Abstract
This study aimed to investigate the efficacy and safety of drug-eluting beads (DEB) transarterial chemoembolization (TACE) treatment in Chinese intrahepatic cholangiocarcinoma (ICC) patients.
37 ICC patients underwent DEB-TACE treatment in CTILC study (registered on clinicaltrials.gov with registry No. NCT03317483) were included in this present study. Treatment response was assessed according to modified Response Evaluation Criteria in Solid Tumors (mRECIST). Overall survival (OS) was calculated from the time of DEB-TACE operation until the date of death from any causes. Liver function change and adverse events (AEs) were recorded during and after DEB-TACE operation.
3 (8.1%) patients achieved complete response (CR) and 22 (59.5%) patients achieved partial response (PR), with objective response rate (ORR) of 67.6%. After DEB-TACE treatment, mean OS was 376 days (95%CI: 341–412 days). Multivariate logistic regression analysis revealed that Bilobar disease (P = .040, OR: 0.105, 95% CI: 0.012–0.898) and portal vein invasion (P = .038, OR: 0.104, 95% CI: 0.012–0.881) could independently predict less possibility of ORR. Patients with ALB abnormal, TP abnormal, ALT abnormal and AST abnormal were increased at 1-week post DEB-TACE treatment (P = .034, P = .001, P < .001, P = .006, respectively), while returned to the levels at baseline after 1 to 3 months (all P > .050). Besides, most of the AEs were mild including pain, fever, vomiting, and nausea in this study.
DEB-TACE was effective and well tolerated in treating ICC patients, and bilobar disease as well as portal vein invasion were independently correlated with less probability of ORR achievement.
Keywords: drug-eluting beads transarterial chemoembolization, intrahepatic cholangiocarcinoma, overall survival, predictive factors, treatment response
1. 1. Introduction
Cholangiocarcinoma (CCA), with a preferential distribution among men rather than women, consists of intrahepatic, perihilar, and distal extrahepatic carcinomas,[1,2] among which intrahepatic cholangiocarcinoma (ICC) constitutes 5% to 15% of all cases.[3] The prognosis of the ICC patients is dismal, and surgery is the only curative treatment option with survival rates of 5 years ranging from 10% to 40%.[4,5] However, surgical therapy can only be carried out in about 30% of the cases due to most patients present at moderate to advanced stages with unspecific clinical symptoms.[6,7] Over the last decade, transarterial chemoembolization (TACE) as a palliative choice in ICC patients who are ineligible to receive curative treatments has become increasingly accepted and there is growing evidence for the ability of TACE to achieve high tumor response rates.[6,8]
TACE, of which the technology includes the obstruction of the tumor-supplying artery and infusion of chemotherapeutic, was mainly made up of conventional TACE (cTACE) and drug-eluting beads TACE (DEB-TACE).[9,10] DEB-TACE, in which the beads diameter varies from 100 μm to 900 μm, has not been commercially available until 2006.[11] Since then, DEB-TACE has become an option for unresectable liver cancer in many centers worldwide. Compared with cTACE, DEB-TACE reduces the risk of systemic chemotherapeutic distribution and increases intratumoral drug concentration. Despite of those benefits of DEB-TACE, little is known about the efficacy and safety of DEB-TACE treatment in Chinese ICC patients. Therefore, this study aimed to investigate the efficacy and safety of DEB-TACE treatment in Chinese ICC patients.
2. Material and methods
2.1. Patients
This study was a part of CTILC study (Chinese CalliSpheres Transarterial chemoembolization In Liver Cancer) which was a multi-center, prospective cohort study aiming to investigate the efficacy and safety of DEB-TACE treatment by CalliSpheres in Chinese patients and to improve the prognosis and patients satisfaction. The inclusion criteria of CTILC were as follows:
-
1.
Diagnosed as primary HCC, primary ICC or secondary liver cancer confirmed by pathological findings, clinical features, or radiographic examinations according to American Association for the Study of the Liver Diseases (AASLD) guidelines;
-
2.
Age above 18 years;
-
3.
About to receive DEB-TACE treatment with CalliSpheres according to clinical needs and patients’ willing.
-
4.
Able to be followed up regularly; (5) Life expectancy above 12 months.
The exclusions were as follows:
-
1.
History of liver transplantation;
-
2.
History of hematological malignances;
-
3.
Severe hepatic failure or renal failure;
-
4.
Contraindication for angiography, embolization procedure, or artery puncture;
-
5.
Patients with cognitive impairment, or unable to understand the study consents.
-
6.
Women in gestation or lactation period.
Other detailed information of CTILC study was available on clinicaltrials.gov with registry No. NCT03317483. 37 ICC patients underwent DEB-TACE treatment from 2015/11/12 to 2016/11/04 in CTILC study were included in this present study. This study was approved by Ethics committee of Zhejiang Cancer Hospital. All the patients or their legal guardian provided the written informed consents. This study was conducted according to the Declaration of Helsinki.
2.2. DEB-TACE procedure
DEB-TACE was performed using transfemoral arterial access route with a micro-puncture system by placing a 5F vascular introducer (Boston Scientific, Natick, MA, United States). CalliSpheres Beads (Jiangsu Hengrui Medicine Co, Ltd., Jiangsu, China) with the diameter between 100 μm to 300 μm were used as carriers. Beads were loaded with Adriamycin drug 50 to 80 mg, the mean dose was 60 mg for patients with ICC patients. Celiac and/or superior mesenteric arteriography was carried out to assess the arterial anatomy, tumor supplying vessel and patency of the portal vein. The lobar/segmental hepatic artery supplying the tumor was selectively cannulated with a microcatheter and embolized with DEB, which was loaded with the mixture of chemotherapy reagent solution and nonionic iodinated contrast material in a ratio of 1:1. The end point for embolization was stasis of blood flow in the tumor feeding artery.
2.3. Response assessment and follow ups
Modified Response Evaluation Criteria in Solid Tumors (mRECIST) was used to assess tumor response using enhanced computerized tomography (CT) or magnetic resonance imaging (MRI)[12]:
-
1.
Complete response (CR): no existence of arterial enhancement of targeted tumors;
-
2.
Partial response (PR): the decrease in diameter of targeted tumor (with arterial enhancement) <=30%;
-
3.
Stable disease (SD): the decrease in diameter of targeted tumor (with arterial enhancement) did not achieve PR or less than PD;
-
4.
Progressive disease (PD): the increase in diameter of targeted tumor (with arterial enhancement) > = 20% or new tumor existed. Objective response rate (ORR) was defined as the portion of patients achieved CR and PR (CR + PR).
Overall survival (OS) was calculated from the time of DEB-TACE operation to the date of death or last follow-up. Safety was assessed according to the change of liver function and the count and percentage of AEs during and after DEB-TACE. The median follow-up duration was 175 (range from 134 to 251) days, and the last follow-up date was December 27th, 2016.
2.4. Liver function and AEs
All patients were discharged after a brief observation period (48–72 hours). Clinical evaluation and assessment of liver function including albumin (ALB), total protein (TP), total bilirubin (TBIL), total bile acid (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were recorded on an outpatient basis during 1 week and 1 to 3 months after DEB-TACE. AEs including pain, fever, nausea, vomiting, bone marrow toxicity, and other AEs were defined as treatment related if occurred during operations or within 1 month of treatment.
2.5. Statistics
Statistical analysis was performed using SPSS 22.0 software (IBM, USA). Data was presented as count (%), mean ± standard deviation or median (25th–75th). Comparison between each visit was determined by McNemar test. K–M curve was drawn to analyze OS. Factors affecting ORR achievement in ICC patients were determined by 1 step enter univariate and multivariate logistic regression analysis. Factors affecting OS in ICC patients were determined by 1 step enter univariate Cox proportional hazards regression model analysis. P < .05 was considered significant.
3. Results
3.1. Baseline characteristics
37 ICC patients aged 62.9 ± 13.4 years with 9 females and 28 males were included in this study. As to stages for ICC, 33 (89.2%) patients were categorized into child-pugh A stage while 4 (10.8%) patients were B stage, and 9 (24.3%), 11 (29.7%) as well as 17 (45.9%) patients were at BCLC stage A, B, and C respectively. In addition, 13 (35.1%) patients had history of HB. The other detailed information about clinicopathological features, biochemical indexes, previous treatments were presented in Table 1.
Table 1.
Baseline characteristics of 37 ICC patients.
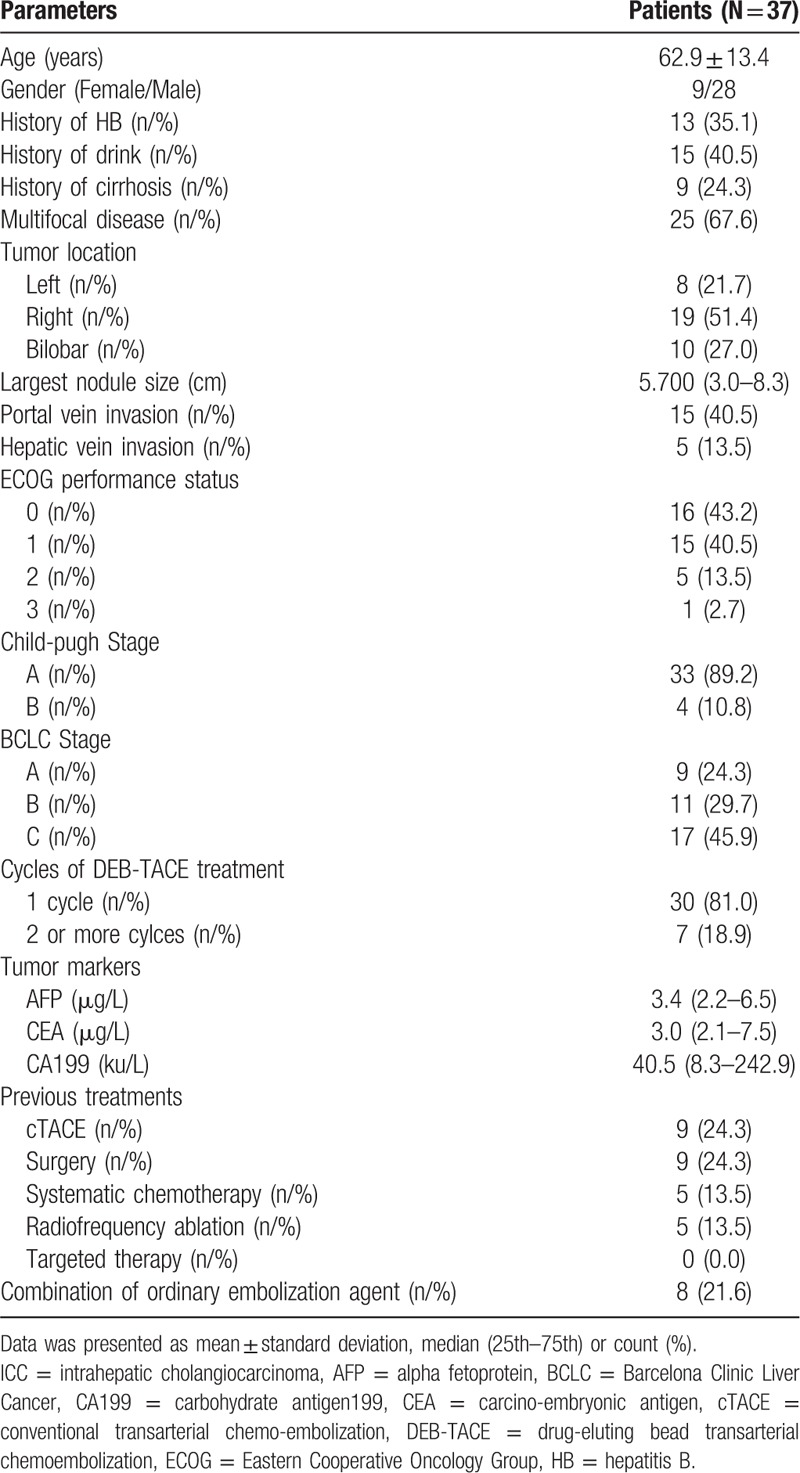
3.2. Treatment response of DEB-TACE treatment
As shown in Figure 1A, 3 (8.1%), 22 (59.5%), 9 (24.3%) and 3 (8.1%) patients achieved CR, PR, SD, and PD, respectively, and ORR was 67.6%. As to treated nodules (Fig. 1B), 4 (8.9%), 26 (57.8%), 10 (22.2%), and 5 (11.1%) nodules achieved CR, PR, SD, and PD respectively, and ORR was 66.67%.
Figure 1.
Treatment response of DEB-TACE in ICC patients. (A) Treatment response of DEB-TACE in patients. (B) Treatment response of DEB-TACE in treated nodules. Comparison among groups was determined by Chi-Squared test. P < .05 was considered significant.
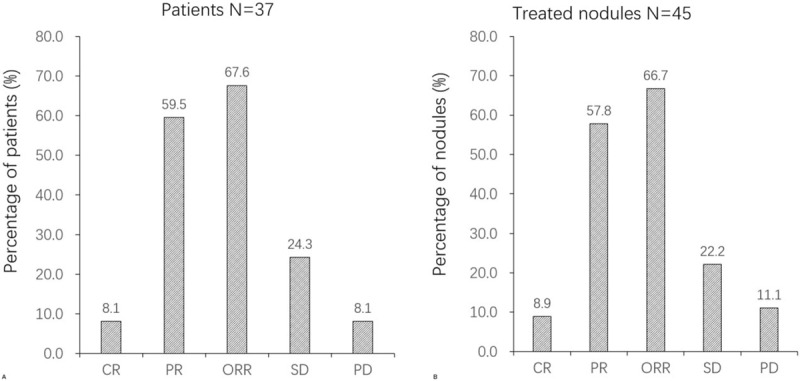
3.3. OS analysis
As presented in Figure 2, after DEB-TACE treatment, mean OS of ICC patients was 376 days (95%CI: 341–412 days), which revealed a great therapeutic effect for ICC patients by using DEB-TACE.
Figure 2.
OS of DEB-TACE treatment in ICC patients. K–M curve was performed to evaluate the OS.
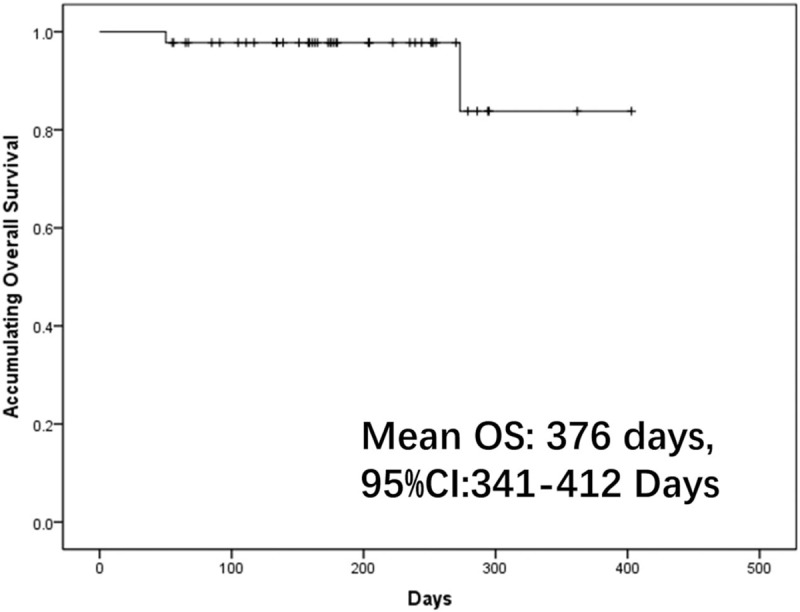
3.4. Comprehensive analysis of factors predicting ORR
Drink (P = .031, OR: 0.194, 95% CI: 0.044–0.858), bilobar disease (P = .037, OR: 0.190, 95% CI: 0.040–0.904) and portal vein invasion (P = .031, OR: 0.194, 95% CI: 0.044–0.858) were predictors for less probability of ORR in univariate logistic regression analysis. Factors with P < .100 were further detected by multivariate logistic regression analysis which illuminated that bilobar disease (P = .040, OR: 0.105, 95% CI: 0.012–0.898) and portal vein invasion (P = .038, OR: 0.104, 95% CI: 0.012–0.881) could independently predict less possibility of ORR (Table 2).
Table 2.
Factors affecting ORR achievement to DEB-TACE treatment in ICC patients by logistic regression model analysis.
3.5. Comprehensive analysis of factors predicting OS
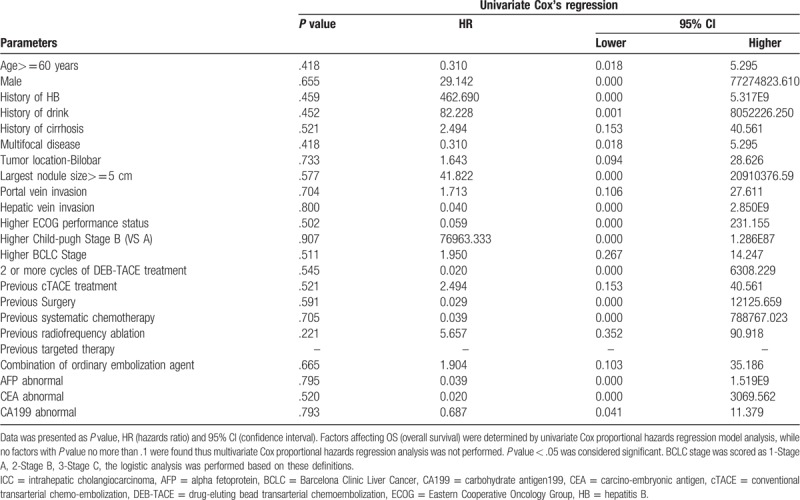
In order to investigate influence of the baseline factors on OS, Cox proportional hazards regression model analysis was performed and the results were showed in Table 3. However, no association was observed between each factor and OS in univariate Cox regression (all P > .050), thus multivariate Cox regression was not performed.
Table 3.
Factors affecting OS to DEB-TACE treatment in ICC patients by Cox proportional hazards regression model analysis.
3.6. Comparison of liver function before and after DEB-TACE treatment
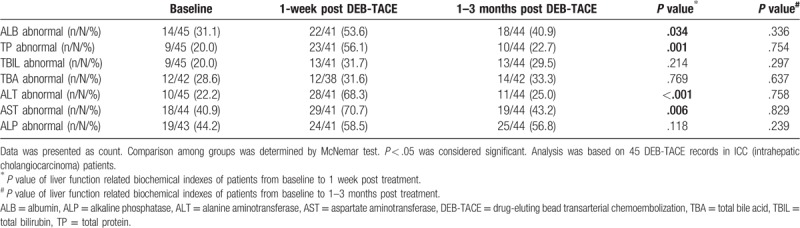
The percentage of ALB abnormal, TP abnormal, ALT abnormal and AST abnormal patients were increased at 1-week post DEB-TACE treatment (P = .034, P = .001, P < .001, P = .006, respectively), while returned to the levels at baseline after 1 to 3 months (all P > .050). No difference of other liver function indexes between each visit were observed (Table 4).
Table 4.
Liver function before and after DEB-TACE treatment (45 DEB-TACE records in cholangiocarcinoma patients).
3.7. AEs of 45 DEB-TACE records
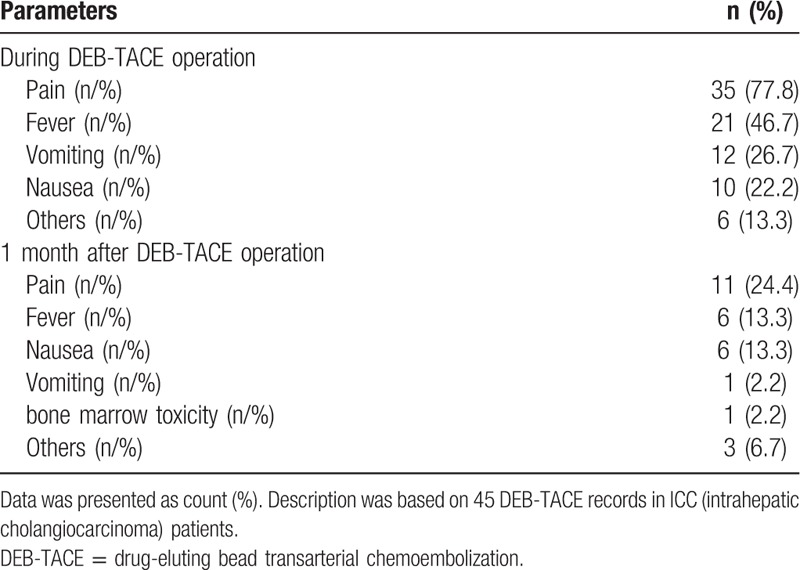
AEs of 45 records during operation and at 1 month post DEB-TACE operation were presented in Table 5. During operation, 35 (77.8%) patients felt pain, 21 (46.7%) patients had fever, 12 (26.7%) patients had vomiting, 10 (22.2%) patients had nausea and 6 (13.3%) patients with other AEs. While at 1-month post DEB-TACE treatment, pain occurred in 11 (24.4%) patients, fever in 6 (13.3%) patients, nausea in 6 (13.3%) patients, vomiting in 1 (2.2%) patients, bone marrow toxicity in 1 (2.2%) patients and other AEs in 3 (6.7%) patients.
Table 5.
Adverse events of DEB-TACE treatment (45 DEB-TACE records in cholangiocarcinoma patients).
3.8. Description of 2 typical cases
In patient 1, tumor-supplying arteries were completely embolized by DEB-TACE according to DSA images (Fig. 3A and B), and the tumor was totally necrotic after DEB-TACE (Fig. 3C and D). In patient 2, arteries were greatly embolized by DEB-TACE (Fig. 3E and F), and tumor was necrotic post DEB-TACE operation (Fig. 3G and H). The results showed a good effect of DEB-TAEC in treating ICC patients.
Figure 3.
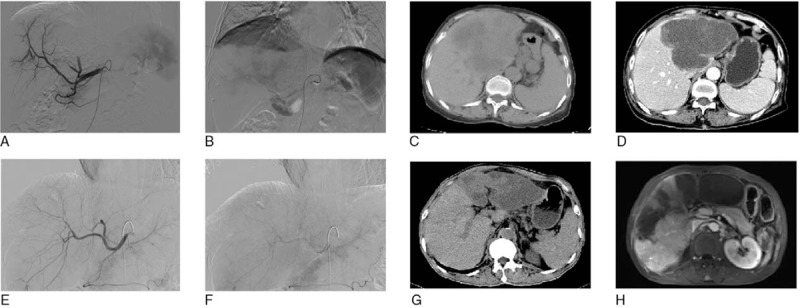
CT and DSA images of 2 patients at pre-operation and post-operation. (A–D) CT and DSA images of 1 patient at preoperation and postoperation. (E–H) CT and DSA images of another patient at preoperation and postoperation. Scan parameters were that: tube voltage was 120 kv; automatic pipe technology to adjust current; collimator width was 128 × 0. 625 mm; the reconstruction was 5 mm; a thick layer of reconstruction interval was 5 mm; screw pitch was 0.914. Enhanced scan USED MEDRAD double cylinder of high-pressure syringe injected iodine alcohol (containing iodine 300 mg/ml) from peripheral vein intravenous (dose of 1.5 ml/kg). When abdominal aorta CT value arrive to 100 HU, it delays after 5 seconds and begins to scan, and after arterial phase late 30 seconds and 150 seconds, respectively, it began to scan portal venous phase and delayed phase.
4. Discussion
In this study, we found:
-
1.
8.1% and 67.6% ICC patients achieved CR and ORR respectively by DEB-TACE treatment, and mean OS was 376 days (95%CI: 341–412 days).
-
2.
Bilobar disease and portal vein invasion were independent factors for predicting less probability of ORR;
-
3.
DEB-TACE was well tolerated in treating ICC patients regarding to liver function change and mild AEs.
ICC, counts for 10% to 20% of all primary liver cancers, is the second most common primary liver malignancy after hepatocellular carcinoma (HCC).[13] The incidence of ICC has been increasing worldwide at a growing rate greater than that of HCC.[14,15] The disease often presents symptoms in an advanced state, which precludes surgical resection in about 50% to 70% of patients at the time of diagnosis. DEB-TACE, a novel drug delivery system, uses microspheres as embolic agents and ensures the slow and sustained release of the drug locally in addition to causing ischemic injury to the tumor.[11,16] Some studies illustrate that, compared to cTACE, DEB-TACE does not improve the treatment response or survival rate but achieves less liver toxicity and better tolerance in HCC patients,[17,18] while several recent meta-analysis articles disclose DEB-TACE could achieve a higher response rate and survival profiles.[19–21]
A great number of studies reveal that DEB-TACE shows good efficacy in treating HCC patients, and the ORR is 64% or higher,[22–24] which is similar to our results that ORR of ICC patients was 67.6%. However, a study that is conducted on patients with HCC receiving DEB-TACE therapy elucidates a CR rate of 58%,[25] which is better compared to ours (8.1%). The reasons might be that ICC is very different from HCC in the type of vascularization, tumor dimensions and sensitivity to the toxic drug, which lead to more dismal outcome than HCC.[26]
Studies investigating DEB-TACE treatment in ICC patients were seldom reported, a prospectively cohort study enrolling 20 ICC patients, among which 11 cases choose the TACE with microspheres treatment and other 9 cases choose palliative care or chemotherapy, discloses that TACE with microspheres achieves extremely high response rate with 10% CR and 90% PR, and realized a favorable OS (median 13 months) compared to palliative care or chemotherapy patients.[27] Another recent published study which recruits 109 ICC patients underwent DEB-TACE treatment reveals the CR, ORR, disease control rate (DCR) are 0%, 7% and 95% respectively.[28] While in this present study, 8.1% and 67.6% ICC patients achieved CR and ORR and mean OS was 376 days (95%CI: 341–412 days) by DEB-TACE treatment. The primary cause of this controversy might result from the gap of technical ability between operators and differences of the population.
In order to improve the prognosis of ICC patients, it is essential to explore novel and convincing biomarker for both treatment response and survival in ICC patients by DEB-TACE treatment. A prospective historical cohort illustrates that nodule size 5 cm and tumor location in the segments 1 and 4 are correlated with more probability of CR in HCC patients.[29] And several retrospective studies reveal that portal vein tumor thrombus is an independent prognostic factor for survival according to uni- and multivariate analysis in TCAE treated patients.[30,31] These studies suggest that tumor location and portal vein invasion could predict less probability of CR, and our results suggested that drink, bilobar disease, and portal vein invasion were associated with less possibility of ORR. The possible explanation of the predictive value of these factors might be: drink could induce pancreatitis, which is a relatively rare but potentially lethal complication after DEB-TACE[32]; (2) severe disease condition with poor liver function including bilobar disease and portal vein invasion led to a worse treatment response.
As to liver function before and after DEB-TACE, a prospective and single-center study illustrate that liver function is not remarkably affected by DEB-TACE in most HCC patients assessed by the image with 2 years follow-up.[33] And a comprehensive review reveals that DEB-TACE has remarkably reduced liver toxicity.[34] In our study, the liver function indexes such as ALB, TBIL, TBA, and ALP became better after DEB-TACE, with no change of ALT at 1week and 1 to 3 months post DEB-TACE compared to baseline, which was consistent with those 2 former studies. However, the percentage of patients with abnormal liver function at 1 to 3 months post DEB-TACE seemed to be larger than that at 1 week. The reason might be that ICC was a malignant tumor leading to persistent deterioration of liver function, of which the speed was higher than that of liver recovery treated by DEB-TACE. Besides, our study illustrated a good safety with mild AEs during operation and 1 month after DEB-TACE operation, which was consistent with the previous study of DEB-TACE treatment in ICC patients which illuminates that the most common AEs are pain, fever, nausea.[28]
Some limitations still existed in our study:
-
1.
lack of a proper control group of ICC patients (for instance, ICC patients with cTACE treatment only or ICC patients who did not accept any treatment) was still a main limitation in this study. Further study is needed.
-
2.
Owning to that most ICC patients enrolled in this study were ecdemic patients, the majority of them received 1 time of DEB-TACE or multiple times of continue DEB-TACE in our hospital. After receiving DEB-TACE, some patients would continue to receive consolidation therapy according to their own conditions in our hospital; whereas most patients would go back to the local place, and they would receive additional treatments in the local hospital when their disease progressed. Thus, we could not collect the detailed information about their tumor progression free survival, recurrence with CR as well as metastasis. Further study investigating the efficacy of DEB-TACE on progression free survival, recurrence with CR as well as metastasis in ICC patients is greatly needed.
-
3.
whether other multiple approaches (eg cTACE, surgery, RFA, etc) after DEB-TACE affect the effiacy and safty of DEB-TACE was not explored in this study, further study is needed.
-
4.
sample size with 37 ICC patients was relatively small and study with a larger sample size is needed in the future;
-
5.
most patients (75.7%) in our study had treatment history before DEB-TACE, of which the efficacy and safety in treatment-naive ICC patients could not be evaluated.
-
6.
The follow-up duration was relatively small thus long-term benefit of DEB-TACE in ICC patients was not assessed.
In conclusion, DEB-TACE was effective and well tolerated in treating ICC patients, and bilobar disease as well as portal vein invasion were independantly correlated with less probability of ORR achievement.
Author contributions
Conceptualization: Jun Luo, Jiaping Zheng, Changsheng Shi, Huanhai Xu, Jiansong Ji, Wenhao Hu.
Data curation: Jun Luo, Jiaping Zheng, Zhiyi Peng, Jing Huang, Junhui Sun, Guanhui Zhou, Tiefeng Li, Dedong Zhu, Qinming Hou, Shihong Ying, Zhichao Sun, Jun Han, Hongjie Hu, Tingyang Hu, Yutang Chen, Jiansong Ji.
Formal analysis: Jun Luo, Jiaping Zheng, Guanhui Zhou, Dedong Zhu, Ling Li.
Funding acquisition: Guanhui Zhou, Huanhai Xu, Jiansong Ji, Wenhao Hu.
Investigation: Jun Luo, Jiaping Zheng, Changsheng Shi, Jian Fang, Zhiyi Peng, Jing Huang, Junhui Sun, Guanhui Zhou, Dedong Zhu, Huanhai Xu, Zhichao Sun, Haijun Du, Xiaoxi Xie, Guohong Cao, Wenbin Ji, Jun Han, Wenjiang Gu, Jian Zhou, Wenqiang Yu, Ling Li, Hongjie Hu, Tingyang Hu, Xia Wu, Yutang Chen, Jiansong Ji, Wenhao Hu.
Methodology: Jun Luo, Jiaping Zheng, Zhiyi Peng, Jing Huang, Wenhao Hu.
Project administration: Jun Luo, Jiaping Zheng, Wenhao Hu.
Resources: Junhui Sun, Jiansong Ji.
Software: Jian Fang, Jing Huang, Junhui Sun, Guanhui Zhou, Tiefeng Li, Dedong Zhu, Huanhai Xu, Qinming Hou, Haijun Du, Xiaohua Guo, Guoliang Shao, Xin Zhang, Hongjie Hu, Xia Wu.
Supervision: Jun Luo, Jian Fang, Wenbin Ji, Jiansong Ji.
Validation: Jun Luo, Jiaping Zheng, Zhihai Yu, Jiansong Ji, Wenhao Hu.
Visualization: Jun Luo, Jiaping Zheng, Changsheng Shi, Junhui Sun, Shihong Ying, Haijun Du, Zhihai Yu, Wenhao Hu.
Writing–original draft: Jun Luo, Jiaping Zheng, Changsheng Shi, Jian Fang, Zhiyi Peng, Jing Huang, Junhui Sun, Dedong Zhu, Zhichao Sun, Haijun Du, Xiaoxi Xie, Guohong Cao, Wenbin Ji, Jun Han, Wenjiang Gu, Jian Zhou, Wenqiang Yu, Yutang Chen, Jiansong Ji, Wenhao Hu.
Writing–review & editing: Jun Luo, Jiaping Zheng, Changsheng Shi, Jian Fang, Zhiyi Peng, Jing Huang, Junhui Sun, Guanhui Zhou, Dedong Zhu, Huanhai Xu, Qinming Hou, Zhichao Sun, Wenbin Ji, Jun Han, Xiaohua Guo, Guoliang Shao, Zhihai Yu, Xin Zhang, Tingyang Hu, Jiansong Ji, Wenhao Hu.
Footnotes
Abbreviations: AASLD = American Association for the Study of the Liver Diseases, ALB = albumin, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CCA = Cholangiocarcinoma, CR = complete response, CR+PR = CR and PR, CT = computerized tomography, cTACE = conventional TACE, DEB-TACE = drug-eluting beads TACE, HCC = hepatocellular carcinoma, ICC = intrahepatic cholangiocarcinoma, mRECIST = Modified Response Evaluation Criteria in Solid Tumors, MRI = magnetic resonance imaging, ORR = objective response rate, OS = overall survival, PD = progressive disease, PR = partial response, SD = stable disease, TACE = transarterial chemoembolization, TBA = total bile acid, TBIL = total bilirubin, TP = total protein.
How to cite this article: Luo J, Zheng J, Shi C, Fang J, Peng Z, Huang J, Sun J, Zhou G, Li T, Zhu D, Xu H, Hou Q, Ying S, Sun Z, Du H, Xie X, Cao G, Ji W, Han J, Gu W, Guo X, Shao G, Yu Z, Zhou J, WY, Zhang X, Li L, Hu H, Hu T, Wu X, Chen Y, Ji J, Hu W. Drug-eluting beads transarterial chemoembolization by CalliSpheres is effective and well tolerated in treating intrahepatic cholangiocarcinoma patients: a preliminary result from CTILC study. Medicine. 2020;99:12(e19276).
JL and JZ contributed equally to this work.
This work was supported by the National Nature Science Foundation of China (81371658) and Zhejiang Provincial Natural Science Foundation of China (LZ18H180001).
The authors have no conflicts of interests to disclose.
References
- [1].Choi J, Ghoz HM, Peeraphatdit T, et al. Aspirin use and the risk of cholangiocarcinoma. Hepatology 2016;64:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Njei B. Changing pattern of epidemiology in intrahepatic cholangiocarcinoma. Hepatology 2014;60:1107–8. [DOI] [PubMed] [Google Scholar]
- [3].Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Savic LJ, Chapiro J, Geschwind JH. Intra-arterial embolotherapy for intrahepatic cholangiocarcinoma: update and future prospects. Hepatobiliary Surg Nutr 2017;6:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology 2013;144:829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut 2012;61:1657–69. [DOI] [PubMed] [Google Scholar]
- [7].Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 2016;122:758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Currie BM, Soulen MC. Decision making: intra-arterial therapies for cholangiocarcinoma-TACE and TARE. Semin Intervent Radiol 2017;34:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee S, Kim KA, Park MS, et al. MRI findings and prediction of time to progression of patients with hepatocellular carcinoma treated with drug-eluting bead transcatheter arterial chemoembolization. J Korean Med Sci 2015;30:965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baur J, Ritter CO, Germer CT, et al. Transarterial chemoembolization with drug-eluting beads versus conventional transarterial chemoembolization in locally advanced hepatocellular carcinoma. Hepat Med 2016;8:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gaba RC, Emmadi R, Parvinian A, et al. Correlation of doxorubicin delivery and tumor necrosis after drug-eluting bead transarterial chemoembolization of rabbit VX2 liver tumors. Radiology 2016;280:752–61. [DOI] [PubMed] [Google Scholar]
- [12].Seyal AR, Gonzalez-Guindalini FD, Arslanoglu A, et al. Reproducibility of mRECIST in assessing response to transarterial radioembolization therapy in hepatocellular carcinoma. Hepatology 2015;62:1111–21. [DOI] [PubMed] [Google Scholar]
- [13].Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268–89. [DOI] [PubMed] [Google Scholar]
- [14].Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McConnell J. Icaac/Icc 2015. Lancet Infect Dis 2015;15:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Prajapati HJ, Spivey JR, Hanish SI, et al. mRECIST and EASL responses at early time point by contrast-enhanced dynamic MRI predict survival in patients with unresectable hepatocellular carcinoma (HCC) treated by doxorubicin drug-eluting beads transarterial chemoembolization (DEB TACE). Ann Oncol 2013;24:965–73. [DOI] [PubMed] [Google Scholar]
- [17].Duan F, Wang EQ, Lam MG, et al. Superselective chemoembolization of HCC: comparison of short-term safety and efficacy between drug-eluting LC beads, quadraspheres, and conventional ethiodized oil emulsion. Radiology 2016;278:612–21. [DOI] [PubMed] [Google Scholar]
- [18].Megias Vericat JE, Garcia Marcos R, Lopez Briz E, et al. Trans-arterial chemoembolization with doxorubicin-eluting particles versus conventional trans-arterial chemoembolization in unresectable hepatocellular carcinoma: a study of effectiveness, safety and costs. Radiologia (Roma) 2015;57:496–504. [DOI] [PubMed] [Google Scholar]
- [19].Huang K, Zhou Q, Wang R, et al. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 2014;29:920–5. [DOI] [PubMed] [Google Scholar]
- [20].Ni JY, Xu LF, Wang WD, et al. Conventional transarterial chemoembolization vs microsphere embolization in hepatocellular carcinoma: a meta-analysis. World J Gastroenterol 2014;20:17206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen P, Yuan P, Chen B, et al. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2017;41:75–85. [DOI] [PubMed] [Google Scholar]
- [22].Liu YS, Ou MC, Tsai YS, et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol 2015;16:125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arabi M, BenMousa A, Bzeizi K, et al. Doxorubicin-loaded drug-eluting beads versus conventional transarterial chemoembolization for nonresectable hepatocellular carcinoma. Saudi J Gastroenterol 2015;21:175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rahman FA, Naidu J, Ngiu CS, et al. Conventional versus doxorubicin-eluting beads transarterial chemoembolization for unresectable hepatocellular carcinoma: a tertiary medical centre experience in malaysia. Asian Pac J Cancer Prev 2016;17:4037–41. [PubMed] [Google Scholar]
- [25].Manini MA, Sangiovanni A, Martinetti L, et al. Transarterial chemoembolization with drug-eluting beads is effective for the maintenance of the Milan-in status in patients with a small hepatocellular carcinoma. Liver Transpl 2015;21:1259–69. [DOI] [PubMed] [Google Scholar]
- [26].Wengert GJ, Bickel H, Breitenseher J, et al. Primary liver tumors: hepatocellular versus intrahepatic cholangiocellular carcinoma. Radiologe 2015;55:27–35. [DOI] [PubMed] [Google Scholar]
- [27].Aliberti C, Benea G, Tilli M, et al. Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol 2008;31:883–8. [DOI] [PubMed] [Google Scholar]
- [28].Aliberti C, Carandina R, Sarti D, et al. Chemoembolization with drug-eluting microspheres loaded with doxorubicin for the treatment of cholangiocarcinoma. Anticancer Res 2017;37:1859–63. [DOI] [PubMed] [Google Scholar]
- [29].Vesselle G, Quirier-Leleu C, Velasco S, et al. Predictive factors for complete response of chemoembolization with drug-eluting beads (DEB-TACE) for hepatocellular carcinoma. Eur Radiol 2016;26:1640–8. [DOI] [PubMed] [Google Scholar]
- [30].Zhu K, Chen J, Lai L, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib--a retrospective controlled study. Radiology 2014;272:284–93. [DOI] [PubMed] [Google Scholar]
- [31].Tawada A, Chiba T, Ooka Y, et al. Efficacy of transarterial chemoembolization targeting portal vein tumor thrombus in patients with hepatocellular carcinoma. Anticancer Res 2014;34:4231–7. [PubMed] [Google Scholar]
- [32].She WH, Chan AC, Cheung TT, et al. Acute pancreatitis induced by transarterial chemoembolization: a single-center experience of over 1500 cases. Hepatobiliary Pancreat Dis Int 2016;15:93–8. [DOI] [PubMed] [Google Scholar]
- [33].Luz JH, Luz PM, Martin HS, et al. DEB TACE for Intermediate and advanced HCC - initial experience in a Brazilian Cancer Center. Cancer Imaging 2017;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol 2012;39:503–9. [DOI] [PubMed] [Google Scholar]


