Abstract
Background:
We performed a systematic review and meta-analysis of the efficacy and safety of teriparatide and bisphosphonates in managing postmenopausal osteoporosis.
Methods:
PubMed, EMBASE, Web of Science, and China National Knowledge Infrastructure were searched for relevant randomized controlled trials that were published before April 2018 and compared teriparatide and bisphosphonates in treating postmenopausal osteoporosis. Stata 12.0 was used for the meta-analysis. The pooled risk ratio (RR) or weighted mean difference and 95% confidence interval (CI) were calculated using a fixed effects or random effects meta-analysis.
Results:
A total of 14 randomized controlled trials were included in this meta-analysis. The teriparatide group was associated with a lower total occurrence of vertebral fractures (RR = 0.55, 95% CI: 0.40–0.77; P = .001) and nonvertebral fractures (RR = 0.65, 95% CI: 0.46–0.90; P = .009) than the bisphosphonate group. Moreover, compared with the bisphosphonate group, the teriparatide group had improved bone mineral density at the lumbar spine and femoral neck at the final follow-up (P < .05). There was no significant difference between the teriparatide and bisphosphonate groups in terms of complications (RR = 1.05, 95% CI: 0.90, 1.22, P = .516).
Conclusions:
Teriparatide significantly reduced the occurrence of vertebral and nonvertebral fractures in osteoporosis patients. More studies should focus on the quality of life of patients using these 2 drugs.
Keywords: bisphosphonates, meta-analysis, teriparatide, vertebral fracture
1. Introduction
Postmenopausal osteoporosis is a public health problem that is common in older women.[1] Approximately 30% of postmenopausal women have osteoporosis, according to the World Health Organization definition of osteoporosis.2,3 Postmenopausal osteoporosis is associated with a risk for fractures, and fractures of this type do not heal easily.
The most common interventions for the treatment of osteoporosis and the prevention of fractures are oral bisphosphonates and injections with teriparatide. Bisphosphonates include alendronate, etidronate, ibandronate, and risedronate.4,5 Teriparatide is a parathyroid hormone (PTH) that is approved for use in individuals with osteoporosis.[6] Teriparatide is also considered a skeletal anabolic drug approved for use in postmenopausal women with osteoporosis who are at high risk for fractures.[7]
We pooled the results from the current clinical studies on teriparatide and bisphosphonates for the treatment of postmenopausal osteoporosis in a meta-analysis. The purpose of this meta-analysis was to determine whether teriparatide is associated with
-
(1)
a lower occurrence of vertebral and nonvertebral fractures,
-
(2)
a higher BMD in the lumbar and femoral neck, and
-
(3)
fewer complications in postmenopausal patients.
2. Materials and methods
This meta-analysis was conducted in accordance with the guidelines of the preferred reporting items for systematic reviews and meta-analysis statement.[8] There was no registered protocol for the current meta-analysis.
2.1. Literature search
Published articles comparing teriparatide and bisphosphonates for improving bone mineral density (BMD) in postmenopausal osteoporosis patients between January 1990 and March 2019 were retrieved. The searchable databases included PubMed, EMBASE, Web of Science, and China National Knowledge Infrastructure, and the following keywords were used: (1) (hPTH (1–34) OR Teriparatide Acetate OR Teriparatide) AND (Bisphosphonates OR “Diphosphonates” [Mesh]). All these words were assembled with the Boolean operator “and” in the search strategy. No restrictions regarding the publication language were used in the literature retrieval step. To maximize the search specificity and sensitivity, authors also examined the reference lists of studies searched to identify additional relevant studies that were not identified through the retrieval strategy. Although the present study involved human participants, ethical approval and informed consent from participants were not required because all data were acquired from previously published studies and analyzed anonymously without any potential harm to the participants. After the initial online search, relevant articles and their bibliographies were manually reviewed.
2.2. Selection criteria
After the primary selection of the studies, the text of the studies that were potentially relevant were reviewed, and the studies included were required to meet the following inclusion criteria:
-
(1)
Randomized controlled trials (RCTs) comparing teriparatide and bisphosphonates;
-
(2)
Studies involving elderly patients with osteoporosis;
-
(3)
Studies reporting the total occurrence of vertebral and nonvertebral fractures, the mean BMD in the lumbar spine and femoral neck at the final follow-up and the complications;
-
(4)
Studies with full text access.
Studies were excluded on the basis of the following exclusion criteria:
-
(1)
Nonrandomized studies;
-
(2)
Studies involving patients with other types of fractures;
-
(3)
Studies lacking available data or comparable results.
2.3. Data extraction
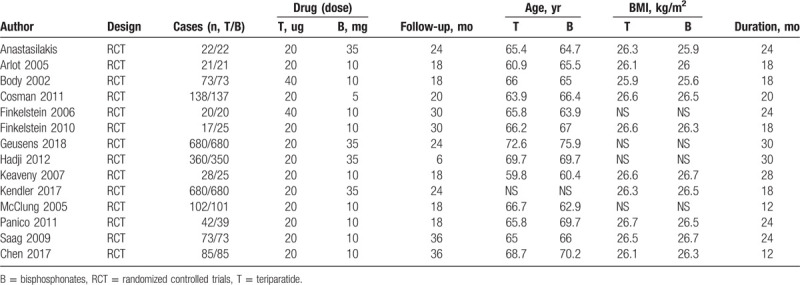
Relevant data from the included studies were compiled in a table. The indicators consisted of the first author's name, study type, cases (teriparatide vs bisphosphonates), drug dose of teriparatide and bisphosphonates, follow-up duration, age of the teriparatide and bisphosphonate groups, body mass index of the teriparatide and bisphosphonates groups and treatment duration.
2.4. Risk of bias
Two authors (Guiyong Fan and Qun Zhao) independently assessed the risk of bias using the Cochrane risk-of-bias tool. We reviewed each trial and scored it as having high, low, or unclear risk of bias according to the following criteria: random sequence generation; allocation concealment; blinding of the participants and personnel to the study protocol; blinding of the outcome assessors; incomplete outcome data; selective reporting; and other bias. Trials with a high risk of bias for >1 key domains were considered to have a high risk of bias, whereas trials with a low risk of bias for all key domains were considered to have a low risk of bias; all others were considered to have an unclear risk of bias.
2.5. Statistical analysis
Stata 12.0 (Stata Corp., College Station, TX) was adopted to estimate the effects of the outcomes among the selected reports. For the continuous outcomes, the mean difference was calculated using the mean difference method. Heterogeneity across studies was calculated using the I 2 statistic, a quantitative measure of inconsistency across studies. Studies with an I 2 of 25% to 50% were considered to have low heterogeneity, I 2 of 50% to 75% indicated moderate heterogeneity, and I 2 > 75% indicated high heterogeneity. If I 2 > 50%, potential sources of heterogeneity were tested by a sensitivity analysis conducted by removing 1 study sequentially and investigating the influence of each study on the combined estimates. Furthermore, when heterogeneity was observed, a random effects model was adopted; if it was absent, a fixed effects model was utilized. Funnel plots were used to examine the potential publication bias.
3. Results
3.1. Search results and general characteristics
The literature search identified 369 studies. There were 328 studies that remained after the duplicates were removed. Of these, 314 were excluded from further assessments when the titles and abstracts were screened, and 14 studies underwent full-text screening (Fig. 1). Finally, 14 RCTs9,10,11,12,13,14,15,16,17,18,19,20,21,22 were included in the meta-analysis. When there were no data on the total occurrence of vertebral fractures, the data were obtained by contacting the authors.
Figure 1.
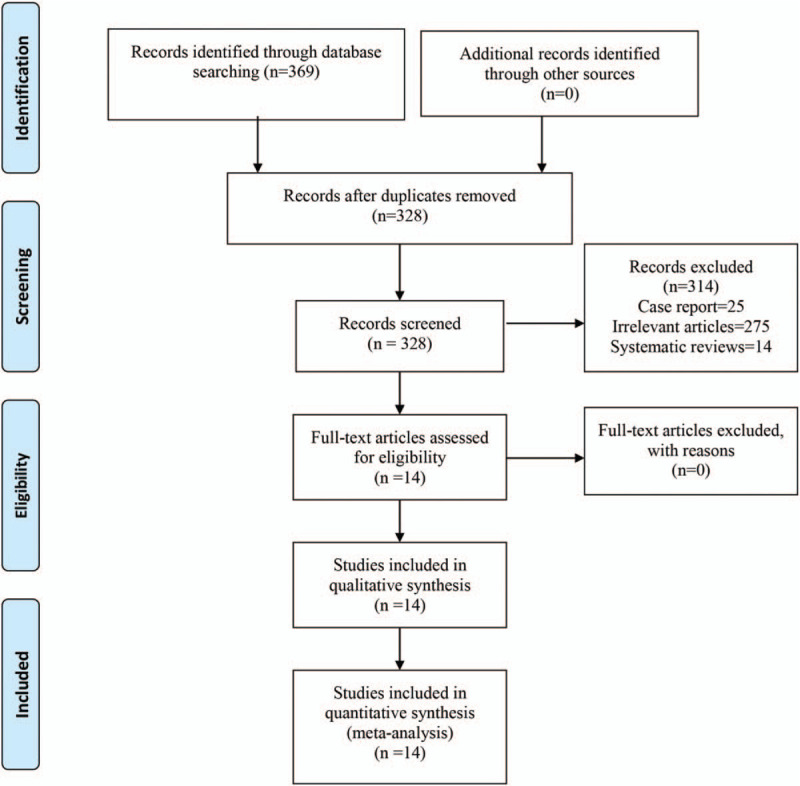
The flow diagram of the study selection process.
The main characteristics of the included trials are summarized in Table 1. All of the included studies were RCTs, and the sample size ranged from 17 to 680. The drug dose of teriparatide ranged from 20 μg to 40 μg, and the dose of bisphosphonates ranged from 10 to 35 mg. There were a total of 3 categories of bisphosphonates, including risedronate,9,14,15,17 alendronate,10,11,13,16,18,19 and zoledronic acid.[12] The follow-up duration ranged from 12 to 36 months. The mean age of the participants in the included studies ranged from 60.9 to 75.9 years.
Table 1.
Details of the randomized clinical trials.
3.2. Risk of bias assessment
Figures 2 and 3 show the risk of bias summary and risk of bias graph. Three trials were categorized as having an unclear risk of bias, and 6 were categorized as having an unclear risk of bias regarding allocation concealment. Two studies were categorized as having a high risk of bias regarding allocation concealment.
Figure 2.
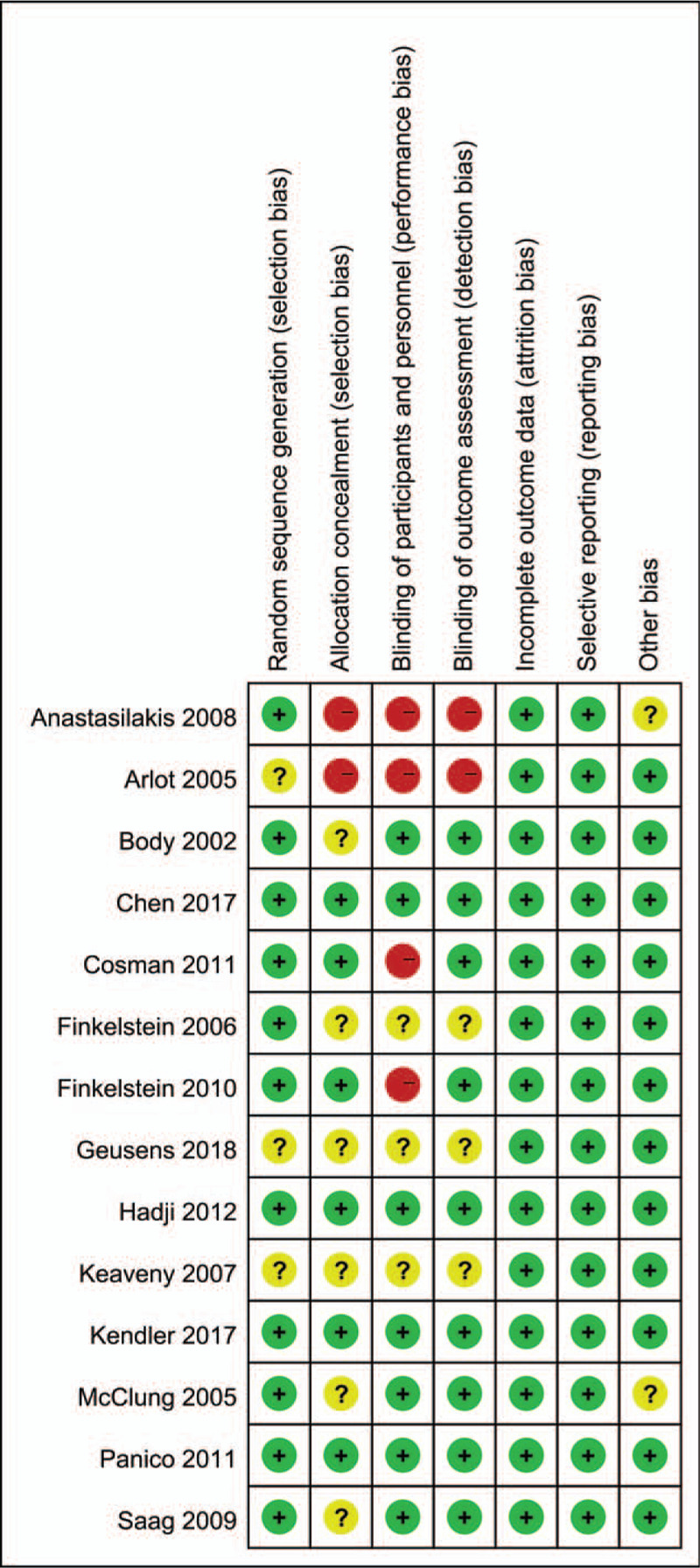
Risk of bias summary for the included studies. +, no bias; –, bias; ?, bias unknown.
Figure 3.
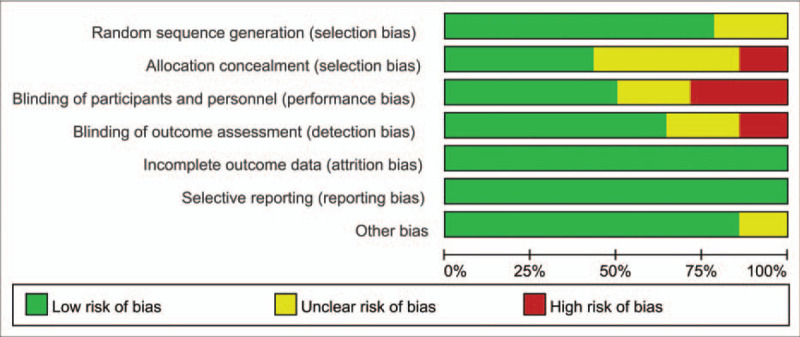
Risk of bias graph of the included studies.
4. Primary outcome
4.1. Total occurrence of vertebral and nonvertebral fractures
Six studies of 3862 patients reported the occurrence of vertebral fractures. The teriparatide group was associated with a lower total occurrence of vertebral fractures than the bisphosphonate group (risk ratio [RR] = 0.55, 95% confidence interval [CI]: 0.40–0.77; P = .001; Fig. 4), which corresponded to a reduction of 5.8% in the vertebral fracture incidence.
Figure 4.
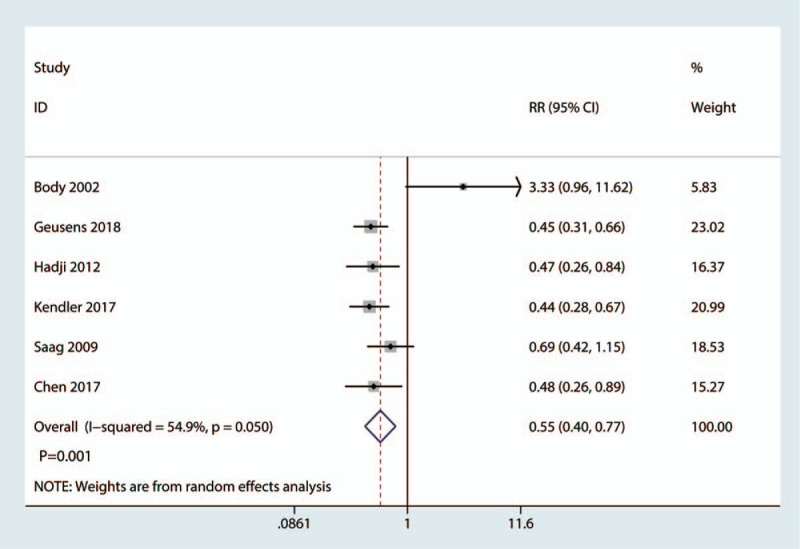
Forest plot for comparing teriparatide versus bisphosphonates in terms of the total occurrence of vertebral fractures.
Six studies of 2419 patients reported the occurrence of nonvertebral fractures. The teriparatide group was associated with a lower total occurrence of nonvertebral fractures than the bisphosphonate group (RR = 0.65, 95% CI: 0.46–0.90; P = .009; Fig. 5), which corresponded to a reduction of 4.2% in the nonvertebral fracture incidence.
Figure 5.
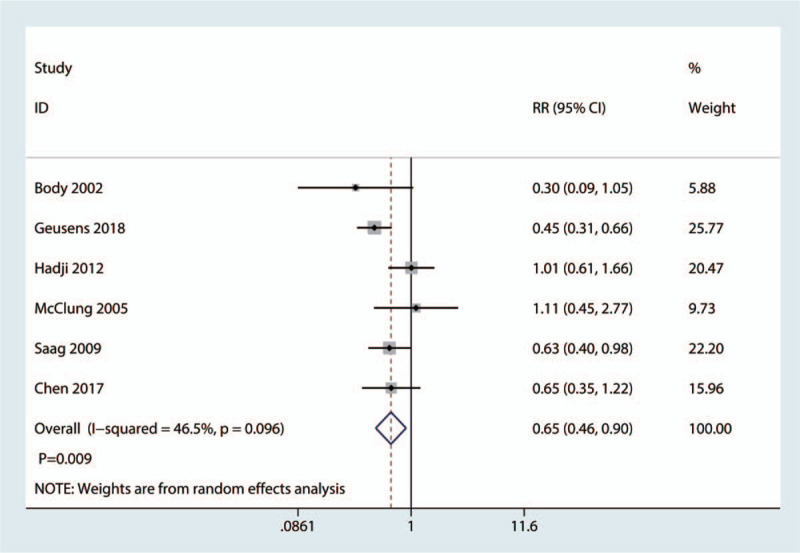
Forest plot for comparing teriparatide versus bisphosphonates in terms of the total occurrence of nonvertebral fractures.
4.2. Mean BMD in the lumbar spine at the final follow-up
Eight trials involving 714 patients provided data about the BMD in the lumbar spine at the final follow-up. Compared with the bisphosphonate group, the teriparatide group had improved BMD in the lumbar spine (weighted mean difference = 1.03, 95% CI: 0.65, 1.40, P = .000, Fig. 6), which was considered clinically important.
Figure 6.
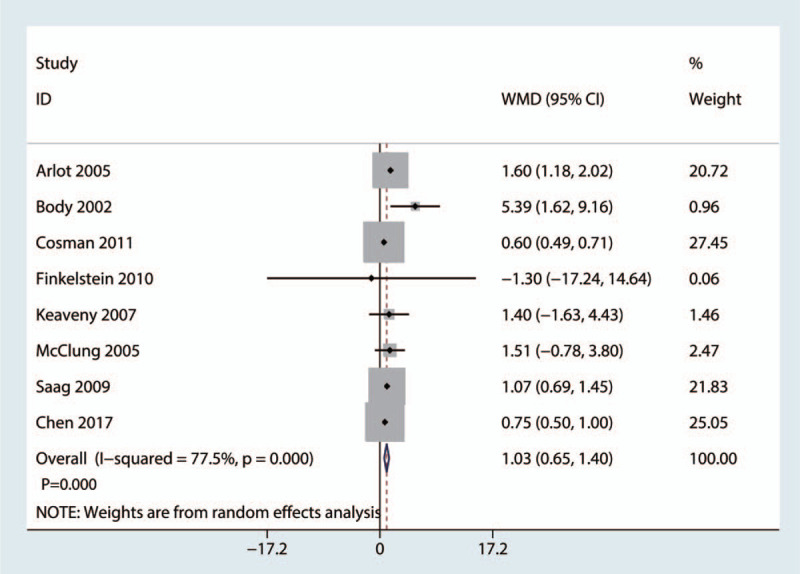
Forest plot for comparing teriparatide versus bisphosphonates in terms of the mean BMD in the lumbar spine at the final follow-up. BMD = bone mineral density.
4.3. Mean BMD in the femoral neck at the final follow-up
Five studies with 1084 patients reported the mean BMD in the femoral neck at the final follow-up. Compared with the bisphosphonate group, treatment with teriparatide was associated with an increase in the mean BMD in the femoral neck at the final follow-up (weighted mean difference = 0.85, 95% CI: 0.65, 1.06, P = .000, Fig. 7), which was considered clinically important.
Figure 7.
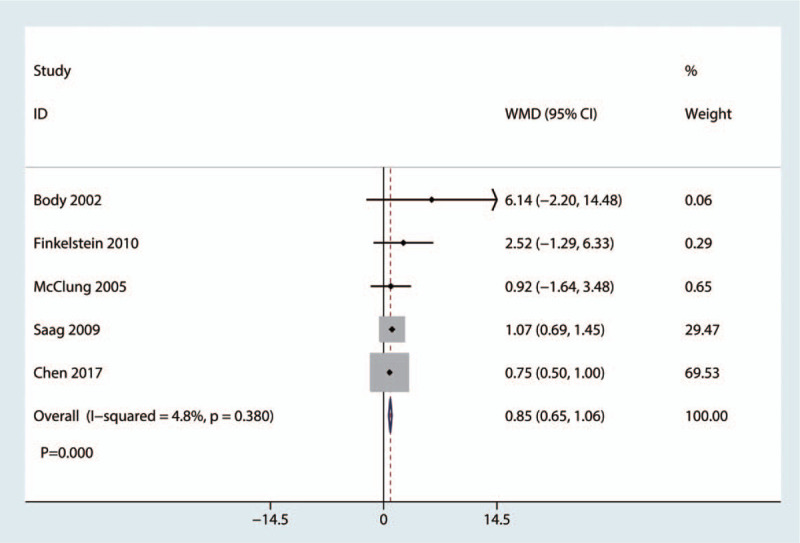
Forest plot for comparing teriparatide versus bisphosphonates in terms of the mean BMD in the femoral neck at the final follow-up. BMD = bone mineral density.
4.4. Complications
Nine studies with 2981 patients reported complications. There was no significant difference between the teriparatide and bisphosphonate groups in terms of the complications (RR = 1.05, 95% CI 0.90, 1.22, P = .516, Fig. 8).
Figure 8.
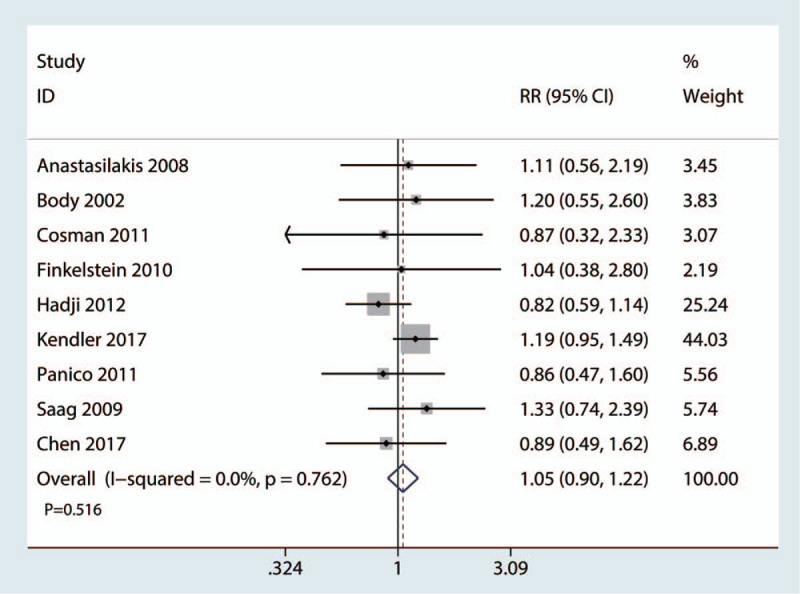
Forest plot for comparing teriparatide versus bisphosphonates in terms of the adverse events.
4.5. Publication bias and sensitivity analysis
A comparison-adjusted funnel plot was used to test the publication bias. The results are presented in Figure 9. All studies lie inside the 95% CIs, with an even distribution around the vertical axis, indicating no obvious publication bias. Publication bias was tested using Egger and Begg tests. The Egger test result was P = .25, and the Begg test result was P = .18 for the total occurrence of vertebral fractures.
Figure 9.
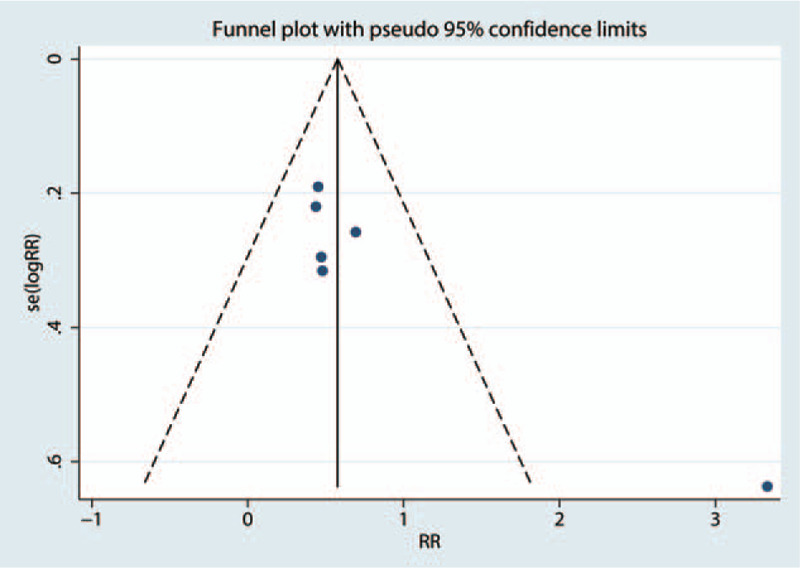
Funnel plot of the total occurrence of vertebral fractures.
A sensitivity analysis was performed by omitting each study sequentially. We found that after omitting each study sequentially, the overall effects were in the upper and lower estimates (Fig. 10).
Figure 10.
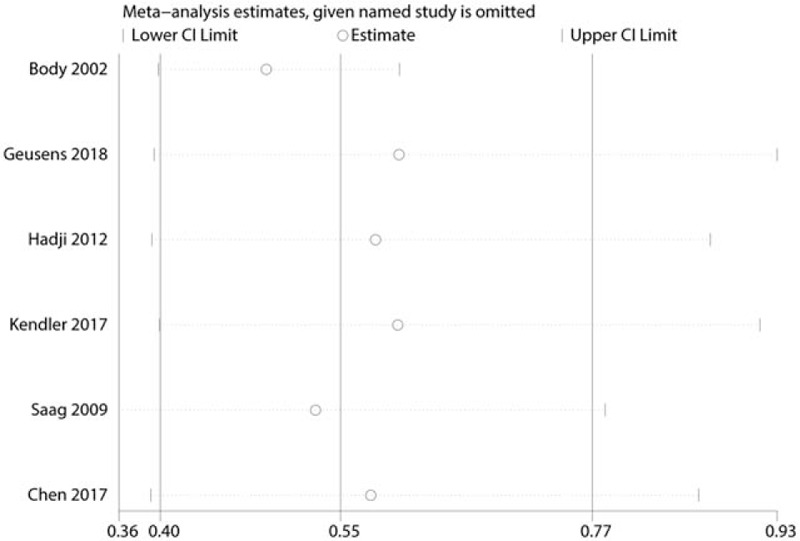
Sensitivity analysis of the total occurrence of vertebral fractures.
5. Discussion
The approved treatments for postmenopausal osteoporosis include antiresorptive and bone-forming drugs.[23] Antiresorptive drugs mainly target osteoclast-mediated bone resorption, thereby reducing bone loss and the occurrence of fractures.[24] Teriparatide (recombinant human PTH) is a bone-forming medication that preferentially stimulates osteoblasts to produce new bone tissue, thereby increasing bone mass and strength.25,26 The current meta-analysis showed that teriparatide was associated with a reduction in the occurrence of vertebral fractures compared with bisphosphonates and that this reduction is not of clinical importance. Moreover, teriparatide was superior to bisphosphonates in improving the BMD of the lumbar spine and femoral neck, which is also clinically important. There was no significant difference between the total incidence of adverse events.
First, we identified the incidence of vertebral fractures as the primary outcome because vertebral fractures are associated with a heavy economic burden. The results showed that compared with bisphosphonates, teriparatide was associated with a reduction in the incidence of vertebral fractures. We measured the incidence of nonvertebral fractures between the teriparatide and bisphosphonate groups. The results showed that there was no statistically significant difference in the incidence of nonvertebral fractures. Yuan et al[27] conducted a meta-analysis on the use of teriparatide for improving BMD in osteoporosis patients. The results showed that teriparatide could significantly decrease the occurrence of vertebral fractures. Chen et al[28] performed a review and revealed that teriparatide was safe and was not associated with an increased rate of adverse events compared with other drugs. However, the authors revealed that teriparatide was effective for the overall prevention of nonvertebral fractures in osteoporotic patients but not for the prevention of site-specific nonvertebral fractures at the wrist and hip. Freemantle et al[29] conducted a network meta-analysis and found that all treatments except for etidronate significantly reduced the risk of vertebral fractures compared with a placebo. Han et al[30] found that teriparatide significantly increased spine and hip BMD, but the improvement was considered conservative due to statistical heterogeneity. Compared with treatment with bisphosphonates, treatment with teriparatide was associated with an increase in the mean BMD in the lumbar and femoral neck at the final follow-up. We also found that after discontinuation of therapy, the BMD of the patients was also increased.
We measured the adverse events between the teriparatide and bisphosphonate groups. The results showed that there was no significant difference between these 2 drugs in terms of complications. Liu et al[26] also found that teriparatide is an effective option for the treatment of osteoporosis (odds ratio = 1.15, 95% CI: 0.71–1.85, P = .570).
There were several limitations of this meta-analysis:
-
(1)
some of the included studies had a small sample size (<50), and thus there was selection bias.
-
(2)
Quality of life was not compared between the bisphosphonate and teriparatide groups; thus, more studies should focus on this outcome.
-
(3)
Since there were many types and doses of bisphosphonates and the antiosteoporosis treatments with these drugs differed, more studies should focus on teriparatide versus 1 type of bisphosphonate.
-
(4)
The diagnoses of osteoporosis were different, and thus clinical bias existed in this meta-analysis.
-
(5)
The follow-up durations in the included studies were relatively short, and thus, the complications in bisphosphonate groups were underestimated.
6. Conclusion
In patients with postmenopausal osteoporosis, compared with bisphosphonates, teriparatide decreases the risk for vertebral fractures and increases the change in BMD in the lumbar spine and femoral neck. The complication rates in the teriparatide and bisphosphonate groups were comparable.
Author contributions
Conceptualization: Guiyong Fan.
Data curation: Hao Chen.
Formal analysis: Jinlian Liu.
Funding acquisition: Guiyong Fan, Qun Zhao.
Software: Weixiao Guo.
Validation: Wei Tan, Chaoqun Liu.
Writing – original draft: Qun Zhao, Pei Lu, Chaoqun Liu.
Writing – review and editing: Hao Chen, Jinlian Liu.
Footnotes
Abbreviations: BMD = bone mineral density, CI = confidence interval, PTH = parathyroid hormone, RCTs = randomized controlled trials, RR = risk ratio.
How to cite this article: Fan G, Zhao Q, Lu P, Chen H, Tan W, Guo W, Liu C, Liu J. Comparison between teriparatide and bisphosphonates for improving bone mineral density in postmenopausal osteoporosis patients: a meta-analysis. Medicine. 2020;99:15(e18964).
The authors have no funding and conflicts of interest to disclose.
References
- [1]. Watts NB, Hattersley G, Fitzpatrick LA, et al. Abaloparatide effect on forearm bone mineral density and wrist fracture risk in postmenopausal women with osteoporosis. Osteoporos Int 2019;30:1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Kahwati LC, Weber RP, Pan H, et al. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: evidence report and systematic review for the US preventive services task force. JAMA 2018;319:1600–12. [DOI] [PubMed] [Google Scholar]
- [3]. Edwards BJ, Brooks ER, Langman CB. Osteoporosis screening of postmenopausal women in the primary care setting: a case-based approach. Gend Med 2004;1:70–85. [DOI] [PubMed] [Google Scholar]
- [4]. Zhou J, Ma X, Wang T, et al. Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: a systematic review with network meta-analyses. Osteoporos Int 2016;27:3289–300. [DOI] [PubMed] [Google Scholar]
- [5]. Nayak S, Greenspan SL. A systematic review and meta-analysis of the effect of bisphosphonate drug holidays on bone mineral density and osteoporotic fracture risk. Osteoporos Int 2019;30:705–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Lyritis GP, Georgoulas T, Zafeiris CP. Bone anabolic versus bone anticatabolic treatment of postmenopausal osteoporosis. Ann N Y Acad Sci 2010;1205:277–83. [DOI] [PubMed] [Google Scholar]
- [7]. Diez-Perez A, Marin F, Eriksen EF, et al. Effects of teriparatide on hip and upper limb fractures in patients with osteoporosis: a systematic review and meta-analysis. Bone 2019;120:1–8. [DOI] [PubMed] [Google Scholar]
- [8]. Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 2013;8: e83138-e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Anastasilakis AD, Goulis DG, Polyzos SA, et al. Head-to-head comparison of risedronate vs. teriparatide on bone turnover markers in women with postmenopausal osteoporosis: a randomised trial. Int J Clin Pract 2008;62:919–24. [DOI] [PubMed] [Google Scholar]
- [10]. Arlot M, Meunier PJ, Boivin G, et al. Differential effects of teriparatide and alendronate on bone remodeling in postmenopausal women assessed by histomorphometric parameters. J Bone Miner Res 2005;20:1244–53. [DOI] [PubMed] [Google Scholar]
- [11]. Body JJ, Gaich GA, Scheele WH, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab 2002;87:4528–35. [DOI] [PubMed] [Google Scholar]
- [12]. Cosman F, Eriksen EF, Recknor C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res 2011;26:503–11. [DOI] [PubMed] [Google Scholar]
- [13]. Finkelstein JS, Wyland JJ, Lee H, et al. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab 2010;95:1838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Geusens P, Marin F, Kendler DL, et al. Effects of teriparatide compared with risedronate on the risk of fractures in subgroups of postmenopausal women with severe osteoporosis: the VERO trial. J Bone Miner Res 2018;33:783–94. [DOI] [PubMed] [Google Scholar]
- [15]. Hadji P, Zanchetta JR, Russo L, et al. The effect of teriparatide compared with risedronate on reduction of back pain in postmenopausal women with osteoporotic vertebral fractures. Osteoporos Int 2012;23:2141–50. [DOI] [PubMed] [Google Scholar]
- [16]. Keaveny TM, Donley DW, Hoffmann PF, et al. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res 2007;22:149–57. [DOI] [PubMed] [Google Scholar]
- [17]. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2017;391:230–40. [DOI] [PubMed] [Google Scholar]
- [18]. McClung MR, San Martin J, Miller PD, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med 2005;165:1762–8. [DOI] [PubMed] [Google Scholar]
- [19]. Panico A, Lupoli GA, Marciello F, et al. Teriparatide vs. alendronate as a treatment for osteoporosis: changes in biochemical markers of bone turnover, BMD and quality of life. Med Sci Monit 2011;17:Cr442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Chen C, Liang YN. Effects of teriparatide and alendronate in women with postmenopausal osteoporosis. J Clin Med Literature 2017;4:9350–1. [Google Scholar]
- [21]. Finkelstein JS, Leder BZ, Burnett SM, et al. Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab 2006;91:2882–7. [DOI] [PubMed] [Google Scholar]
- [22]. Saag KG, Zanchetta JR, Devogelaer JP, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum 2009;60:3346–55. [DOI] [PubMed] [Google Scholar]
- [23]. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 2018;391:230–40. [DOI] [PubMed] [Google Scholar]
- [24]. Horikawa A, Miyakoshi N, Hongo M, et al. A prospective comparative study of intravenous alendronate and ibandronate for the treatment of osteoporosis. Medicine (Baltimore) 2019;98: e14340-e14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Ishtiaq S, Fogelman I, Hampson G. Treatment of post-menopausal osteoporosis: beyond bisphosphonates. J Endocrinol Invest 2015;38:13–29. [DOI] [PubMed] [Google Scholar]
- [26]. Liu CL, Lee HC, Chen CC, et al. Head-to-head comparisons of bisphosphonates and teriparatide in osteoporosis: a meta-analysis. Clin Invest Med 2017;40:E146–57. [DOI] [PubMed] [Google Scholar]
- [27]. Yuan F, Peng W, Yang C, et al. Teriparatide versus bisphosphonates for treatment of postmenopausal osteoporosis: a meta-analysis. Int J Surg 2019;66:1–1. [DOI] [PubMed] [Google Scholar]
- [28]. Chen Y, Liu R, Hettinghouse A, et al. Clinical application of teriparatide in fracture prevention: a systematic review. JBJS Rev 2019;7: e10-e10. [DOI] [PubMed] [Google Scholar]
- [29]. Freemantle N, Cooper C, Diez-Perez A, et al. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int 2013;24:209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Han SL, Wan SL. Effect of teriparatide on bone mineral density and fracture in postmenopausal osteoporosis: meta-analysis of randomised controlled trial. Int J Clin Pract 2012;66:199–209. [DOI] [PubMed] [Google Scholar]


