Abstract
Background:
We performed a meta-analysis of the efficacy and safety of stem cell therapy as a clinical treatment of knee osteoarthritis. This meta-analysis is expected to provide evidence of the efficacy of stem cell therapy, which is currently controversial, as a conservative treatment for knee osteoarthritis.
Methods:
An online search for relevant articles was conducted in the PubMed, EMBASE, and Cochrane Library databases. The search terms were “stem cells” and “osteoarthritis.” We conducted a quality assessment of the included articles and extracted the following indicators: Visual Analogue Scale (VAS) score, Subjective International Knee Documentation Committee (IKDC) score, Western Ontario and McMaster Universities (WOMAC) subscales, and adverse events. The RevMan5.3 software was used for determining effect sizes.
Results:
Nine randomized controlled trials involving 339 patients were included. VAS score and IKDC score from baseline to 24 months were improved in the stem cell therapy group compared to those in the control group. However, no significant difference was observed between the 2 groups in IKDC score changes from baseline to 6 and 12 months, as well as in WOMAC-Pain, WOMAC-Stiffness, and WOMAC-Physical Function score changes at each visit point.
Conclusion:
Stem cell therapy is certainly superior to traditional treatments in the conservative treatment of KOA; it considerably reduces pain with no obvious additional side effects.
Keywords: knee osteoarthritis, meta-analysis, stem cell therapy
1. Introduction
Knee osteoarthritis is a chronic degenerative bone metabolic disease that commonly occurs in middle-aged and older adults; it affects patients’ daily activities and even causes disability.[1,2] Its clinical features mainly include cartilage degenerative lesions, with clinical manifestations such as joint swelling, pain, and deformity. Thus, the main therapeutic purposes of knee osteoarthritis are to reduce or eliminate pain, correct joint deformities, and improve joint function through cartilage repair.[3]
In recent years, replacement of damaged articular cartilage by chondrocytes or cartilage tissue has been considered a potential approach for treating knee osteoarthritis. Studies have shown that it is feasible to induce human pluripotent stem cells to differentiate into chondrocytes; therefore, stem cell therapy has become a new method for local treatment of knee osteoarthritis. For example, mesenchymal stem cells (MSCs) have multi-directional differentiation potential and can be differentiated into osteoblasts and chondrocytes under specific induction conditions in vitro and in vivo, thereby repairing bone and articular cartilage.[4,5] However, there is still a dispute on the clinical effects of stem cells,[6–8] for which a multitude of clinical trials and meta-analyses have been conducted.[9,10]
We herein present a meta-analysis of the controversial efficacy and safety of stem cell therapy as a clinical treatment of knee osteoarthritis. This study is markedly distinguished from previous meta-analyses[9,10] because it focused on bone marrow MSCs, peripheral blood stem cells, and amniotic fluid free stem cells. In addition, we used updated data from several latest high-level randomized controlled trials (RCTs).[11,12] This meta-analysis is expected to provide an evidence of the efficacy of stem cell therapy as a conservative treatment of knee osteoarthritis.
2. Methods
All analyses were based on previous published studies; thus, no ethical approval and patient consent are required.
2.1. Study selection
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement,[13] 2 researchers independently screened the literature, as well as extracted and cross-checked the relevant data. If disagreements occurred, a decision regarding data extraction was made by a third researcher.
2.2. Search strategy
We conducted the search in PubMed (1970-May 2019), Embase (1970-May 2019), and The Cochrane Library (1970-May 2019) databases for relevant articles, with “stem cells” and “osteoarthritis” as search terms. We also manually screened relevant Chinese and English language journals and reference lists to include potential studies. The search strategy for PubMed is detailed herein as an example: (((“Stem Cells”[Mesh]) OR ((((((((((((((Cell, Stem[Title/Abstract]) OR Stem Cell[Title/Abstract]) OR Progenitor Cells[Title/Abstract]) OR Cell, Progenitor[Title/Abstract]) OR Cells, Progenitor[Title/Abstract]) OR Progenitor Cell[Title/Abstract]) OR Mother Cells[Title/Abstract]) OR Cell, Mother[Title/Abstract]) OR Cells, Mother[Title/Abstract]) OR Mother Cell[Title/Abstract]) OR Colony-Forming Unit[Title/Abstract]) OR Colony Forming Unit[Title/Abstract]) OR Colony-Forming Units[Title/Abstract]) OR Colony Forming Units[Title/Abstract]))) AND ((((((((((((((Osteoarthritides[Title/Abstract]) OR Osteoarthrosis[Title/Abstract]) OR Osteoarthroses[Title/Abstract]) OR Arthritis, Degenerative[Title/Abstract]) OR Arthritides, Degenerative[Title/Abstract]) OR Degenerative Arthritides[Title/Abstract]) OR Degenerative Arthritis[Title/Abstract]) OR Osteoarthrosis Deformans[Title/Abstract]) OR Polyarthritides[Title/Abstract]) OR Arthritides[Title/Abstract]) OR Polyarthritis[Title/Abstract]) OR Arthritis[Title/Abstract])) OR “Osteoarthritis”[Mesh]).
2.3. Eligibility criteria
The study inclusion criteria included:
-
(1)
studies involving patients with knee osteoarthritis;
-
(2)
studies including stem cell therapy as the test group, as well as placebo, hyaluronic acid, and steroid treatments as the control groups;
-
(3)
RCTs;
-
(4)
studies that used at least one of the following indicators: Visual Analogue Scale (VAS) score, Western Ontario and McMaster Universities (WOMAC) subscale, International Knee Documentation Committee (IKDC) score, and incidence of adverse events.
Studies were ineligible if they met any of the following conditions:
-
(1)
studies that used animals or cadavers as research objects;
-
(2)
studies that were unable to extract or convert valid data;
-
(3)
retrospective studies, literature reviews, or conference papers with no full text.
2.4. Data extraction
Data were extracted independently by 2 researchers using a predesigned data sheet. Valid data were converted as per the Cochrane Handbook for Systematic Reviews of Interventions,[14] in the case where standard deviation could not be acquired. If disagreements occurred, the decision regarding data extraction was done by the third reviewer. Each RCT was concurrently assessed with risk of bias.
2.5. Outcome measures
VAS is a scoring scale that intuitively quantifies the intensity of pain in the knee. A lower score indicates milder pain.
The WOMAC subscale is a rating scale that assesses the structure, stiffness, and function of the knee in pain A lower score indicates better knee condition.
IKDC is a subjective scale for assessing the knee joint. A higher score indicates better symptoms, functions, and physical activities of the knee joint.
Adverse events refer to treatment-related adverse reactions, including joint effusion, stiffness, and pain.
2.6. Statistical analysis
Statistical analysis was conducted using the RevMan 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The chi-square test was used to assess inter-study heterogeneity. I2 > 50% indicated heterogeneity. A random effects model was used; otherwise, a fixed effects model was used. Relative risk and standardized mean difference were used for assessing binary variables and continuous variables, respectively. The 95% confidence interval estimates and hypothesis testing results for each variable were listed in a forest plot. For each endpoint with high heterogeneity, a sensitivity analysis, in which the included studies were removed one at a time, was conducted to screen the source of heterogeneity. A publication bias assessment using a funnel plot was performed if there were no less than 10 studies included.
3. Results
3.1. Literature search
We retrieved 7054 relevant articles, and ultimately included 9 RCTs[11,12,15–21] involving 399 patients (Fig. 1). In the studies by Kuah,[12] Lamo-Espinosa,[16] and Thomas Vangsness et al,[19] there were 2 parallel test groups, namely the high- and low-dose groups, in comparison with the control group. Therefore, for each study mentioned above, we conducted statistical analyses in 2 RCTs: high-dose vs control and low-dose vs control.
Figure 1.
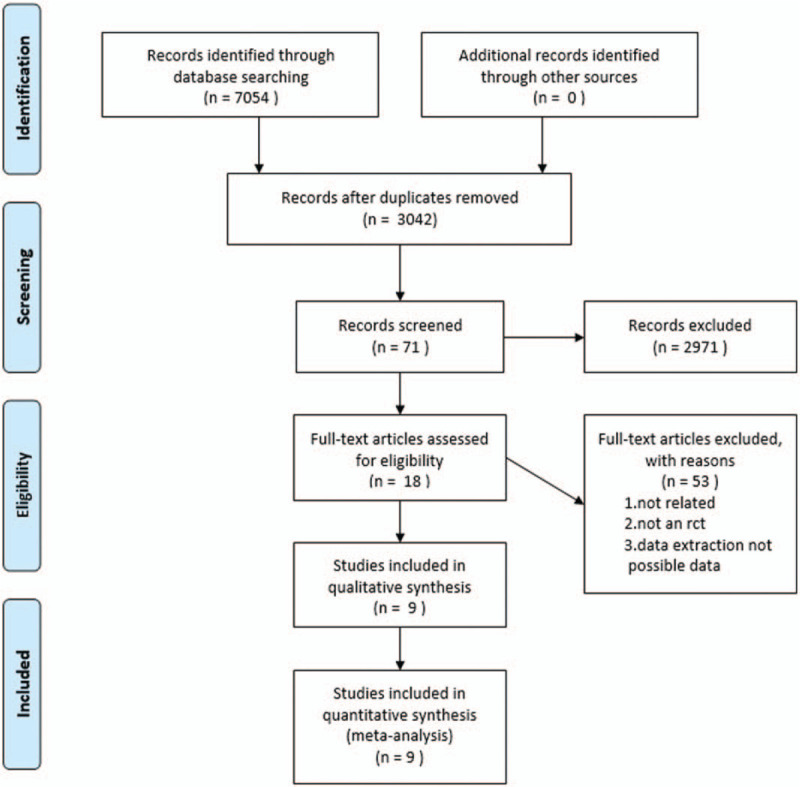
Flowchart of literature retrieval.
3.2. Study characteristics
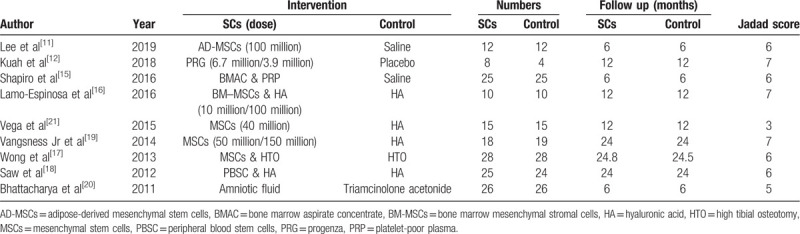
There were 203 patients in the stem cell therapy group and 196 patients in the control group. The specific features and Jadad scores of the patients[22,23] are listed in Table 1. The Jadad scale is a 7-point scale that includes random sequence generation, randomized hiding, blind method, withdrawal, and dropout.
Table 1.
Main characteristics of all the eligible studies included in the analysis.
3.3. Clinical outcomes
3.3.1. VAS
From baseline to 3 months, 4 studies[12,15,16,20] were included, involving 6 RCTs with 87 patients in the stem cell group and 79 patients in the control group Fig. 2. There was no heterogeneity (I2 = 0%) between the studies; thus, the fixed effects model was used for the analysis. According to Figure 2, SMD (standardized mean difference) = −0.36, 95% CI (confidence interval)[−0.67, −0.05], and P = .02. The VAS score in the stem cell group was significantly lower than that in the control group.
Figure 2.
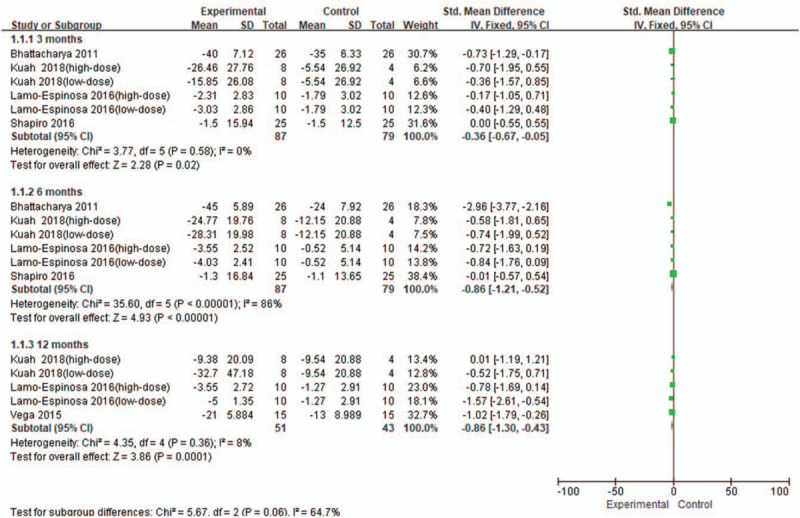
Forest plot of the change of VAS score. VAS = visual analogue scale score.
From baseline to 6 months, 4 studies[12,15,16,20] were included, involving 6 RCTs with 87 patients in the stem cell group and 79 patients in the control group. Because there was a high heterogeneity (I2 = 86%) between the studies, the study by Bhattacharya et al[20] was removed from the sensitivity analysis, and the I2 value was reduced to 0%. The fixed effects model was used. According to Figure 2, SMD = −0.86, 95% CI [−1.21, −0.52], and P < .00001. The VAS score in the stem cell group was significantly lower than that in the control group.
From baseline to 12 months, 3 studies[12,16,21] were included, involving 5 RCTs with 51 patients in the stem cell group and 43 patients in the control group. There was a low heterogeneity (I2 = 8%) between the studies, and thus the fixed effects model was used. According to Figure 2, SMD = −0.86, 95% CI [−1.30, −0.43], and P = 0.0001. The VAS score in the stem cell group was significantly lower than that in the control group.
3.3.2. WOMAC-Pain
From baseline to 3 months, 2 studies[12,16] were included, involving four RCTs with 36 patients in the stem cell group and 28 patients in the control group Fig. 3. There was a low heterogeneity (I2 = 40%) between the studies, and thus the fixed effects model was used. According to Figure 3, SMD = −0.22, 95% CI [−0.73, 0.30], and P = .41. There was no significant difference in WOMAC-Pain score between the groups.
Figure 3.
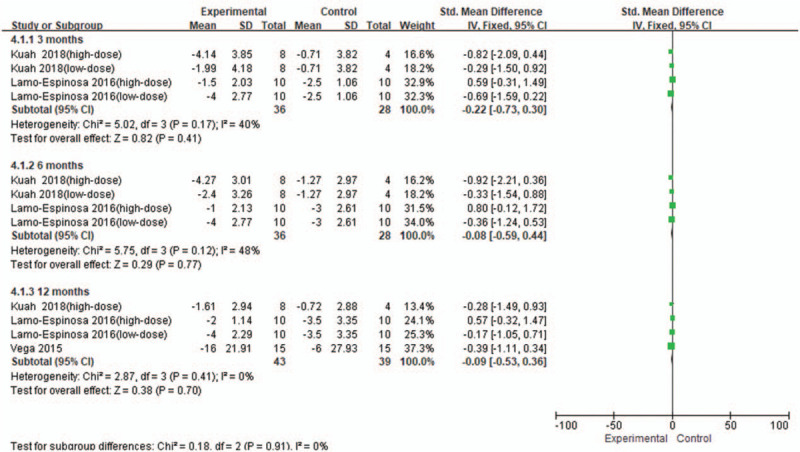
Forest plot of the change of WOMAC-Pain score. WOMAC = Western Ontario and McMaster Universities subscore.
From baseline to 6 months, 2 studies[12,16] were included, involving four RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was a low heterogeneity (I2 = 48%) between the studies, and thus the fixed effects model was used. According to Figure 3, SMD = −0.08, 95% CI [−0.59, 0.44], and P = .77. There was no significant difference in WOMAC-Pain score between the groups.
From baseline to 12 months, 3 studies[12,16] were included, involving 4 RCTs with 43 patients in the stem cell group and 39 patients in the control group. There was no heterogeneity (I2 = 0%) between the studies, and thus the fixed effects model was used. According to Figure 3, SMD = −0.09, 95% CI [−0.53, 0.36], and P = .70. There was no significant difference in WOMAC-Pain score between the groups.
3.3.3. WOMAC-Stiffness
From baseline to 3 months, 2 studies[12,16] were included, involving 4 RCTs with 36 patients in the stem cell group and 28 patients in the control group Fig. 4. There was no heterogeneity (I2 = 0%) between the studies, and the fixed effects model was used. According to Figure 4, SMD = −0.51, 95% CI [−1.02, 0.01], and P = .05. There was no significant difference in WOMAC-Stiffness score between the groups.
Figure 4.
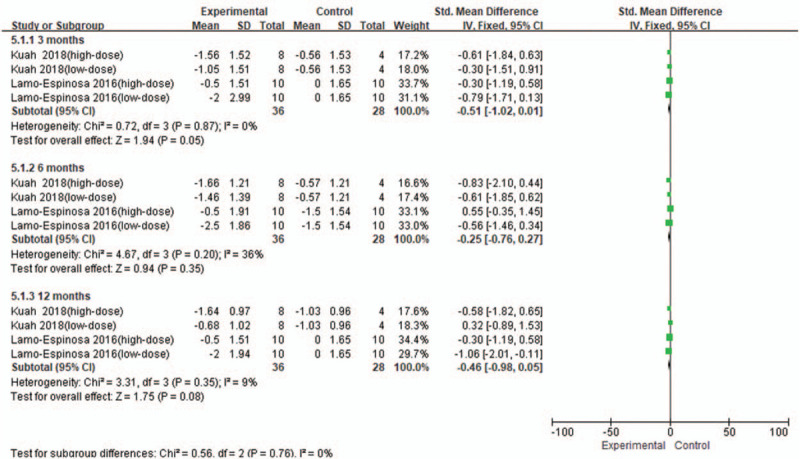
Forest plot of the change of WOMAC-Stiffness score. WOMAC = Western Ontario and McMaster Universities subscore.
From baseline to 6 months, 2 studies[12,16] were included, involving four RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was a low heterogeneity (I2 = 36%) between the studies, and the fixed effects model was used. According to Figure 4, SMD = −0.25, 95% CI [−0.76, 0.27], and P = .35. There was no significant difference in WOMAC-Stiffness score between the groups.
From baseline to 12 months, 2 studies[12,16] were included, involving 4 RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was a low heterogeneity (I2 = 9%) between the studies, and the fixed effects model was used. According to Figure 4, SMD = −0.46, 95% CI [−0.98, 0.05], and P = .08. There was no significant difference in WOMAC-Stiffness score between the groups.
3.3.4. WOMAC-Function
From baseline to 3 months, 2 studies[12,16] were included, involving 4 RCTs with 36 patients in the stem cell group and 28 patients in the control group Fig. 5. There was no heterogeneity (I2 = 0%) between the studies, and the fixed effects model was used. According to Figure 5, SMD = 0.15, 95% CI [−0.35, 0.66], and P = .55. There was no significant difference in WOMAC-Function score between the groups.
Figure 5.
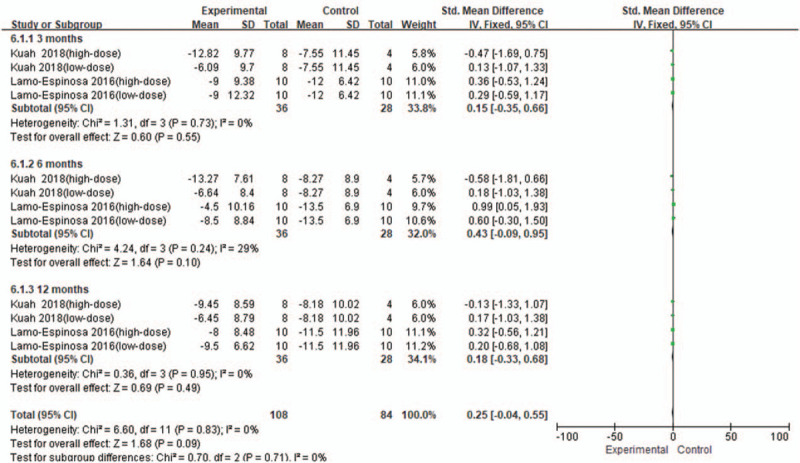
Forest plot of the change of WOMAC-Function. WOMAC = Western Ontario and McMaster Universities subscore.
From baseline to 6 months, 2 studies[12,16] were included, involving four RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was a low heterogeneity (I2 = 29%) between the studies, and the fixed effects model was used. According to Figure 5, SMD = 0.43, 95% CI [−0.09, 0.95], and P = .1. There was no significant difference in WOMAC-Function score between the groups.
From baseline to 12 months, 2 studies[12,16] were included, involving 4 RCTs with 36 patients in the stem cell group and 28 patients in the control group. There was no heterogeneity (I2 = 0%) between the studies, and the fixed effects model was used. According to Figure 5, SMD = 0.18, 95% CI [−0.33, 0.68], and P = .49. There was no significant difference in WOMAC-Function score between the groups.
3.3.5. IKDC
From baseline to 6 months, 2 studies[17,18] were included, involving 2 RCTs with 53 patients in the stem cell group and 52 patients in the control group Fig. 6. There was a high heterogeneity (I2 = 51%) between the studies, and the random effects model was used. According to Figure 6, SMD = 0.16, 95% CI [−0.39,0.72], and P = .56. There was no significant difference in IKDC score between the groups.
Figure 6.
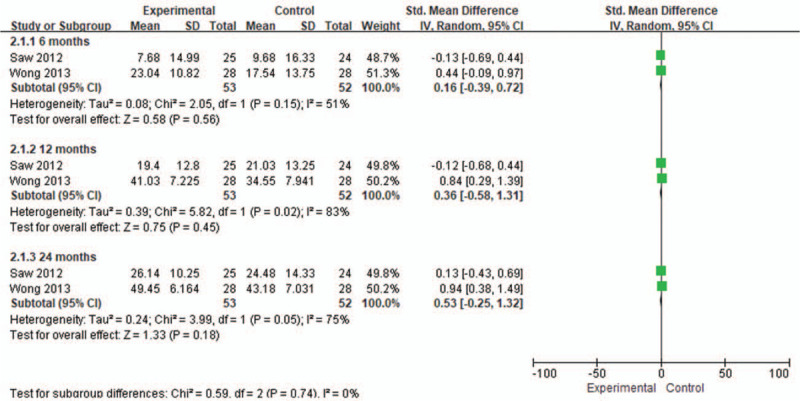
Forest plot of the change of IKDC score. IKDC = International Knee Documentation Committee.
From baseline to 12 months, 2 studies[17,18] were included, involving 2 RCTs with 53 patients in the stem cell group and 52 patients in the control group. There was a high heterogeneity (I2 = 83%) between the studies, and the random effects model was used. According to Figure 6, SMD = 0.36, 95% CI [−0.58, 1.31], and P = .45. There was no significant difference in IKDC score between the groups.
From baseline to 24 months, 2 studies[17,18] were included, involving 2 RCTs with 53 patients in the stem cell group and 52 patients in the control group. There was a high heterogeneity (I2 = 75%) between the studies, and the random effects model was used. According to Figure 6, SMD = 0.53, 95% CI [−0.25, 1.32], and P = .18. There was no significant difference in IKDC score between the groups.
3.3.6. Adverse events
There were 2 included studies,[11,12] involving three RCTs with 28 patients in the stem cell group and 20 patients in the control group Fig. 7. There was a high heterogeneity (I2 = 73%) between the studies, and thus the random effects model was used. According to Figure 7, SMD = 1.54, 95% CI [0.57, 4.19], and P = .40. There was no significant difference in incidence of adverse events between the groups.
Figure 7.
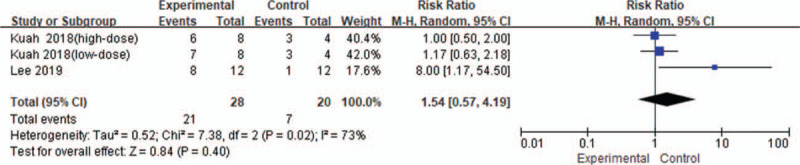
Forest plot of the change of adverse events.
4. Discussion
4.1. Key findings
Changes in VAS and IKDC scores from baseline to 24 months were superior in the stem cell group than in the control group, whereas there were no statistical differences in the changing trend of other indicators between the 2 groups, including the changes in IKDC scores at 6 months, IKDC score at 12 months, WOMAC-Pain score, WOMAC-Stiffness score, WOMAC-Function score, WOMAC-Pain score, and incidence of adverse events.
Pain relief is key to treating knee osteoarthritis, with VAS scores as an important endpoint for pain assessment. Kuah et al[12] found that relative to placebo, stem cell therapy considerably relieved pain at 3, 6, and 12 months after treatment. This conclusion has been confirmed in our meta-analysis. We found that the VAS scores in the stem cell group were significantly reduced at each visit point. Inflammatory response is known as one of the causes of pain. MSCs can release anti-inflammatory factors, thereby relieving pain. Lamo-Espinosa et al[16] believed that stem cells have a paracrine function and their anti-inflammatory properties contribute to pain relief. In addition, studies have found that in an acute renal failure model, MSCs can promote recovery of renal function by releasing anti-inflammatory factors and inhibiting production of pro-inflammatory cytokines, such as interleukin-1β, tumor necrosis factor, and interferon-γ.[24] Similar findings were observed in a pulmonary fibrosis model, in which MSCs release IL-1 receptor antagonist (IL-1RA) to inhibit interleukin-1α-producing T cells and TNF-producing macrophages, indicating that MSCs have anti-inflammatory properties.[25] WOMAC-Pain scores showed no statistical difference between the 2 groups at each visit point, but these data regarding WOMAC-Pain score were obtained only from 3 studies;[12,16,21] therefore, further studies with larger sample sizes are warranted to verify these findings.
Functional improvement of the knee joint is one of the ultimate purposes of knee osteoarthritis treatment. In this study, we used WOMAC-Stiffness, WOMAC-Function, and IKDC scores to comprehensively assess knee joint function. Statistical analysis results showed that there was no significant difference between the 2 groups in IKDC scores at 6 and 12 months, as well as in WOMAC-Stiffness and WOMAC-Function scores at each visit point. Studies have found that mesenchymal stem cell implantation achieves better outcomes in patients with grade 3 knee osteoarthritis than those in patients with grade 4 knee osteoarthritis.[26] We thus concluded that treatment with MSCs are effective in preventing or limiting the progression of knee osteoarthritis at the early stage. In the studies by Kuah[12] and Lamo-Espinosa et al,[16] patients with grade 3 osteoarthritis or higher accounted for 75% and over 80% of the total patients, respectively. Most patients developed knee osteoarthritis at the middle and late stages, for whom treatment with MSCs had no significant efficacy and was not conducive to functional recovery. Moreover, in most tissue engineering methods, MSCs are combined with cell scaffolds containing chondrogenic growth factors to form fully functional hyaline cartilage. Such a regimen is commonly used in small-animal models of surgically induced cartilage or osteochondral defects, but cannot be used for repairing large-area cartilage defects associated with knee osteoarthritis.[27] In addition, Centeno,[28] Emadedin,[29] and Vangsness et al[19] pointed out that treatment with approximately 2 × 107 stem cells can afford good clinical results. Kuah[12] and Lamo-Espinosa et al[16] reported that a stem cell dose of lower or higher than 2 × 107 may also impact the therapeutic efficacy of stem cells. In addition, the change in IKDC score at 24 months was higher in the stem cell group than in the control group; however, these data were extracted from only 2 studies. Therefore, further investigation with a larger sample size is warranted.
For adverse events, we sent an e-mail to the authors of the relevant research[18,19,21] to obtain data on the number of patients who experienced treatment-related adverse events, including arthralgia, joint effusion, and joint stiffness, in both the stem cell and control groups. Because of the lack of response from the other studies, only 2 studies[11,12] were included, involving 3 RCTs. There were no statistical differences in adverse events between the 2 groups, indicating that stem cell treatment has no obvious side effects. A study addressing 87 patients with lupus erythematosus[30] showed no adverse events associated with transplantation after 4 years of follow-up. Similarly, no graft-related adverse events occurred in many patients undergoing stem cell therapy for other diseases.[31–35] These findings indicated that the human body has good tolerance to MSCs, and that stem cell treatment has no significant side effects.
4.2. Limitations
Differences in the original RCT protocols led to insufficient representation of some outcome indicators. Thus, high-quality, large-scale RCTs are required to verify our findings. In addition, there was no uniform standard in the preparation and use of stem cells, which may cause certain heterogeneity.
5. Conclusion
Compared to traditional methods, stem cell treatment has a certain superiority as a conservative treatment of knee osteoarthritis, in terms of markedly reducing pain without inducing side effects.
Author contributions
Conceptualization: Rui Huang.
Data curation: Rui Huang, Wei Li, Ying Zhao.
Formal analysis: Rui Huang, Wei Li, Ying Zhao, Fan Yang.
Investigation: Rui Huang, Wei Li, Ying Zhao.
Methodology: Rui Huang, Wei Li, Fan Yang.
Project administration: Meng Xu.
Software: Rui Huang, Wei Li, Ying Zhao, Fan Yang.
Supervision: Rui Huang, Wei Li, Ying Zhao, Meng Xu.
Validation: Ying Zhao, Fan Yang, Meng Xu.
Visualization: Rui Huang, Wei Li, Fan Yang.
Writing – original draft: Rui Huang, Wei Li.
Writing – review & editing: Ying Zhao, Meng Xu.
Footnotes
Abbreviations: AD-MSCs = adipose-derived mesenchymal stem cells, BMAC = bone marrow aspirate concentrate, BM-MSCs = bone marrow mesenchymal stromal cells, Cl = confidence interval, HA = hyaluronic acid, HTO = high tibial osteotomy, IKDC = International Knee Documentation Committee, IL-1RA = IL-1 receptor antagonist, MSCs = mesenchymal stem cells, PBSC = peripheral blood stem cells, PRG = progenza, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis, PRP = platelet-poor plasma, RCTs = randomized controlled trials, SMD = standardized mean difference, VAS = Visual Analogue Scale, WOMAC = Western Ontario and McMaster Universities.
How to cite this article: Huang R, Li W, Zhao Y, Yang F, Xu M. Clinical efficacy and safety of stem cell therapy for knee osteoarthritis: A meta-analysis. Medicine. 2020;99:11(e19434).
The authors have no conflicts of interests to disclose.
References
- [1].Krasnokutsky S, Samuels J, Abramson SB. Osteoarthritis in 2007. Bulletin of the Nyu Hospital for Joint Diseases 2007;65:222–8. [PubMed] [Google Scholar]
- [2].Clouet J, Vinatier C, Merceron C, et al. From osteoarthritis treatments to future regenerative therapies for cartilage. Drug Discovery Today 2009;14:913–25. [DOI] [PubMed] [Google Scholar]
- [3].Abramson SB, Attur M. Developments in the scientific understanding of osteoarthritis. Arthritis Res Ther 2009;11:227–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wakitani S, Imoto K, Yamamoto T, et al. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage 2002;10:199–206. [DOI] [PubMed] [Google Scholar]
- [5].Davatchi F, Abdollahi BS, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis 2016;14:211–5. [DOI] [PubMed] [Google Scholar]
- [6].Sotiropoulou PA, Perez SA, Maria S, et al. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2010;24:462–71. [DOI] [PubMed] [Google Scholar]
- [7].J Mary M, Kenneth D, Stephen B, et al. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum 2010;46:704–13. [DOI] [PubMed] [Google Scholar]
- [8].Banfi A, Bianchi G, Notaro R, et al. Replicative aging and gene expression in long-term cultures of human bone marrow stromal cells. Tissue Eng 2002;8:901–10. [DOI] [PubMed] [Google Scholar]
- [9].Yubo M, Yanyan L, Li L, et al. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: a meta-analysis. Plos One 2017;12:e0175449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xia P, Wang X, Lin Q, et al. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop 2015;39:2363–72. [DOI] [PubMed] [Google Scholar]
- [11]. Lee WS, Kim HJ, Kim KI, et al. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8:504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kuah D, Sivell S, Longworth T, et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: a randomized double-blind placebo-controlled single ascending dose study. J Transl Med 2018;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Panic N, Leoncini E, De Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and metaanalyses. Stefania Boccia. European Journal of Public Health 2013;23: suppl_1: e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].2001;Jüni P, Altman DG, Egger M. Assessing the quality of randomized controlled trials."Systematic reviews in health care: meta-analysis in context. 87–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shapiro SA, Kazmerchak SE, Heckman MG, et al. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med 2017;45:82–90. [DOI] [PubMed] [Google Scholar]
- [16].Lamo-Espinosa JM, Mora G, Blanco JF, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med 2016;14:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wong KL, Lee KB, Tai BC, et al. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: a prospective, randomized controlled clinical trial with 2 years’ follow-up. Arthroscopy 2013;29:2020–8. [DOI] [PubMed] [Google Scholar]
- [18].Khay-Yong S, Adam A, Caroline SYJ, et al. Articular cartilage regeneration with autologous peripheral blood stem cells versus hyaluronic acid: a randomized controlled trial. Arthroscopy 2013;29:684–94. [DOI] [PubMed] [Google Scholar]
- [19].C Thomas V, Jack F, Joel B, et al. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am 2014;96:90–8. [DOI] [PubMed] [Google Scholar]
- [20].Bhattacharya N. Clinical use of amniotic fluid in osteoarthritis: a source of cell therapy. Transplantation 2011;90:395–403. [Google Scholar]
- [21].Aurelio V, Miguel Angel MF, Francisco DC, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation 2015;99:1681–90. [DOI] [PubMed] [Google Scholar]
- [22].Moher D, Jadad AR, Nichol G, et al. Assessing the quality of randomized controlled trials: an annotated bibliography of scales and checklists. Control Clin Trials 1995;16:62–73. [DOI] [PubMed] [Google Scholar]
- [23].Jadad AR, Rennie D. The randomized controlled trial gets a middle-aged checkup. J Am Med Assoc 1998;279:319–20. [DOI] [PubMed] [Google Scholar]
- [24].Tögel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005;289:F31–42. [DOI] [PubMed] [Google Scholar]
- [25].Ortiz LA, Maria D, Cheryl F, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 2007;104:11002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012;19:902–7. [DOI] [PubMed] [Google Scholar]
- [27].Grässel S, Lorenz J. Tissue-engineering strategies to repair chondral and osteochondral tissue in osteoarthritis: use of mesenchymal stem cells. Curr Rheumatol Rep 2014;16:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Centeno CJ, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Phys 2008;11:343–53. [PubMed] [Google Scholar]
- [29].Emadedin M, Aghdami N, Taghiyar L, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iranian Med 2012;15:422. [PubMed] [Google Scholar]
- [30].Wang D, Zhang H, Liang J, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant 2013;22:2267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Koç ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after confusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000;18:307. [DOI] [PubMed] [Google Scholar]
- [32].Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005;11:389–98. [DOI] [PubMed] [Google Scholar]
- [33].Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308:2369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bernardo ME, Pagliara D, Locatelli F. Mesenchymal stromal cell therapy: a revolution in regenerative medicine? Bone Marrow Transplant 2012;47:164–71. [DOI] [PubMed] [Google Scholar]
- [35].Wang P, Li Y, Huang L, et al. Effects and safety of allogenic mesenchymal stem cell intravenous infusion in active ankylosing spondylitis patients who failed NSAIDs: a 20-week clinical trial. Cell Transplant 2014;23:1293–303. [DOI] [PubMed] [Google Scholar]


