Abstract
We aim to explore the relationship between early-onset diabetes and proliferative diabetic retinopathy (PDR) in type 2 diabetes mellitus (T2DM) patients with microalbuminuria.
A total of 461 T2DM patients with microalbuminuria were enrolled. Subjects were defined as early-onset or late-onset based on the age at which they were diagnosed with diabetes (<40 and ≥40 years, respectively). Medical history, anthropometry, and laboratory indicators were documented. PDR was defined as the presence of any of the following changes on fundus photography: neovascularization, vitreous hemorrhage, or preretinal hemorrhage.
The prevalence of PDR was 6-fold higher in patients with early-onset than late-onset T2DM [(6.1% vs 1.0%), P = .004]. Univariate correlation analysis showed that early-onset diabetes, use of oral hypoglycemic drugs, and insulin therapy were risk factors for PDR. In multivariate logistic analysis, patients with early-onset diabetes exhibited a 7.00-fold [(95% confidence interval 1.40–38.26), P = .019] higher risk of PDR than subjects with late-onset diabetes after adjusting for sex; T2DM duration; systolic blood pressure; total triglyceride; glycated hemoglobin; insulin therapy; and the use of oral hypoglycemic drugs, antihypertensive drugs, and lipid-lowering drugs.
In T2DM patients with microalbuminuria, early-onset diabetes is an independent risk factor for the development of PDR.
Keywords: disease duration, early-onset, microalbuminuria, proliferative diabetic retinopathy, Type 2 diabetes mellitus
1. Introduction
Diabetic retinopathy (DR) is a common vascular complication of diabetes and constitutes one of the most important causes of vision loss worldwide.[1] Early retinopathy does not have a significant effect on vision, but it can progress to a more advanced stage, termed proliferative diabetic retinopathy (PDR), putting patients at a substantially increased risk of serious visual impairment that can result in permanent vision loss.[2] Therefore, prevention, early detection, and effective management of DR and identification of the determinants for DR progression to PDR are very important to improve public health and ease the financial burden of health care.[3]
Besides the common risk factors like chronic hyperglycemia,[4] hypertention,[5] serum lipoprotein,[6] and anemia,[7] diabetes mellitus duration has been recognized as the most important risk factor for the development and progression of DR.[8–11] Furthermore, some researchers observed a subset of patients with longer diabetes duration, characterized by an early age of onset; these patients’ clinical features are significantly different from those of patients with a typical age of onset (middle age or older), with the former subset having a stronger family history, poorer metabolic control, a higher proportion of insulin therapy,[12,13] and a higher risk of premature diabetes-related complications.[12,14–16] Compared with late-onset diabetes, early-onset diabetes (usually defined as type 2 diabetes mellitus [T2DM] that begins before 40 years of age[17]) was associated with an increased prevalence of DR[18–20] and PDR.[21,22] Further study showed that early-onset diabetes increased the risk of DR 1.9-fold, independent of diabetes duration, hemoglobin glycation, blood pressure, and other factors.[20] However, the impact of early age of T2DM diagnosis on the subsequent progression of DR has not yet been carefully examined, particularly the development of sight-threatening PDR. Therefore, in the present study, we explored whether early onset could also affect the risk of developing PDR.
Microalbuminuria is an indicator of generalized endothelial damage[23] and is not only widely accepted as the first clinical sign of diabetic nephropathy but also recognized as a precursor to DR. As microalbuminuria in T2DM patients is related to early signs of diabetic retinopathy, such as decreased retinal microcirculation[24] and inner retinal neurodegeneration,[25] it was not overlooked by clinicians for the early detection of DR.[26] Therefore, we included T2DM patients with microalbuminuria that reflects early diabetic retinal microangiopathy to explore the relationship between early-onset diabetes and the development of PDR. In addition, it is still unclear whether the increased prevalence of complications connected to early-onset diabetes is purely the result of longer disease duration, or on account of the worse metabolic phenotypes, or some inherent factors linked to early-onset diabetes that make tissues more susceptible to hyperglycemic damage. Thus, in the present study, the diabetes duration and other risk factors were adjusted in the model to determine whether early-onset diabetes is an independent risk factor for the development of PDR.
2. Methods
2.1. Subjects
A total of 461 T2DM patients with microalbuminuria were included from January 1, 2015 to June 25, 2018 in the First Affiliated Hospital of Chongqing Medical University. T2DM was diagnosed based on a standard oral glucose tolerance test (OGTT) or previous medical records. Microalbuminuria was defined at least twice out of 3 times the random urinary microalbumin creatinine ratio (UACR) between 30 and 300 mg/g creatinine. The exclusion criteria were as follows: individuals with age <18 or >85 years; type 1 diabetes and other special types of diabetes; definitely diagnosed with other types of chronic renal diseases such as membranous nephropathy or IgA nephropathy; need long-term glucocorticoid treatment for other chronic diseases; and patients with malignant tumor history, immune dysfunction, and severe infection. Subjects were defined as early-onset and late-onset based on the age of diagnosis of diabetes <40 and ≥40 years, respectively.[10,15] Informed consent was obtained from all participants. This study was approved by the Ethical Committee of the First Affiliated Hospital of Chongqing Medical University.
2.2. Clinical procedures
Clinical information regarding family history and medical history was collected through physician interviews. All subjects underwent physical anthropometry measurements, including weight, height, systolic blood pressure (SBP), diastolic blood pressure (DBP), and body mass index (BMI), which was calculated by dividing weight by the height squared. A biochemical analyzer was used to measure plasma glucose levels (BS-380; Mindray Medical International, Shenzhen, China). Glycosylated hemoglobin (HbA1c) was measured using boronate affinity high-performance liquid chromatography (Trinity Biotech, ultra2, Trinity Biotech, Dublin, Ireland). Serum lipids, including total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), were measured enzymatically by an automatic analyzer (Model 7080; Hitachi, Tokyo, Japan). Serum creatinine, urinary creatinine, and albumin were measured with an automatic biochemical analyzer (Modular DDP, Roche). UACR was calculated. PDR was defined as at least the following changes based on fundus photography: neovascularization, vitreous hemorrhage, or preretinal hemorrhage.[27]
2.3. Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science, version 22.0 (IBM SPSS, Armonk, NY). Data were expressed as the mean ± standard deviation (SD) when the sample distribution was approximately normal. If normal assumption was not optimal after checking the quantile–quantile plot of the data, we used median (interquartile range, IQR) for expression. Categorical variables were reported as frequencies (percentages). The χ2 test is used for between-group comparisons. Continuous variables between 2 groups were compared using Student t test or the Mann–Whitney U test. We conducted Spearman correlation analysis to investigate the risk factors of PDR in T2DM patients with microalbuminuria. Multivariate logistic analysis was conducted to determine whether early-onset diabetes could be an independent risk factor for PDR. Four models were performed to adjust for confounding factors: model 1 was adjusted for gender and T2DM duration; model 2 was further adjusted for other traditional metabolic factors (including SBP, TG, and HbA1c) in addition to the variables in model 1; model 3 was further adjusted for drug factors (including oral hypoglycemic drugs, insulin therapy, antihypertensive drugs, and lipid-lowering drugs) on the basis of model 2. Bilateral P values <.05 were considered statistically significant.
3. Results
3.1. Clinical features of the patients with early-onset and late-onset T2DM
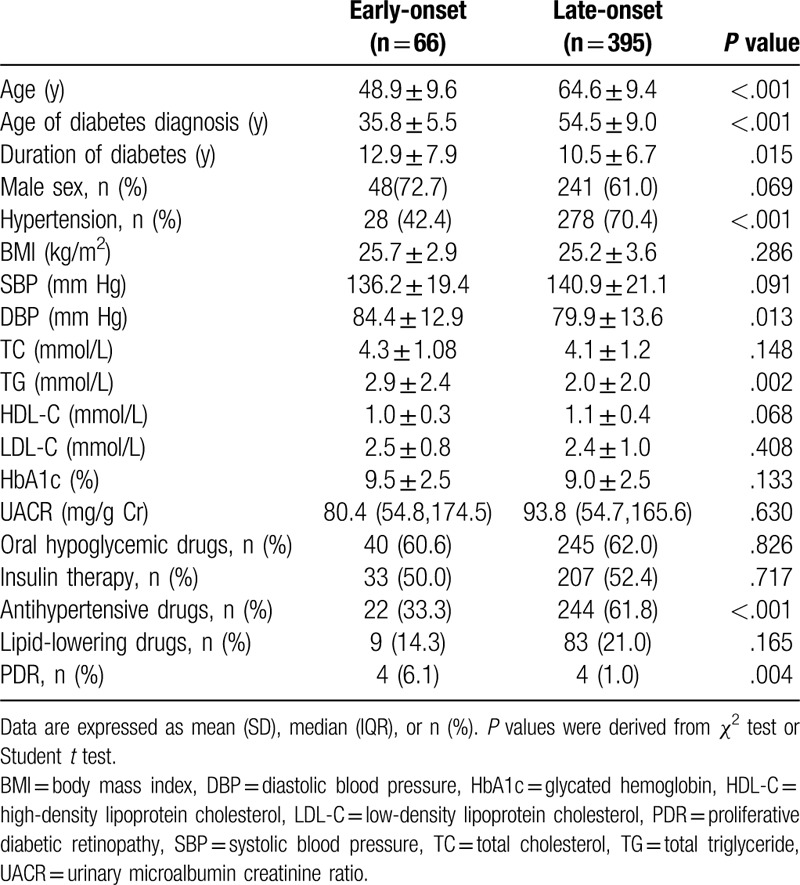
The mean age of the 461 T2DM patients with microalbuminuria was 62.4 (SD: 10.9) years, with 11.8 (SD: 7.2) years of duration of diabetes. The clinical characteristics of early-onset and late-onset T2DM are reported in Table 1. Patients with early-onset diabetes were more likely to be male and younger. The early-onset group had a longer diabetes duration, and a higher DBP and TG were less likely to be on antihypertensive drugs. Compared with subjects with late-onset T2DM, the prevalence of PDR was elevated in patients with early-onset T2DM [(6.1% vs 1.0%), P = .004].
Table 1.
Clinical characteristics of patients with early-onset versus late-onset type 2 diabetes.
3.2. Risk factors of PDR in patients with microalbuminuria
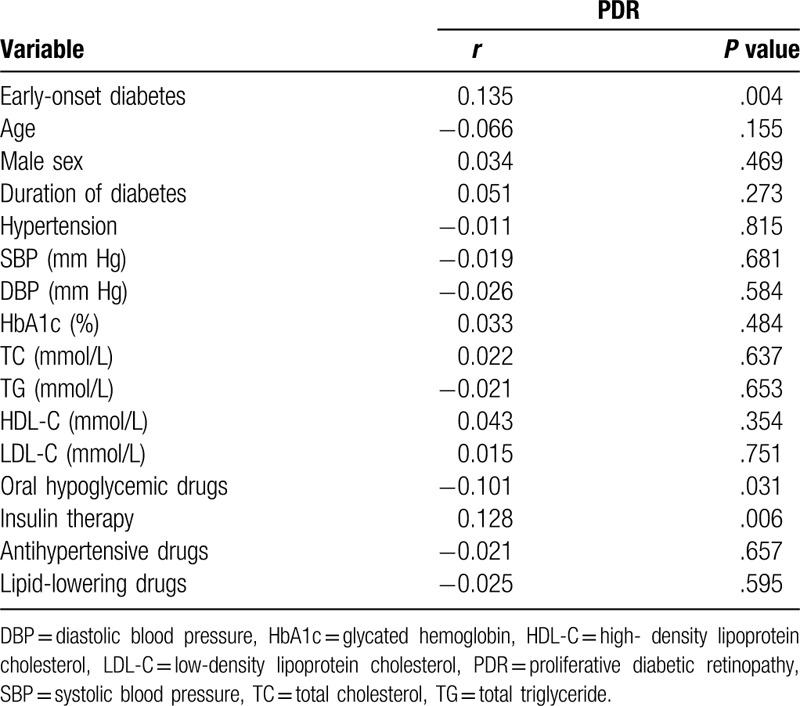
According to Spearman correlation analysis (Table 2), early-onset diabetes (r = 0.135, P = .004), oral hypoglycemic drugs (r = −0.101, P = .031), and insulin treatment (r = 0.128, P = .006) were more correlated with the development of PDR. The factors, such as age, duration of diabetes, and history of hypertension, HbA1c, were not related to the development of PDR in patients with microalbuminuria.
Table 2.
Spearman correlation coefficient for PRD in patients with microalbuminuria.
3.3. Early-onset T2DM: an independent factor for PDR in T2DM patients with microalbuminuria
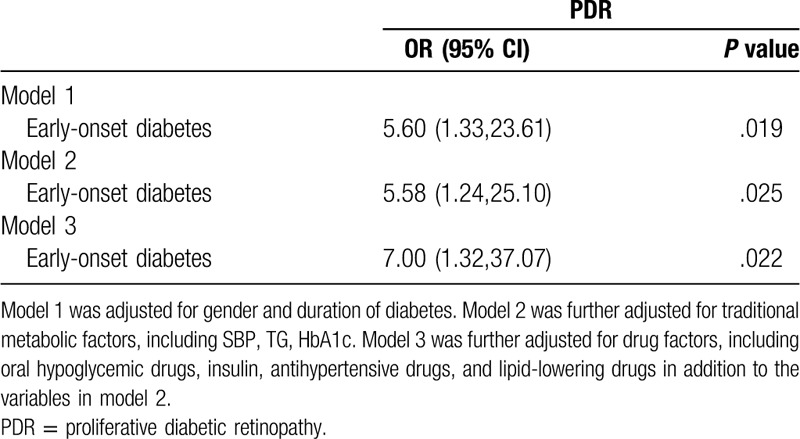
Multivariate logistic analysis was conducted to adjust for confounding factors. Model 1 was adjusted for sex and duration of diabetes. Early-onset T2DM patients had a 5.60-fold (95% CI 1.33–23.61) increase in the risk of PDR (Table 3). Even with further adjustment for other traditional metabolic factors (including SBP, TG, HbAc1), early-onset T2DM still had a marked increased risk of PDR (model 2). Further adjustment for drug factors (including oral hypoglycemic drugs, insulin therapy, antihypertensive drugs, lipid-lowering drugs), early-onset T2DM was associated with a 7.00-fold (95% CI 1.32–37.07) increased risk of PDR in the patients with microalbuminuria (model 3, Table 3).
Table 3.
Odds ratio (OR) of early-onset versus late-onset type 2 diabetes for PDR in patients with microalbuminuria.
4. Discussion
In this study, we demonstrated for the first time that early-onset diabetes increases the risk of PDR in T2DM patients with microalbuminuria. Notably, this finding remains significant after adjusting for diabetes duration and other risk factors, suggesting that early-onset diabetes is an independent risk factor for the development of PDR.
Type 2 diabetes is increasingly diagnosed in children, adolescents, and young adults.[28] Several epidemiological studies have reported a consistent increase in the prevalence of early-onset type 2 diabetes across most regions of the world, for example, in the UK,[29] Finland,[30] and China.[31,32] According to the International Diabetes Federation report, approximately 63 million young adults aged 20 to 39 years had type 2 diabetes worldwide in 2013, which accounted for 16% of total adults with type 2 diabetes.[33]
Many patients with early-onset type 2 diabetes develop PDR. A longitudinal observational study showed that of the 1065 early-onset T2DM patients (diabetes diagnosed before 30 years of age), 299 (28%) individuals developed PDR, and nearly half of them developed PDR before the age of 35 years.[21] It was observed in the same cohort that nearly one-third of patients with early-onset diabetes progressed from nonproliferative DR to PDR during a mean follow-up of 7.1 years.[22] The rate of progression from nonproliferative DR to PDR found in patients with early-onset diabetes[22] was considerably higher than that found in late-onset type 2 diabetic patients.[34–36] These studies suggest that early-onset diabetes may be at high risk for the development of PDR. Our study confirmed that the prevalence of PDR was 6-fold higher in early-onset T2DM patients with microalbuminuria compared with the late-onset phenotype. Of note, we focused on T2DM patients with microalbuminuria, which has been recognized as a marker of generalized endothelial (including retinal microvascular) damage.[23] On the pathological basis of early retinal microangiopathy, the relationship between early-onset diabetes and the development of PDR was identified, and we found that early-onset T2DM had a 7-fold higher risk for PDR compared with the late-onset group, even adjusted for common risk factors.
The mechanisms by which early-onset diabetes increases the risk of PDR have not been fully elucidated. Early-onset diabetes may indicate a longer diabetes duration. Relative to late-onset diabetes, early development of disease imposes a longer exposure to chronic hyperglycemia and increases the likelihood of progression to PDR even before reaching middle age. Notably, our study and other studies have found that early-onset T2DM was still associated with chronic complications even adjusted for diabetes duration.[16,20] This result suggests that there are additional factors hitherto unexplored in early-onset individuals that predispose them to retinopathy. Several studies have observed that early-onset diabetes has a strong family history of diabetes,[12] which may be related to genetic variation. Kong investigated the genetic factors of early-onset T2DM through genetic risk scores (GRSs) and found that early-onset diabetes was associated with genetic variants linked to beta-cell function.[37] One study on the genetic factors of early-onset T2DM through GRSs found that early-onset diabetes was associated with genetic variants linked to beta-cell function. Previous studies have reported that decreased insulin secretion by islet beta cells is a risk factor for severe DR. The prevalence of PDR among those with type 2 diabetes who require insulin was 5 times higher than those who do not.[38] Therefore, we can speculate that the genetic variants linked to beta-cell function in early-onset diabetes lead to a decrease in the insulin secretion ability of beta cells, which further aggravates the progression of DR. In addition, an allele of the (A–C) dinucleotide repeat sequence near the promoter region of the aldose reductase gene appeared in 50% of T2DM patients with DR aged less than 40 years.[39] Conceivably, the genetic factors contributing to early-onset diabetes and its role in the development of vascular complications would be an interesting avenue for future research.
We demonstrated for the first time that early-onset diabetes is an independent risk factor for the development of PDR in T2DM patients with microalbuminuria. With the increased prevalence of early-onset diabetes, our research had important clinical implications: further attention must be paid to the subset of diabetic retinopathy patients with younger diabetes onset, and the metabolic targets applied before middle-age should be made more stringent. The following limitations should be noted. First, our study was a cross-sectional study. Large multicenter prospective cohort studies to explore the cause-effect association between early-onset T2DM and PDR in patients with microalbuminuria are needed in the future. Second, this study was conducted in a single-tertiary hospital center, and some economically disadvantaged patients or patients with mild type 2 diabetes might not have sought medical treatment and thus might have been missed. Third, the precise onset age of T2DM is difficult to determine because of the insidious onset of this disease. The observed onset age may differ from the real onset age.
5. Conclusion
In conclusion, our study provides information on the effect of age at diabetes diagnosis on the development of PDR in patients with microalbuminuria. These perspectives highlight the growing need to direct attention toward early-onset T2DM and to apply effective interventions before middle aged.
Acknowledgments
The authors thank the Chongqing Medical University for their support toward publishing this article. The authors also thank the staff and patients of the First Affiliated Hospital of Chongqing Medical University for their contributions during the study. Finally, they thank Qinying Zhao, Yangmei Zhou, and Wenjin Luo for helping with data collection for the study.
Author contributions
All authors have contributed significantly. ZW and XC designed the study. XL and XR conducted the data analysis and wrote the manuscript. TL, JH, and ZL oversaw the data collection and contributed to the study design. XC and SH contributed to the data analysis. LZ, YT, QZ, YZ, WL, LY, and XL contributed to the data collection. YW and QZ contributed to the writing of the manuscript. SH and QL edited the manuscript. ZW are the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors are in agreement with the content of the manuscript.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, DBP = diastolic blood pressure, DR = diabetic retinopathy, GRSs = genetic risk scores, HbA1c = glycosylated hemoglobin, HDL-C = high-density lipoprotein cholesterol, IQR = interquartile range, LDL-C = low-density lipoprotein, OGTT = glucose tolerance test, OR = odds ratio, PDR = proliferative diabetic retinopathy, SBP = systolic blood pressure, SD = standard deviation, TC = total cholesterol, TG = triglycerides, UACR = urinary microalbumin creatinine ratio.
How to cite this article: Lv X, Ran X, Chen X, Luo T, Hu J, Wang Y, Liu Z, Zhen Q, Liu X, Zheng L, Tang Y, Zhao Q, Han S, Zhou Y, Luo W, Yang L, Li Q, Wang Z. Early-onset type 2 diabetes: A high-risk factor for proliferative diabetic retinopathy (PDR) in patients with microalbuminuria. Medicine. 2020;99:19(e20189).
XL and XR should be considered as joint first author.
This work was supported by National Natural Science Foundation of China [grant number 81670785]; Technological Innovation and Application Development Project of Chongqing [grant number cstc2019jscx-msxmX0207]; Chongqing Science and Health Joint Medical Research Project [grant number 2018GDRC004]. High-end Medical Talents of Middle-aged and Young People in Chongqing[yuweiren[2015]49].
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
References
- [1].Robert N, Frank MD. Diabetic retinopathy. N Engl J Med 2004;350:48–58. [DOI] [PubMed] [Google Scholar]
- [2].Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet 2010;376:124–36. [DOI] [PubMed] [Google Scholar]
- [3].Sabanayagam C, Banu R, Chee ML, et al. Incidence and progression of diabetic retinopathy: a systematic review. Lancet Diabetes Endocrinol 2019;7:140–9. [DOI] [PubMed] [Google Scholar]
- [4].UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–53. [PubMed] [Google Scholar]
- [5].UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- [6].Tu WJ, Liu H, Liu Q, et al. Association between serum lipoprotein (a) and diabetic retinopathy in Han Chinese patients with type 2 diabetes. J Clin Endocrinol Metab 2017;102:2525–32. [DOI] [PubMed] [Google Scholar]
- [7].Chung JO, Park SY, Chung DJ, et al. Relationship between anemia, serumbilirubin concentrations, and diabetic retinopathy in individuals with type 2 diabetes. Medicine (Baltimore) 2019;98:e17693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].From the Diabetes Control and Complications Trial Research Group. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. Ophthalmology 1995;102:647–61. [DOI] [PubMed] [Google Scholar]
- [9].Aldington SJ, Kohner EM, Meuer S, et al. Methodology for retinal photography and assessment of diabetic retinopathy: the EURODIAB IDDM complications study. Diabetologia 1995;38:437–44. [DOI] [PubMed] [Google Scholar]
- [10].Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105:1801–15. [DOI] [PubMed] [Google Scholar]
- [11].Manaviat MR, Afkhami M, Shoja MR. Retinopathy and microalbuminuria in type II diabetic patients. BMC Ophthalmol 2004;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yeung RO, Zhang Y, Luk A, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol 2014;2:935–43. [DOI] [PubMed] [Google Scholar]
- [13].Song SH, Gray TA. Early intensive cardiovascular risk management in young people with type 2 diabetes. Diabetes Res Clin Pract 2011;92:e70–2. [DOI] [PubMed] [Google Scholar]
- [14].Pavkov ME, Bennett PH, Knowler WC, et al. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 2006;296:421–6. [DOI] [PubMed] [Google Scholar]
- [15].Li L, Ji L, Guo X, et al. Prevalence of microvascular diseases among tertiary care Chinese with early versus late onset of type 2 diabetes. J Diabetes Complications 2015;29:32–7. [DOI] [PubMed] [Google Scholar]
- [16].Huo X, Gao L, Guo L. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol 2016;4:115–24. [DOI] [PubMed] [Google Scholar]
- [17].Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci 2013;1281:64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Henricsson M, Nilsson A, Groop L, et al. Prevalence of diabetic retinopathy in relation to age at onset of the diabetes, treatment, duration and glycemic control. Acta Ophthalmol Scand 1996;74:523–7. [DOI] [PubMed] [Google Scholar]
- [19].Song SH, Gray TA. Early-onset type 2 diabetes: high risk for premature diabetic retinopathy. Diabetes Res Clin Pract 2011;94:207–11. [DOI] [PubMed] [Google Scholar]
- [20].Wong J, Molyneaux L, Constantino M, et al. Timing is everything: age of onset influences long-term retinopathy risk in type 2 diabetes, independent of traditional risk factors. Diabetes Care 2008;31:1985–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yokoyama H, Okudaira M, Otani T, et al. Existence of early-onset NIDDM Japanese demonstrating severe diabetic complications. Diabetes Care 1997;20:844–7. [DOI] [PubMed] [Google Scholar]
- [22].Okudaira M, Yokoyama H, Otani T, et al. Slightly elevated blood pressure as well as poor metabolic control are risk factors for the progression of retinopathy in early-onset Japanese Type 2 diabetes. J Diabetes Complications 2000;14:281–7. [DOI] [PubMed] [Google Scholar]
- [23].Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, et al. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989;32:219–26. [DOI] [PubMed] [Google Scholar]
- [24].Nagaoka T, Yoshida A. Relationship between retinal blood flow and renal function in patients with type 2 diabetes and chronic kidney disease. Diabetes Care 2013;36:957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Farias LB, Lavinsky D, Benfica CZ, et al. Microalbuminuria is associated with early retinal neurodegeneration in patients with type 2 diabetes. Ophthalmic Surg Lasers Imaging Retina 2018;49:e36–43. [DOI] [PubMed] [Google Scholar]
- [26].Lee MK, Han KD, Lee JH, et al. Normal-to-mildly increased albuminuria predicts the risk for diabetic retinopathy in patients with type 2 diabetes. Sci Rep 2017;7:11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Haneda S, Yamashita H. International clinical diabetic retinopathy disease severity scale. Nihon Rinsho 2010;68: suppl 9: 228–35. [PubMed] [Google Scholar]
- [28].Lascar N, Brown J, Pattison H, et al. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol 2018;6:69–80. [DOI] [PubMed] [Google Scholar]
- [29].Holden SH, Barnett AH, Peters JR, et al. The incidence of type 2 diabetes in the United Kingdom from 1991 to 2010. Diabetes Obes Metab 2013;15:844–52. [DOI] [PubMed] [Google Scholar]
- [30].Lammi N, Taskinen O, Moltchanova E, et al. A high incidence of type 1 diabetes and an alarming increase in the incidence of type 2 diabetes among young adults in Finland between 1992 and 1996. Diabetologia 2007;50:1393–400. [DOI] [PubMed] [Google Scholar]
- [31].Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–59. [DOI] [PubMed] [Google Scholar]
- [32].Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090–101. [DOI] [PubMed] [Google Scholar]
- [33].International Diabetes Federation. IDF Diabetes Atlas 6th Edition, Available at: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/19-atlas-6th-edition.html; 2013. Accessed August 15, 2019. [Google Scholar]
- [34].Lee ET, Lee VS, Lu M, et al. Development of proliferative retinopathy in NIDDM. A follow-up study of American Indians in Oklahoma. Diabetes 1992;41:359–67. [DOI] [PubMed] [Google Scholar]
- [35].Klein R, Klein BE, Moss SE, et al. Is blood pressure a predictor of the incidence or progression of diabetic retinopathy? Arch Intern Med 1989;149:2427–32. [PubMed] [Google Scholar]
- [36].Kim HK, Kim CH, Kim SW, et al. Development and progression of diabetic retinopathy in Koreans with NIDDM. Diabetes Care 1998;21:134–8. [DOI] [PubMed] [Google Scholar]
- [37].Kong X, Xing X, Zhang X, et al. Early-onset type 2 diabetes is associated with genetic variants of β-cell function in the Chinese Han population. Diabetes Metab Res Rev 2019;29:e3214. [DOI] [PubMed] [Google Scholar]
- [38].Klein R, Davis MD, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. A comparison of retinopathy in younger and older onset diabetic persons. Adv Exp Med Biol 1985;189:321–35. [DOI] [PubMed] [Google Scholar]
- [39].Ko BC, Lam KS, Wat NM, et al. An (A-C)n dinucleotide repeat polymorphic marker at the 5’ end of the aldose reductase gene is associated with early-onset diabetic retinopathy in NIDDM patients. Diabetes 1995;44:727–32. [DOI] [PubMed] [Google Scholar]


