonetheless, when needed, Necklite moldable neck brace seems to be a reasonable option.
Registration: ClinicalTrials database (www.clinicaltrials.gov, NCT03609879).
Keywords: cervical collar, intracranial pressure, medical simulation, optic nerve sheath diameter, ultrasonography
1. Introduction
On the basis of the expert and consensus opinion, multiple guidelines suggest utilizing cervical collar as an aid for immobilization following potential cervical spine injury.[1–4] Immobilization of the cervical spine in trauma patients appears reasonable, as immobilization intends to limit secondary injuries due to unintended cervical spine movements.[5,6] However, there is considerable concern that especially prolonged cervical spine immobilization is associated with negative outcomes, including increased risk of aspiration, difficult airways and, impaired venous return secondary leading to increased intracranial pressure (ICP).[7–12] Some authors also suggest to limit the use of cervical collars, or even not to use them at all.[13]
Elevated ICP is a major clinical concern, as it is clearly associated with mortality and poor neurologic outcomes.[14–16] Perhaps, consequently, elevated ICP related to a cervical collar is primarily attributed to venous compression followed by impaired drainage leading to brain milieu imbalances. As a consequence, transmitted pressures rapidly displace subarachnoid fluid into the rich trabeculation of the optic nerve sheath, thereby increasing its thickness.[17] Ultrasound imaging test of the optic nerve sheath diameter (ONSD) is highly sensitive and helpful noninvasive surrogate for ICP[18–23] (Fig. 1).
Figure 1.
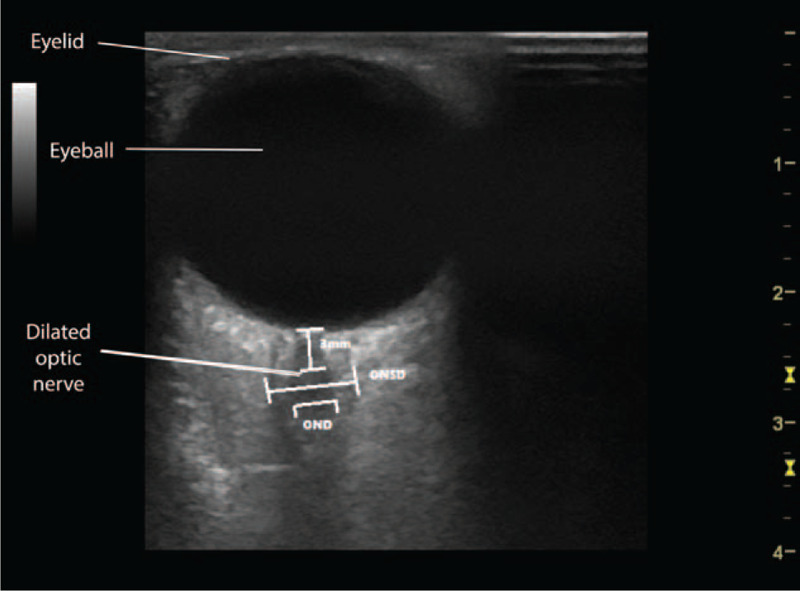
View of optic nerve sheath diameter view.
Previous work suggests that cervical collar application resulted in an increase in ONSD among healthy volunteers.[24,25] Different types of cervical collars immobilize the cervical spine variably well and may presumably differently influence the venous compression and hence the ICP. Which cervical collar is chosen presumably matters in head trauma patients because of variable effect on ICP.[7] These comparisons nonetheless may not be feasible in a vulnerable population, we therefore aimed to evaluate the influence of cervical spine immobilization with 5 different types of cervical collars on ONSD measured noninvasively by ultrasound on healthy volunteers.
2. Methods
The study protocol was approved by the Institutional Review Board of the Polish Society of Disaster Medicine (Approval No. 15/02/2017.IRB) and a priori registered in the ClinicalTrials database (www.clinicaltrials.gov, NCT03609879). Before the study, all participants were informed about the purpose of the study and written informed consent was obtained from each participant. Sixty participants were included in the study. Inclusion criteria composed of healthy subjects between 18 and 50 years of age who had the carotid sinus ultrasound assessed for pathological changes. Exclusion criteria consisted of head injury within 6 months preceding the examination, pathological changes in the cervical sinus, or refusal to participate in the study.
The study was designed as a randomized, crossover study. The study evaluated 5 widely available and used cervical collars by 5 separate producers:
-
(1)
Ambu Perfit ACE (AMBU; Ambu A/S, Ballerup, Denmark);
-
(2)
Philly One-Peace Collar (PHILLY; Armstrong Medical Industries, Inc., Lincolnshire, IL);
-
(3)
Necloc Collar (NECLOC; Össur Americas, Foothill Ranch, CA);
-
(4)
NexSplit Plus (NexSplit; IV Tactical Ltd, Buckinghamshire, UK);
-
(5)
New NECKLITE moldable neck brace (FLAMOR SRL, San Pietro Mosezzo, Italy).
Before the study, a control assessment of the optic nerve sheath thickness was performed. Each eye was scanned in both sagittal and transverse planes. ONSD was measured with probe in transverse plane and then in vertical plane at 3 mm distal to origin of optic nerve. The average of those 2 values was calculated and was a final diameter. For this purpose, an ultrasound device EZONO 5000 (eZono AG, Jena, Germany) was used equipped with a using a 13-6 MHz linear-array probe. Both eyes were examined. During the test, the cervical collar was placed in accordance with the manufacturer's instructions, adjusted to the size of the patient. The patient was placed on a transport stretcher for a period of 20 minutes; the ONSD measurement was performed in the 5th and 20th minute of the collar placement. During the next days, the procedure was repeated using the second type of cervical collar. Both the order of use of the neck collars and the order of the participants were random. For this purpose, the Research Randomizer program (randomizer.org) was used. A detailed randomization procedure is shown on Fig. 3. Simple Regression analysis was utilized to determine the relationship of an independent variable (age) on a dependent variable (delta change in ONSD at 5 and 20 minutes) with the assumption that all other variables remain fixed.
Figure 3.
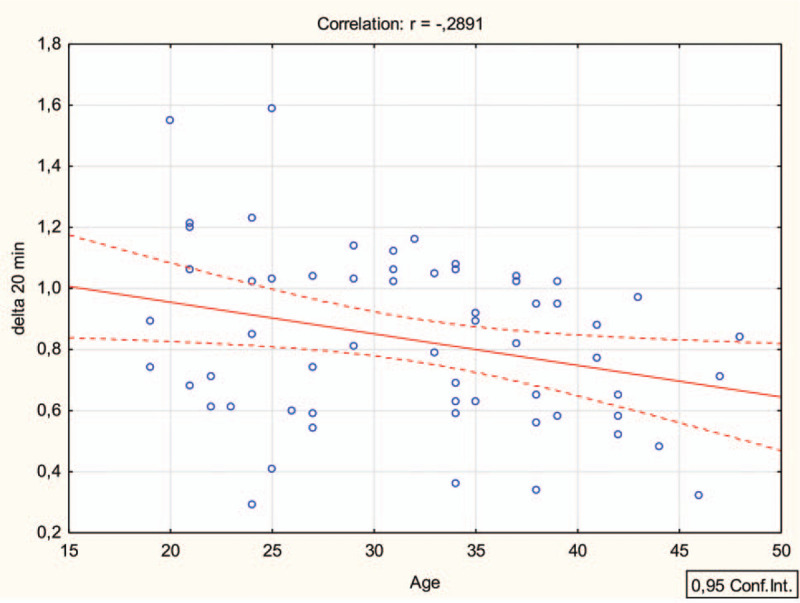
Simple linear regression analysis (Pearson) delta 20 min (right ONSD) and age.
2.1. Statistical analysis
On the basis of previous studies, we calculated the necessary sample size of at least 45 participants using the G∗ Power 3.1 software (Heinrich-Heine-Universität, Düsseldorf, Germany) (2-tailed t-test; Cohen d, 0.8; alpha error, 0.05; power, 0.95). In order to increase the power of the study, we decided to qualify 60 participants.
Results were blinded at the stage of statistical analysis. Statistical analysis was performed with the Statistica software version 13.3 for Windows (Tibco Inc., Tulsa). The level of significance was set at the value of P < .05. Data are presented as median [interquartile range (IQR)], as appropriate. Nonparametric tests were used for the data that did not have a normal distribution, which was tested with the Lilliefors test and the Shapiro–Wilk test. All statistical tests were 2-sided. The 1-way analysis of variance (ANOVA) on ranks was applied to compare the different times and to determine the statistical difference for each group (post-hoc).
3. Results
Sixty healthy volunteers were included in our study (Table 1). Control assessment of both the optic nerve sheath thickness was performed using the standard measurement method. ONSD left diameter [mm] for the baseline was 3.8 (IQR: 3.65–3.93) mm. Using AMBU after 5 minutes, ONSD was changed up to 4.505 (IQR 4.285–4.61) mm and to 4.655 (IQR: 4.41–4.82) after 20 minutes. The largest change at 5 minutes and 20 minutes was using Philly 4.73 (IQR: 4.49–4.895) and 4.925 (IQR: 4.65–5.06), respectively. Necklite reported the lowest change in ONSD: 3.92 (IQR: 3.795–4;) mm in 5 minutes and 3.995 (IQR: 3.875–4.1) mm in 20 minutes, while Necloc 4.27 (IQR: 4.15–4.395) mm in 5 minutes and 4.415 (IQR: 4.27–4.55) and NexSplint 4.705 (IQR: 4.52–4.935) mm in 5 minutes and 4.92 (IQR: 4.68–5.115), Table 2.
Table 1.
Participants baseline characteristics.
Table 2.
ONSD left.
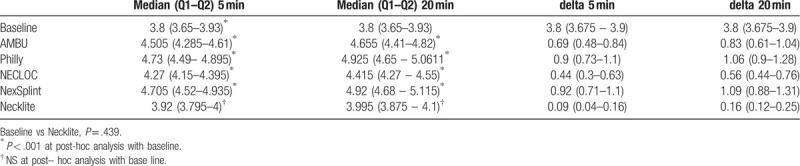
ONSD right diameter [mm] for the baseline was 3.8 (IQR: 3.675–3.9) mm. Using AMBU after 5 minutes, ONSD was changed up to 4.5 (IQR: 4.21–4.6) mm and to 4.615 (IQR: 4.375–4.805) after 20 minutes. The largest change at 5 minutes and 20 minutes was using Philly 4.705 (IQR: 4.455–4.9) and 4.93 (IQR: 4.645–5.075), respectively. Necklite reported the lowest change in ONSD: 33.9 (IQR: 3.795–3.99) mm in 5 minutes and 3.995 (IQR: 3.86–4.09) mm in 20 minutes, while Necloc 4.29 (IQR: 4.14–4.375) mm in 5 minutes and 4.38 (IQR: 4.265–4.52) and NexSplint 4.72 (IQR: 4.5–4.92) mm in 5 minutes and 4.9 (IQR: 4.67–5.1), Table 3, Fig. 2.
Table 3.
ONSD right.
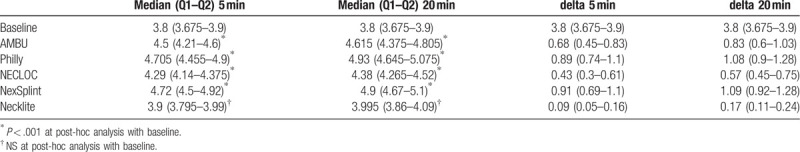
Figure 2.
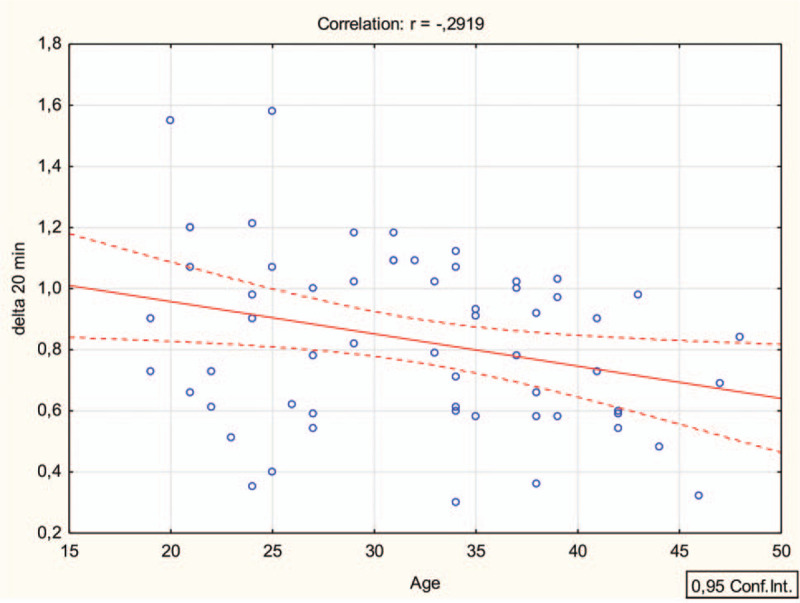
Simple linear regression analysis (Pearson) delta 5 min (right ONSD) and age.
The 1-way ANOVA confirmed the existence of statistically significant differences (all P for trend < .05) between age and delta 5 minutes and 20 minutes after ONSD baseline, Fig. 3.
4. Discussion
We utilized 5 different types of cervical collars to assess and compare the difference in change in ONSD from the baseline. Our analysis demonstrated a statistically significant increase in the ONSD from the baseline among healthy volunteers at 5 and 20-minute interval, respectively. The rise in ONSD is primarily related to the restrictive ability of the cervical collar around the neck vasculature.[26] Previous work suggests that all cervical collars—irrespective of the types—are restrictive in nature.[26] Similarly, all cervical collars in our study were found to be restrictive and likewise an increase of ONSD was noted. However, among all, Philly cervical collar appears to cause the maximum increase in ONSD. Our findings basically confirm previous findings by Tescher et al[26] who measured craniofacial tissue—interface pressure and cervical range of motion to assess the restrictive ability of various collars. The authors concluded that the Philadelphia collar was the most restrictive among other collars such as Aspen, Miami J/Occian. Similarly, other studies also reported the similar results for Philadelphia collars.[27,28]
In our study, Necklite cervical collar was reported to have the lowest change in ONSD and no statistical or clinical meaningful difference was noted between baseline and delta change in ONSD at 5 and 20 minutes, probably because of its pliable and relatively less restrictive nature. This finding extends the results of our pilot study in which we reported a statistically significant reduction in the pressure on the mastoid process, subjective pain scores, improvement in degree of mouth opening with Necklite, when compared with AMBU cervical collar.[29] Necklite was also noted to have reduced increase in ONSD when compared with Patroit collar in our other preliminary study.[30] Inherent cervical collar design characteristics may partly explain the significant differences in outcomes. Moldable collars (such as Necklite) allow full adjustment to accommodate each patient's under chain anatomical features, whereas stiff collars (such as AMBU, Philly, Nexsplint, Necloc) primarily immobilize spine by mandible compression that inadvertently compresses jugular veins.[20]
Surprisingly, no significant difference was noted between ONSD measurements at 5 and 20 minutes. In contrast, one would expect an exponential rise in ICP as a function of time when compensatory mechanisms (such as cerebral autoregulation) are exhausted. Importantly, the exponential rise in ONSD may not be evident in a healthy young volunteer, probably due to intact autoregulation mechanism. We caution, though, that rise in ICP may be more detrimental in traumatic brain injury patients when compensatory mechanisms are impaired—and presumably becomes more pronounced with a cervical collar.
Positioning in head trauma patients is another consideration. Our patient remained supine throughout the 20-minute interval. However, head trauma patients oftentimes are positioned in reverse Trendelenburg position to reduce ICP without jeopardizing cerebral perfusion.[31–33] A study by Romagnuolo et al[34] showed that ONSD measurement does not change significantly with position in healthy supine patients. It, therefore, seems unlikely that supine position per se played a role in the rise in ONSD in our healthy patients. Certainly, it does not imply that supine position as opposed to reverse Trendelenburg is safe; however, it is still unknown whether high ICP has any effect on position change in head trauma patients. Nevertheless, position change does have a potential plausible role in the management of ICP.[16,35–37]
We found a weak negative correlation between age and change in ONSD at 5 and 20 minutes of cervical collar placement. Although comparable, no clinically meaningful difference was noted in right and left ONSD and between 5 and 20 minutes. Notably, very few individuals account for 45 to 50 years age group and moreover demonstrable decline in cerebrovascular reactivity may not be evident at this age. How much pressure change a cervical collar can impart on elderly traumatic brain injury patients is still unknown. This is important because the incidence of traumatic brain injury is high among elderly with poorer survival and functional outcomes.[38]
Our study had several limitations. The study participants were healthy, relatively young volunteers and therefore limit the external validity of our results to actual head trauma patients. ONSD measurements are subjective and significant inter-evaluator variability may be observed. For instance, the measurements are taken at millimeter distance and slight change may result in differences in readings. In addition, the measurement of distance from the globe is not defined uniformly in the existing literature.[39–41] The evaluators were not blinded in our study.
In conclusion, our results demonstrated a statistically significant increase in the ONSD from baseline after cervical collar placement among healthy volunteers at 5 minutes and 20 minutes interval, respectively. Out of 5 cervical collars, Philly cervical collar appears to have maximum, while Necklite reported the lowest change in ONSD. In addition, no significant difference was noted between ONSD measurements at 5 and 20 minutes. This increase may be much more detrimental to traumatic brain injury patients. Future trials are needed to assess the ICP change by the cervical collar in traumatic brain injury patients. In the interim, clinicians should take proactive steps to assess the actual need for a cervical collar, on a case-by-case basis. However, when needed, Necklite seems to be a reasonable option.
Author contributions
Conceptualization: Michal Ladny, Lukasz Szarpak, Kurt Ruetzler.
Data curation: Michal Ladny, Lukasz Szarpak.
Formal analysis: Michal Ladny, Lukasz Szarpak.
Investigation: Michal Ladny, Jacek Smereka, Lukasz Szarpak.
Methodology: Michal Ladny, Lukasz Szarpak, Kurt Ruetzler.
Project administration: Lukasz Szarpak, Jerzy Robert Ladny.
Resources: Michal Ladny, Jacek Smereka, Lukasz Szarpak, Jerzy Robert Ladny.
Software: Lukasz Szarpak, Jerzy Robert Ladny.
Supervision: Lukasz Szarpak.
Validation: Sanchit Ahuja, Lukasz Szarpak, Kurt Ruetzler.
Visualization: Lukasz Szarpak.
Writing – original draft: Michal Ladny, Jacek Smereka, Lukasz Szarpak, Kurt Ruetzler, Jerzy Robert Ladny.
Writing – review & editing: Michal Ladny, Jacek Smereka, Sanchit Ahuja, Lukasz Szarpak, Kurt Ruetzler, Jerzy Robert Ladny.
Lukasz Szarpak orcid: 0000-0002-0973-5455
Footnotes
Abbreviations: ANOVA = Analysis of variance, ICP = Intracranial pressure, IQR = Interquartile range, IRB = Institutional Review Board, ONSD = Optic nerve sheath diameter.
How to cite this article: Ladny M, Smereka J, Ahuja S, Szarpak L, Ruetzler K, Ladny JR. Effect of five different cervical collar on optic nerve sheath diameter: A randomized crossover trial. Medicine. 2020;99:16(e19740).
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
The authors have no conflict of interests to disclose.
References
- [1].American College of Surgeons. Committee on trauma. ATLS: advanced trauma life support for doctors (student course manual). In: Sharon Henry MKB, Stewart RM, eds. 10th ed. Chicago, IL: 2018 American College of Surgeons; 2008. [Google Scholar]
- [2].Hadley MN, Walters BC, Grabb PA, et al. Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg 2002;49:407–98. [PubMed] [Google Scholar]
- [3].Toscano J. Prevention of neurological deterioration before admission to a spinal cord injury unit. Paraplegia 1988;26:143–50. [DOI] [PubMed] [Google Scholar]
- [4].Johansson J, Blomberg H, Svennblad B, et al. Prehospital Trauma Life Support (PHTLS) training of ambulance caregivers and impact on survival of trauma victims. Resuscitation 2012;83:1259–64. [DOI] [PubMed] [Google Scholar]
- [5].Oosterwold JT, Sagel DC, van Grunsven PM, et al. The characteristics and pre-hospital management of blunt trauma patients with suspected spinal column injuries: a retrospective observational study. Eur J Trauma Emerg Surg 2017;43:513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Deasy C, Cameron P. Routine application of cervical collars – What is the evidence? Injury 2011;42:841–2. [DOI] [PubMed] [Google Scholar]
- [7].Kwan I, Bunn F, Roberts I. Spinal immobilisation for trauma patients. Cochrane Database Syst Rev 2001;Cd002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Abram S, Bulstrode C. Routine spinal immobilization in trauma patients: what are the advantages and disadvantages? Surgeon 2010;8:218–22. [DOI] [PubMed] [Google Scholar]
- [9].Benger J, Blackham J. Why do we put cervical collars on conscious trauma patients? Scand J Trauma Resusc Emerg Med 2009;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hauswald M, Braude D. Spinal immobilization in trauma patients: is it really necessary? Curr Opin Crit Care 2002;8:566–70. [DOI] [PubMed] [Google Scholar]
- [11].Sporer KA. Why we need to rethink C-spine immobilization: we need to reevaluate current practices and develop a saner cervical policy. EMS world 2012;41:74–6. [PubMed] [Google Scholar]
- [12].Aoi Y, Inagawa G, Hashimoto K, et al. Airway scope laryngoscopy under manual inline stabilization and cervical collar immobilization: a crossover in vivo cinefluoroscopic study. J Trauma 2011;71:32–6. [DOI] [PubMed] [Google Scholar]
- [13].Sundstrøm T, Asbjørnsen H, Habiba S, et al. Prehospital use of cervical collars in trauma patients: a critical review. J Neurotrauma 2014;31:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miller JD, Becker DP, Ward JD, et al. Significance of intracranial hypertension in severe head injury. J Neurosurg 1977;47:503–16. [DOI] [PubMed] [Google Scholar]
- [15].Vik A, Nag T, Fredriksli OA, et al. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg 2008;109:678–84. [DOI] [PubMed] [Google Scholar]
- [16].Hoefnagel AL, Rajan S, Martin A, et al. Cognitive aids for the diagnosis and treatment of neuroanesthetic emergencies: consensus guidelines on behalf of the Society for Neuroscience in Anesthesiology and Critical Care (SNACC) Education Committee. J Neurosurg Anesthesiol 2019;31:7–17. [DOI] [PubMed] [Google Scholar]
- [17].Stone MB, Tubridy CM, Curran R. The effect of rigid cervical collars on internal jugular vein dimensions. Acad Emerg Med 2010;17:100–2. [DOI] [PubMed] [Google Scholar]
- [18].Hassen GW, Bruck I, Donahue J, et al. Accuracy of optic nerve sheath diameter measurement by emergency physicians using bedside ultrasound. J Emerg Med 2015;48:450–7. [DOI] [PubMed] [Google Scholar]
- [19].Ohle R, McIsaac SM, Woo MY, et al. Sonography of the optic nerve sheath diameter for detection of raised intracranial pressure compared to computed tomography: a systematic review and meta-analysis. J Ultrasound Med 2015;34:1285–94. [DOI] [PubMed] [Google Scholar]
- [20].Ferguson J, Mardel SN, Beattie TF, et al. Cervical collars: a potential risk to the head-injured patient. Injury 1993;24:454–6. [DOI] [PubMed] [Google Scholar]
- [21].Wang J, Li K, Li H, et al. Ultrasonographic optic nerve sheath diameter correlation with ICP and accuracy as a tool for noninvasive surrogate ICP measurement in patients with decompressive craniotomy. J Neurosurg 2019;1–7. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [22].Robba C, Cardim D, Tajsic T, et al. Ultrasound non-invasive measurement of intracranial pressure in neurointensive care: a prospective observational study. PLoS Med 2017;14:e1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Raffiz M, Abdullah JM. Optic nerve sheath diameter measurement: a means of detecting raised ICP in adult traumatic and non-traumatic neurosurgical patients. Am J Emerg Med 2017;35:150–3. [DOI] [PubMed] [Google Scholar]
- [24].Woster CM, Zwank MD, Pasquarella JR, et al. Placement of a cervical collar increases the optic nerve sheath diameter in healthy adults. Am J Emerg Med 2018;36:430–4. [DOI] [PubMed] [Google Scholar]
- [25].Yard J, Richman PB, Leeson B, et al. The influence of cervical collar immobilization on optic nerve sheath diameter. J Emerg Trauma Shock 2019;12:141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tescher AN, Rindflesch AB, Youdas JW, et al. Range-of-motion restriction and craniofacial tissue-interface pressure from four cervical collars. J Trauma 2007;63:1120–6. [DOI] [PubMed] [Google Scholar]
- [27].Sandler AJ, Dvorak J, Humke T, et al. The effectiveness of various cervical orthoses. An in vivo comparison of the mechanical stability provided by several widely used models. Spine (Phila Pa 1976) 1996;15(21):1624–9. [DOI] [PubMed] [Google Scholar]
- [28].Gavin TM, Carandang G, Havey R, et al. Biomechanical analysis of cervical orthoses in flexion and extension: a comparison of cervical collars and cervical thoracic orthoses. J Rehabil Res Dev 2003;40:527–37. [DOI] [PubMed] [Google Scholar]
- [29].Ladny M, Szarpak L. Ladny JR: assessment of the cervical collar application impact on the conditions of intubation and the feelings of patients: pilot study. Disaster Emerg Med J 2018;3:1–4. [Google Scholar]
- [30].Szarpak L, Pyda S, Madziala M, et al. Comparison of two cervical collars on the intracranial pressure measured indirectly based on the thickness of the optic nerve sheath. Preliminary data. Post N Med 2018;31:68–71. [Google Scholar]
- [31].Mavrocordatos P, Bissonnette B, Ravussin P. Effects of neck position and head elevation on intracranial pressure in anaesthetized neurosurgical patients: preliminary results. J Neurosurg Anesthesiol 2000;12:10–4. [DOI] [PubMed] [Google Scholar]
- [32].Meixensberger J, Baunach S, Amschler J, et al. Influence of body position on tissue-pO2, cerebral perfusion pressure and intracranial pressure in patients with acute brain injury. Neurol Res 1997;19:249–53. [DOI] [PubMed] [Google Scholar]
- [33].Rolighed Larsen JK, Haure P, Cold GE. Reverse Trendelenburg position reduces intracranial pressure during craniotomy. J Neurosurg Anesthesiol 2002;14:16–21. [DOI] [PubMed] [Google Scholar]
- [34].Romagnuolo L, Tayal V, Tomaszewski C, et al. Optic nerve sheath diameter does not change with patient position. Am J Emerg Med 2005;23:686–8. [DOI] [PubMed] [Google Scholar]
- [35].Czosnyka M, Balestreri M, Steiner L, et al. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg 2005;102:450–4. [DOI] [PubMed] [Google Scholar]
- [36].Fjell AM, Walhovd KB, Fennema-Notestine C, et al. One-year brain atrophy evident in healthy aging. J Neurosci 2009;29:15223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kinoshita K. Traumatic brain injury: pathophysiology for neurocritical care. J Intensive Care 2016;4:29–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Karibe H, Hayashi T, Narisawa A, et al. Clinical characteristics and outcome in elderly patients with traumatic brain injury: for establishment of management strategy. Neurol Med Chir (Tokyo) 2017;57:418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Passi N, Degnan AJ, Levy LM. MR imaging of papilledema and visual pathways: effects of increased intracranial pressure and pathophysiologic mechanisms. AJNR Am J Neuroradiol 2013;34:919–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Frumin E, Schlang J, Wiechmann W, et al. Prospective analysis of single operator sonographic optic nerve sheath diameter measurement for diagnosis of elevated intracranial pressure. West J Emerg Med 2014;15:217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kimberly HH, Noble VE. Using MRI of the optic nerve sheath to detect elevated intracranial pressure. Crit Care (London, England) 2008;12:181. [DOI] [PMC free article] [PubMed] [Google Scholar]


