Supplemental Digital Content is available in the text
Keywords: calibration, discrimination, distant metastasis, nomogram, pancreatic neuroendocrine tumor
Abstract
As a rare malignant tumor, pancreatic neuroendocrine tumor (pNET) has very low incidence. However, most of the pNET patients would develop the distant metastasis, which significantly reduces patients’ survival rate. Therefore, it is very important to construct a prognostic model of pNET patients with distant metastasis based on a large database to guide clinical application and treatment. The aim of this study is to establish nomograms for cancer-specific survival (CSS) and overall survival (OS) of patients with distant metastatic pNET based on the Surveillance, Epidemiology, and End Results (SEER) database.
SEER were reviewed and the patients with pNET diagnosed between 1973 and 2015 were selected. After screening, a total of 624 cases were included in the study. Patients were randomly divided into a training cohort (n = 416) and a validation cohort (n = 208). Cox proportional hazard analysis revealed that age at diagnosis of ≥80 years, year of diagnosis, histological grade, and primary site surgery were independent factors both for CSS and OS. The nomograms indicated good accuracy in predicting 1-, 3-, and 5-year survival, with a C-index of 0.777 (95% confidence interval [CI], 0.743–0.811) for CSS and 0.772 (95% CI 0.738–0.806) for OS in training cohort. In the validation cohort, the C-index was 0.798 (95% CI 0.755–0.841) for CSS and 0.797 (95% CI 0.753–0.841) for OS. The calibration curves showed satisfactory consistency between predicted and actual survival.
The study establishes excellent prognostic nomograms for CSS and OS for pNET patients with distant metastasis. They can be used to accurately predict survival rate, and provide useful information to physicians and patients.
1. Introduction
As a rare neoplasm, neuroendocrine tumor (NET) may occur in various organs, including lung, gastrointestinal tract, pancreas, etc.[1,2] Pancreatic neuroendocrine tumor (pNET) originates from pancreatic neuroendocrine cells and represents approximately 7% of all NETs and accounts for 1% to 2% of all pancreatic malignancies.[1–3] Over the past several decades, the annual incidence of pNET has been increasing in the United States.[4] pNETs have great variance in biological behavior. Most pNETs have low malignant behavior and slow growth rate, but some of them possess high ability of invasiveness.[5] Up to 60% to 80% of patients develop distant metastasis during the course of pNET.[6,7] Based on the data from Surveillance Epidemiology and End Results (SEER) database from 1973 to 2015 in the present study, 50.0% of patients (4212/8422) were diagnosed combining distant metastasis.
Previous studies have revealed that the presence of distant metastasis is one of the strongest predictors for patients’ survival. The 5-year survival rate was significantly worse than for patients without distant metastasis.[8] Most existing studies reported the outcome of distant metastasis from different origins, including colon, pancreas, lung, thymus, and stomach. However, few studies have exclusively focused on the pNET with distant metastasis, and the factors associated with survival outcome have not been clearly elucidated. The characteristics and survival outcomes of pNET with distant metastasis is yet to be answered. Thus, the aim of current study is to explore and elucidate the factors associated with survival in patients with metastatic pNET with nomograms.
Nomogram is a mathematical model that combines characteristics to predict specific endpoints. Nomogram allows predictions to be obtained easily and quickly in practice and can visualize the results of regression analysis. Furthermore, nomogram can provide personalized estimates of the probability of events, such as individual disease recurrence or death by integrating various factors. Nomogram has been widely used as a practical predictive tool for survival analysis for malignancies.[9–11] However, the nomogram applicable for metastatic pNET has not been constructed. In the present study, we aim to develop and validate the nomograms predicting cancer-specific survival (CSS) and overall survival (OS) for patients with metastatic pNET based on a population data from National Cancer Institute SEER database.
2. Materials and methods
2.1. Ethical approval
This study does not contain any studies with human participants or animals performed by any of the authors. In addition, according to the guidelines of the government of the United States, data released through the SEER database does not require informed patient consent.
2.2. Data source and patient selection
The data in this study were obtained from the SEER database, using SEER∗stat software (version 8.3.5). The SEER database collects the information of cancer incidence, prevalence, mortality, demographics, cancer characteristics, and treatment, and covers approximately 27.8% of the U.S. population (based on the 2010 census) from 1973 to 2015.
The data of patients with pNET diagnosed between 1973 and 2015 were retrieved, and the patients with distant metastatic pNET were selected. PNET was defined as including the following International Classification of Diseases for Oncology, third edition (ICD-O3) codes: 8150/3, 8151/3, 8152/3, 8153/3, 8155/3, 8156/3, 8240/2, 8240/3, 8241/3, 8242/3, 8243/3, 8246/2, 8246/3, 8249/3. All pancreatic anatomical sites were included (C25.0–C25.9) in our study. All the data were screened carefully, and the exclusion criteria are discussed in the next section (see Fig. 1).
Figure 1.
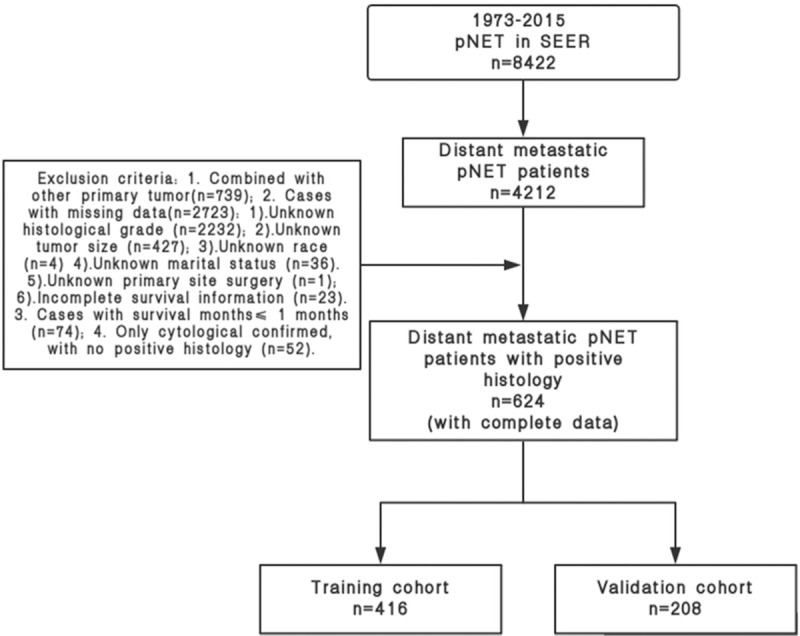
Patients’ screening process.
2.2.1. Exclusion criteria
-
1)
Combined with other primary tumors (n = 739);
-
2)
Cases with missing data (n = 2723);
-
(I)
Unknown histological grade (n = 2232)
-
(II)
Unknown tumor size (n = 427)
-
(III)
Unknown race (n = 4)
-
(IV)
Unknown marital status (n = 36)
-
(V)
Unknown primary site surgery (n = 1)
-
(VI)
Incomplete survival information (n = 23)
-
(I)
-
3)
Cases with survival months ≤ 1 month (n = 74);
-
4)
Only cytological confirmed, with no positive histology (n = 52).
After exclusion, a total 624 patients with distant pNET were confirmed, and the patients were randomized to 2 groups (training cohort, n = 416; validation cohort, n = 208).
2.3. Data collection and outcome measurement
Variables of age at diagnosis, gender, race, year of diagnosis, site of the tumor, histological grade of tumor, tumor size, marital status, and information of primary site surgery were collected. In addition, the survival months and the cause of death were also confirmed.
The 2 primary outcomes were CSS and OS. Patients were followed until date of death or censored at the end of 2015. CSS was defined as the duration from diagnosis to death attributable to the pNET. OS was defined as the duration from diagnosis to death from any cause.
2.4. Selection of the independent factors and the construction of the nomograms
The model of nomograms was established based on the training cohort. A total of 416 patients in training set were included in the univariate Cox proportional hazard analysis for CSS and OS. The variables with P < .1 were furtherly analyzed by multivariate Cox proportional hazard model. According to the results of multivariate Cox proportional hazard analysis, nomograms combining all the independent prognostic factors were constructed for predicting 1-, 3-, and 5-year CSS and OS.
2.5. Validation of the nomograms
The prognostic performance of the nomograms was validated with discrimination and calibration. The discrimination of nomograms was assessed by Harrell C-index.[12] The Harrell C-index can estimate the probability between the observed and predicted survival outcome, and higher C-index indicates more precise prediction of survival outcome. The Kaplan–Meier method and bootstraps with 1000 resamples were used to assess calibration; the predicted probabilities produced by the nomograms were compared with actual probabilities.
2.6. Statistical analysis
The univariate and multivariate Cox proportional hazard analysis was performed using IBM SPSS Statistics 22.0 (SPSS Inc., Chicago, IL). The construction and validation of nomograms were conducted by R version 3.4.4 software (Institute for Statistics and Mathematics, Vienna, Austria; http://www.r-project.org/). The variables with P < .1 in univariate Cox analysis were further analyzed by multivariate Cox proportional hazard model. P values were 2-sided and P < .05 was regarded as statistically significance.
3. Results
3.1. Clinicopathologic characteristics of the patients and survival outcome
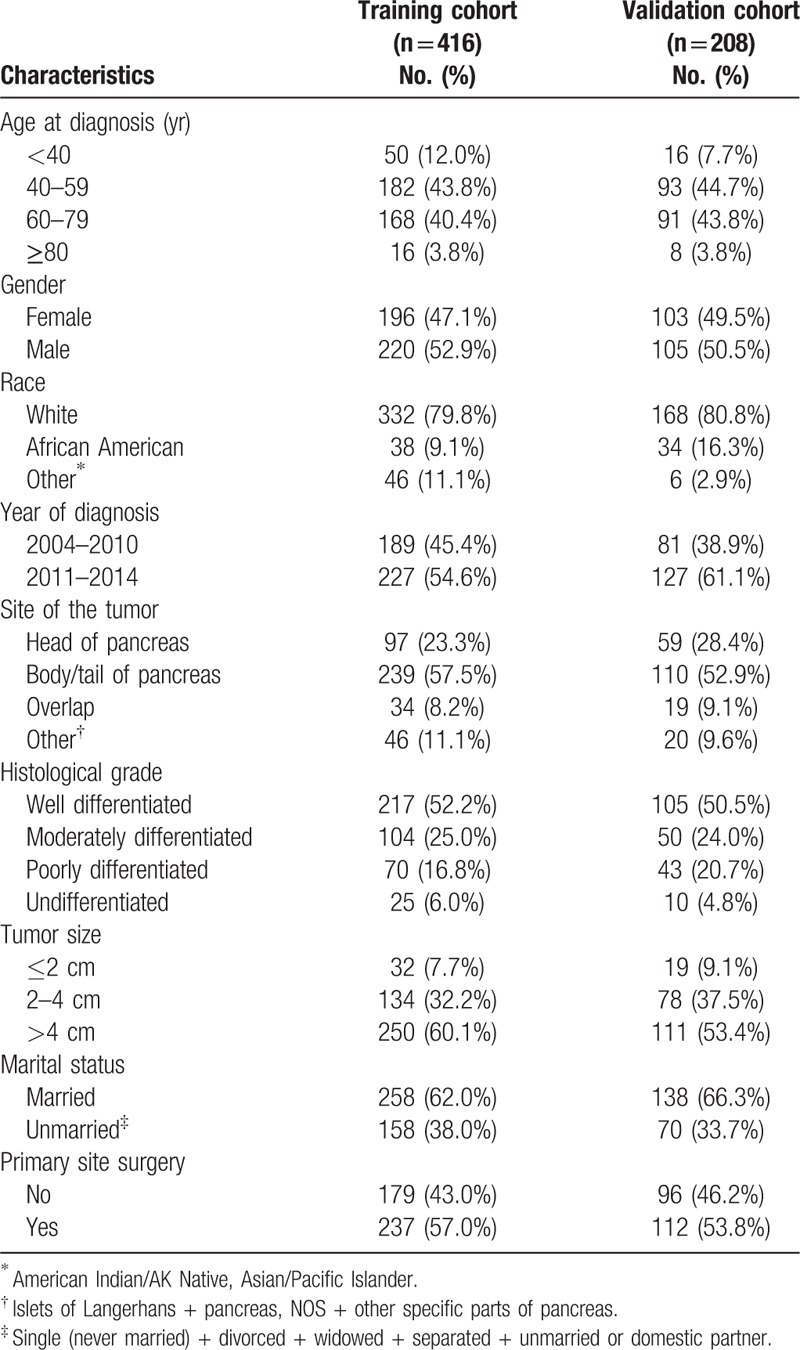
A total of 8422 patients with pNET were identified from SEER database. After screening, 624 cases were included in the study. Patients were randomly divided into a training cohort (n = 416) and a validation cohort (n = 208). The clinicopathologic characteristics of patients in the training and validation cohorts are listed in Table 1. The median CSS was 70 months (95% CI, 50.8–89.4 months) in the training cohort, and the 1-, 3-, and 5-year CSS rates were 82.2%, 62.9%, and 51.0%, respectively. The median OS was 56 months (95% CI, 41.6–70.4 months) in the training cohort, and the 1-, 3-, and 5-year OS rates were 81.3%, 61.0%, and 48.7%, respectively.
Table 1.
Characteristics of training cohort and validation cohort.
3.2. Independent prognostic factors in the training cohort and nomogram development
Univariate Cox proportional hazard analysis was performed for CSS and OS in the training cohort, and the variables with P < .1 were further analyzed by multivariate Cox proportional hazard model. As shown in Tables 2 and 3, age at diagnosis, year of diagnosis, histological grade, and primary site surgery were independent prognostic factors for CSS and OS (P < .05). The result of univariate and multivariate Cox proportional hazard analysis for CSS is listed in Table 2, and the result of univariate and multivariate Cox proportional hazard analysis for OS is listed in in Table 3. The development of nomograms was based on the above independent prognostic factors in the training cohort. The prognostic nomogram for 1-, 3-, and 5-year CSS and OS is shown in Figure 2.
Table 2.
Univariate and multivariate analysis for cancer-specific survival of the training cohort.
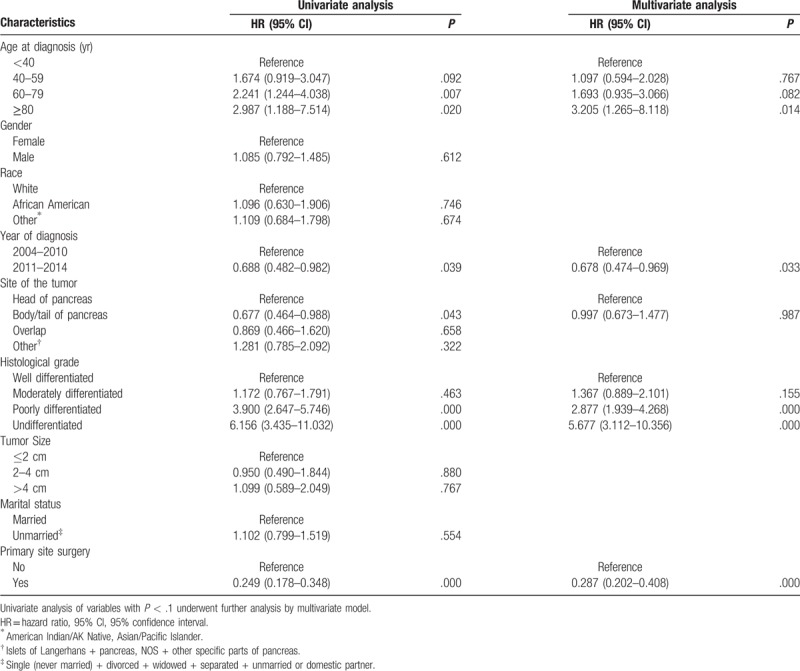
Table 3.
Univariate and multivariate analysis for overall survival of the training cohort.
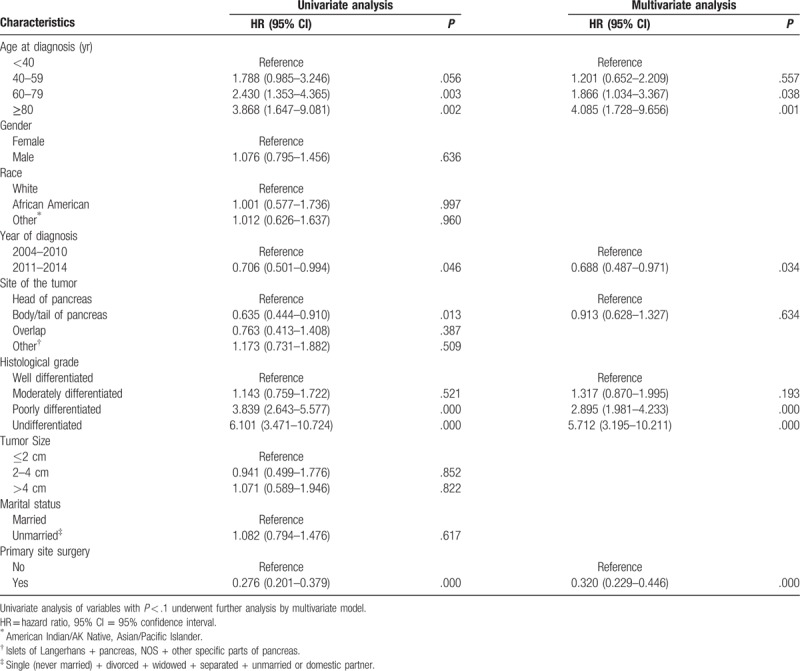
Figure 2.
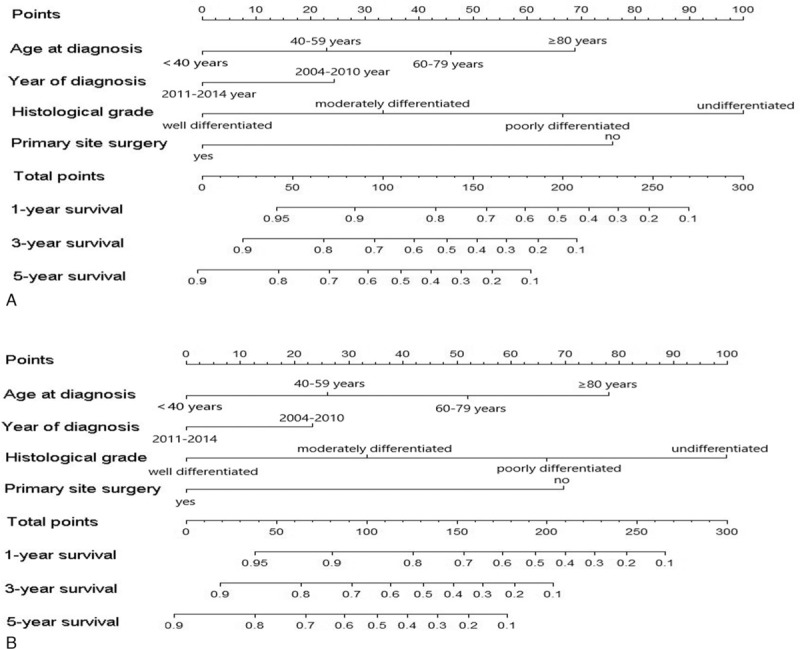
Nomograms for predicting the 1-, 3-, and 5-year (A) cancer-specific survival and (B) overall survival of pancreatic neuroendocrine tumor patients with distant metastasis.
3.3. Validation of the nomograms
The bootstrap analyses with 1000 resamples were performed internally (training cohort) and externally (validation cohort) to validate the nomograms. As listed in Table 4, analysis of the internal validation cohort (training cohort) showed C-index values of 0.777 (95% CI, 0.743–0.811) for nomogram predictions of CSS and 0.772 (95% CI, 0.738–0.806) for nomogram predictions of OS. In the external validation cohort, the C-index for predicting CSS and OS were 0.772 (95% CI, 0.738–0.806) and 0.797 (95% CI, 0.753–0.841), respectively (Table 4).
Table 4.
C-index for the nomogram to predict cancer-specific survival and overall survival.

The calibration curves of the training cohort and external cohort are presented in Figures 3 and 4. The x-axis represents the survival rate predicted by the nomogram, and the y-axis presents the actual survival rate generated by Kaplan–Meier method. The predicted 1-, 3-, and 5-year CSS and OS demonstrated excellent accordance to the observed values.
Figure 3.
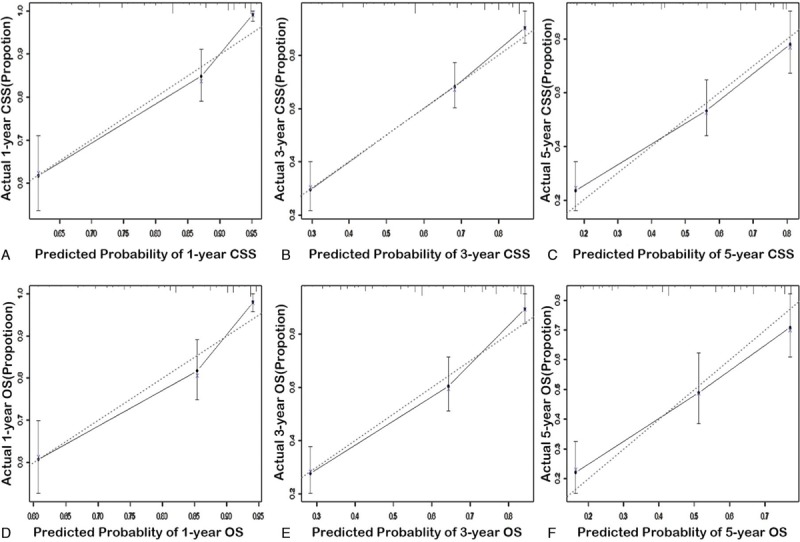
The calibration curves for prediction of 1-, 3-, and 5-year cancer-specific survival (A–C) and overall survival (D–F) in the training cohort (internal calibration). The dashed lines represent perfect agreement between the predicted probabilities (x-axis) and the actual probabilities which were calculated by Kaplan–Meier analysis (y-axis). A perfectly accurate nomogram prediction model would result in a plot where the actual and predicted probabilities fall along the 45° line.
Figure 4.
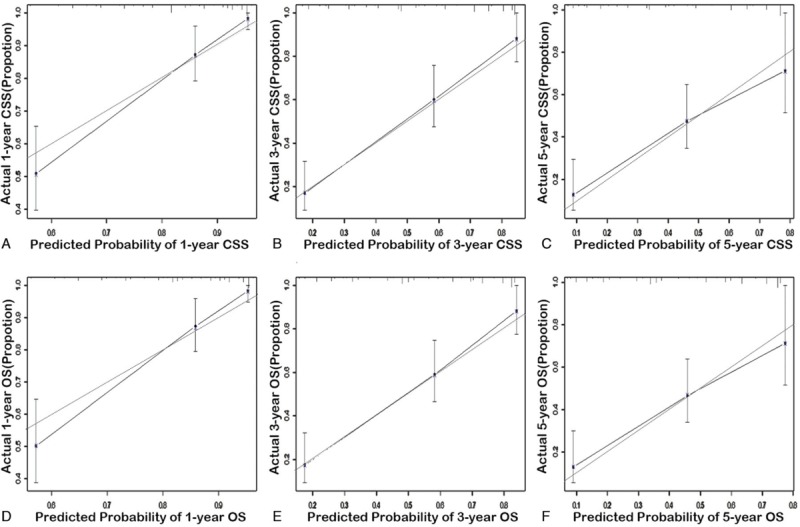
The calibration curves for prediction of 1-, 3-, and 5-year cancer-specific survival (A-C) and overall survival (D-F) in the validation cohort (external calibration). The dashed lines represent perfect agreement between the predicted probabilities (x-axis) and the actual probabilities which were calculated by Kaplan–Meier analysis (y-axis). A perfectly accurate nomogram prediction model would result in a plot where the actual and predicted probabilities fall along the 45° line.
4. Discussion
Nomogram uses disjoint lines to calculate the occurrence probability of events. When using the nomogram, the occurrence probability of events is usually estimated by vertical ruler. In practice, we often use a lot of clinical data and examination results of patients to predict the risk of patients, or to predict the survival probability of cancer patients. Essentially, nomogram is the visualization of the results of regression equation and is often used to display the results of logistic regression or Cox regression. According to the results of regression, multiple disjoint lines are drawn according to a specific proportion from the results of regression equation. By drawing it, the risk or survival probability of an individual can be easily calculated. In addition, researchers are required to have a sufficient number of research object data when making the nomogram, so as to effectively establish a good prediction model. As a rare disease, pNETs are not easy to study in a large population. Therefore, it is more necessary to study the oncological database, such as SEER database.
The prediction model needs to be validated before the application of the nomogram. The common validation processes are internal validation and external validation. Internal validation refers to the use of modeled data to verify the predictive effect of the model. Bootstrap self-sampling method can be used. Bootstrap self-sampling method is to carry out the sample with playback in the study sample, and then use the sample to calculate. External validation uses data from another group of subjects (i.e., external data) to validate the predictive accuracy of the model. Only when the prediction effect of the model has been clearly verified, can the nomogram have a good application value.
pNETs with distant metastasis should be treated mainly by systematic therapy, including surgery, somatostatin analogues, molecular targeted therapy, chemotherapy, and/or peptide receptor radionuclide therapy (PRRT) therapy. However, unfortunately, SEER database could not provide detailed systemic treatment information. From the results of this study, age ≥ 80 is an independent risk factor for stage IV pNET patients. This may be related to the fact that older patients tend to accompany more comorbidities and the choice of treatment options tends to be more conservative. Additionally, according to our previous study, the elderly pNET patients have increased possibility of poorly differentiated tumor, and decreased proportion of primary site surgery, number of removed lymph node, and married status.[13] These factors contribute to the poor prognosis of elderly patients.
In recent years, the research on neuroendocrine tumors has been deepened gradually in the medical field, and the therapeutic schemes have also made great progress. Molecular targeted therapy, immunotherapy, and PRRT therapy have begun to increase gradually for pNET in recent years. As a result, more patients received these treatments after 2011 than those before 2010. This may lead to different prognosis of patients with different years of diagnosis.
On the premise of the same stage, it is an indisputable fact that the higher the grade of tumor differentiation, the worse the prognosis of patients. It was also confirmed in the current study that the histological grade of tumor differentiation is an independent risk factor for prognosis.
Currently, there is still controversy on primary site surgery in stage IV pNET. In 2009, Bettini et al have shown that the benefit of primary tumors resection was to prevent symptoms arising from the tumors such as biliary or gastrointestinal obstruction and symptoms from functional tumors, rather than improving survival.[14] However, in recent years, more and more studies revealed that the primary site surgery can achieve survival improvements in pNET patients with distant metastasis.[15–17] The authors proposed that resection of primary tumor may enhance the efficacy of systemic therapy even in the absence of symptoms. In our study, we also found that primary site surgery improved CSS and OS in pNET patients with distant metastasis. Unfortunately, the functional status of the tumor could not be acquired in the SEER database, which leads to the failure of classification research regarding the tumor function in the present study.
There are several limitations in the current study. First, the variables including patients’ performance status, cormordities, Ki-67, and detailed surgical information (duration, volume of blood loss, postoperative complications, etc) are lacking in the SEER database. Secondly, the management of adjuvant chemotherapy (chemotherapy, targeted therapy, and endocrine therapy) may be helpful for better analysis and this information is also not provided in SEER database. Thirdly, due to the limited information, the location of distant metastasis is not clear, and it is impossible to judge the impact of metastasis location on prognosis.
5. Conclusion
This study establishes prognostic nomograms and validates them internally and externally to predict the prognosis of pNET patients with distant metastasis. They can be used to accurately predict survival rate, and provide useful information to physicians and patients.
Supplemental figure 1
Supplemental table 1
Supplemental table 2
Author contributions
Gang Li: concept, design, literature search, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing
Mao-lin Tian: data analysis, statistical analysis
Yun-tao Bing: literature search, data acquisition
Hang-yan Wang: manuscript preparation, manuscript editing
Chun-hui Yuan: manuscript preparation, manuscript editing
Dian-rong Xiu: concept, design. manuscript preparation, manuscript editing
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CSS = cancer-specific survival, ICD-O3 = International Classification of Diseases for Oncology, third edition, OS = overall survival, pNET = pancreatic neuroendocrine tumor, PRRT = peptide receptor radionuclide therapy, SEER = Surveillance, Epidemiology, and End Results.
How to cite this article: Li G, Tian M-l, Bing Y-t, Wang H-y, Yuan C-h, Xiu D-r. Nomograms predict survival outcomes for distant metastatic pancreatic neuroendocrine tumor: a population based STROBE compliant study. Medicine. 2020;99:13(e19593).
This work was funded by the National Natural Science Foundation-Youth Fund, 81702855.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhu LM, Tang L, Qiao XW, et al. Differences and similarities in the clinicopathological features of pancreatic neuroendocrine tumors in China and the United States: a multicenter study. Medicine 2016;95:e2836–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shimata K, Sugawara Y, Hibi T. Liver transplantation for unresectable pancreatic neuroendocrine tumors with liver metastases in an era of transplant oncology. Gland Surg 2018;7:42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589–97. [DOI] [PubMed] [Google Scholar]
- [5].Jiang Y, Jin JB, Zhan Q, et al. Impact and clinical predictors of lymph node metastases in nonfunctional pancreatic neuroendocrine tumors. Chin Med J 2015;128:3335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bertani E, Fazio N, Botteri E, et al. Resection of the primary pancreatic neuroendocrine tumor in patients with unresectable liver metastases: possible indications for a multimodal approach. Surgery 2014;155:607–14. [DOI] [PubMed] [Google Scholar]
- [7].Spolverato G, Bagante F, Aldrighetti L, et al. Neuroendocrine liver metastasis: prognostic implications of primary tumor site on patients undergoing curative intent liver surgery. J Gastrointest Surg 2017;21:2039–47. [DOI] [PubMed] [Google Scholar]
- [8].Frilling A, Clift AK. Therapeutic strategies for neuroendocrine liver metastases. Cancer 2015;121:1172–86. [DOI] [PubMed] [Google Scholar]
- [9].Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liang W, Zhang L, Jiang G, et al. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J Clin Oncol 2015;33:861–9. [DOI] [PubMed] [Google Scholar]
- [11].Pierorazio PM, Patel HD, Johnson MH, et al. Distinguishing malignant and benign renal masses with composite models and nomograms: a systematic review and meta-analysis of clinically localized renal masses suspicious for malignancy. Cancer 2016;122:3267–76. [DOI] [PubMed] [Google Scholar]
- [12].Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA 1982;247:2543–6. [PubMed] [Google Scholar]
- [13].Li G, Tian ML, Bing YT, et al. Clinicopathological features and prognosis factors for survival in elderly patients with pancreatic neuroendocrine tumor: a STROBE-compliant article. Medicine 2019;98:e14576–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bettini R, Mantovani W, Boninsegna L, et al. Primary tumour resection in metastatic nonfunctioning pancreatic endocrine carcinomas. Dig Liver Dis 2009;41:49–55. [DOI] [PubMed] [Google Scholar]
- [15].Citterio D, Pusceddu S, Facciorusso A, et al. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur J Surg Oncol 2017;43:380–7. [DOI] [PubMed] [Google Scholar]
- [16].Bertani E, Fazio N, Radice D, et al. Assessing the role of primary tumour resection in patients with synchronous unresectable liver metastases from pancreatic neuroendocrine tumour of the body and tail. A propensity score survival evaluation. Eur J Surg Oncol 2017;43:372–9. [DOI] [PubMed] [Google Scholar]
- [17].Keutgen XM, Nilubol N, Glanville J, et al. Resection of primary tumor site is associated with prolonged survival in metastatic nonfunctioning pancreatic neuroendocrine tumors. Surgery 2016;159:311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


