Abstract
To determine the influence of puncture site on aspiration in dealing with pneumothorax following CT-guided lung biopsy.
Two hundred thirty-six pneumothorax patients after CT guided lung biopsies were retrospective analyzed from January 2013 to December 2018. Patients with minor asymptomatic pneumothorax were treated conservatively with monitoring of vital signs and follow-up CT to confirm stability. Ninety of the 236 pneumothorax patients, who underwent manual aspiration, were included in this analysis. In first manual aspiration, the needle from the lesion was retracted back into the pleural space after biopsy, and then aspiration treatment was performed. If the treatment is of unsatisfied result, a second attempt aspiration treatment, which puncture site away from initial biopsy one, was conducted. The efficacy of simple manual aspiration and the new method, changing puncture site for re-aspiration was observed.
Immediate success was obtained in 62 out of the 90 patients in the first attempt. The effective rate and failure rate were 68.9% (62/90) and 31.1% (28/90), respectively. Twenty-eight patients in whom first attempt simple aspiration were unsuccessful underwent a second attempt aspiration, which puncture site away from initial biopsy one, was successful in 13 patients with 15 patients undergoing chest tube placement. The effective rate and failure rate were 46.4% (13/28) and 53.6% (15/28), respectively. Applying the modified procedure, total effective rate of aspiration elevated significantly from 68.9% (62/90) to 83.3% (75/90) (P < .05). No serious side effects were detected in the period of aspiration procedure.
Manual aspiration with puncture site away from initial biopsy one is worth trying to deal with post-biopsy pneumothorax. This modified procedure improved the efficiency of treatment significantly, and reduced the rate of pneumothorax requiring chest tube placement.
Keywords: chest tubes, computed tomography, lung biopsy, pneumothorax
1. Introduction
Computed tomography (CT)-guided lung biopsy is a common diagnostic procedure and pneumothorax is the most frequently associated adverse event.1,2 About 5% to 22.8% of CT-guided biopsy-induced pneumothorax cases require chest tube insertion, which increase the need for hospitalization, cost and discomfort.3,4 Several studies have suggested auxiliary measures to diminish the prevalence: including positioning precautions and injection of saline into the biopsy tract.5,6 Simple manual aspiration was a safe and reliable technique, which was recommended to deal with the pneumothorax after CT-guided biopsy, but the efficiency varies greatly.[7] It was reported that a number of contributing factors may affect the efficiency.8,9 We rarely find studies focus on the puncture site for improving the efficiency. The aim of this retrospective study was to assess the influence of puncture site on aspiration as an initial treatment for biopsy-induced pneumothorax.
2. Materials and methods
This retrospective study was approved by the institutional review board with waiver of the requirement for patients’ informed consent. From January 2013 to December 2018, CT-guided percutaneous lung biopsy was performed in 2350 consecutive patients, and pneumothorax were detected in 236 cases. Total of 146 patients with small and asymptomatic pneumothoraces do not need any treatment and resolve spontaneously, were excluded from this study. Ninety of the 236 pneumothorax patients, who underwent manual aspiration, were included in this analysis. The average age was 58.5 years (range 28–81 years).
All procedures were performed by experienced interventional radiologists with 3 to 10-year experience in performing percutaneous lung biopsies. During the time of biopsy, CT images through the area of interest were obtained in helical scan mode using 3 to 5-mm thick. The biopsy procedure was planned so that the needle trajectory would avoid emphysematous lung and pulmonary vessels, and cross the least number of pleural surfaces. Patients were positioned accordingly, either in a supine, prone, or lateral decubitus position, depending on the location of the lesion to provide the shortest and safe route for lung biopsy. The skin was prepped with an antiseptic solution, and local anesthesia using 1% lidocaine. A 19-gauge puncture needle was introduced, and new tomographic images were obtained so as to confirm and modify the needle position. The biopsies were performed to avoid ribs, bullae, vessels and fissures within a breath-hold. After the procedure, a chest CT scan was routinely done with slices 3 to 5-mm to detect the presence of possible complications such as pneumothorax or intrapulmonary hemorrhage. The biopsy tract in the lung was gently injected with 3 to 5 mL of sterile 0.9% saline while withdrawing the coaxial sheath.
Minor pneumothorax refers to the distance of parietal and visceral pleura was less than or equal to 1 cm, medium if it was greater than 1 cm but less than or equal to 2 cm, or large if it was greater than 2 cm. Patients with minor asymptomatic pneumothorax or intrapulmonary hemorrhage were treated conservatively with monitoring of vital signs and follow-up CT to confirm stability. In first attempt manual aspiration, the needle from the lesion was retracted back into the pleural space after biopsy, accurate localization of the needle tip was confirmed with axial tomographic cuts, and aspiration treatment was performed. If the first treatment was of unsatisfied result, a second attempt aspiration treatment was performed. The second puncture site should be as far away from the initial biopsy one as possible, with largest aspect of the pneumothorax on contiguous transverse tomographic sections. Intercostal vessels injury should be prevented. The position of the needle tip was confirmed with repeat CT and the second aspiration was conducted. The characteristics of the patients, lesions, and biopsy procedures with successful results are summarized in Table 1. After the procedure, a chest CT scan was routinely done to detect pneumothorax or intrapulmonary hemorrhage and also for other complications. The maximum distance between parietal and visceral pleura was measured and compared to the distance before aspiration. A reduction in the distance of the pneumothorax by more than one half and relief of symptoms was deemed to be success. The treatment was considered to be unsuccessful when the pneumothorax persisted to more than one half the distance before the procedure or the symptoms persisted. A chest tube was placed for patients with failed aspiration procedure, who became dyspnoeic, or who developed diminishing oxygen saturation.
Table 1.
Comparison of variables between simple aspiration and re-aspiration with favorable results.
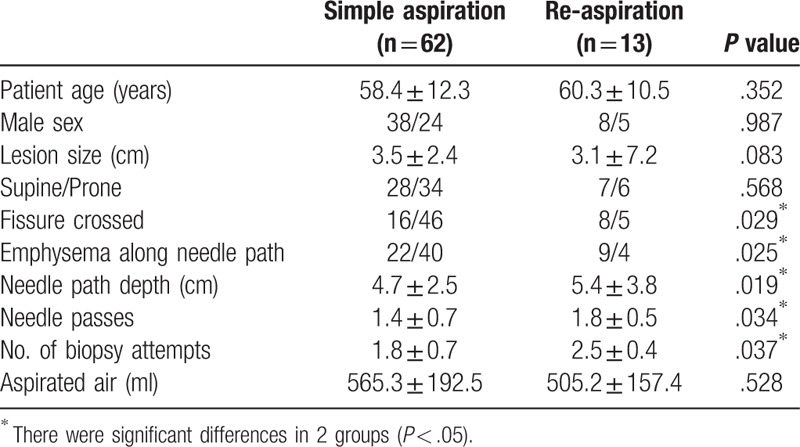
Statistical analysis was performed with PASW Statistics 18.0 (SPSS, Chicago). Fisher exact test or chi-square test and unpaired Student t test were used to assess the statistical significance of the differences between the 2 groups for categorized and continuous variables, respectively. A P value was considered statistically significant when equal to or less than .05. The study flow diagram is listed in Figure 1.
Figure 1.
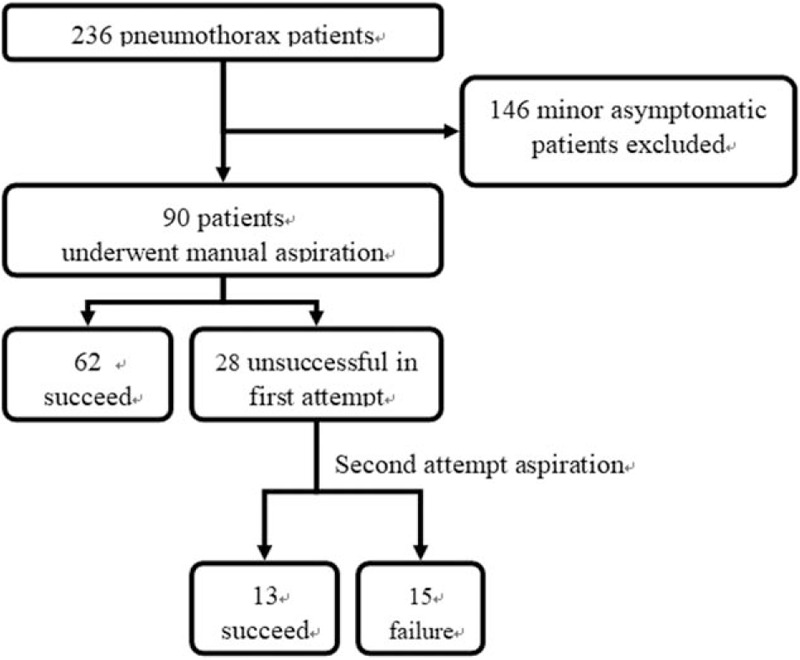
Study flow diagram.
3. Results
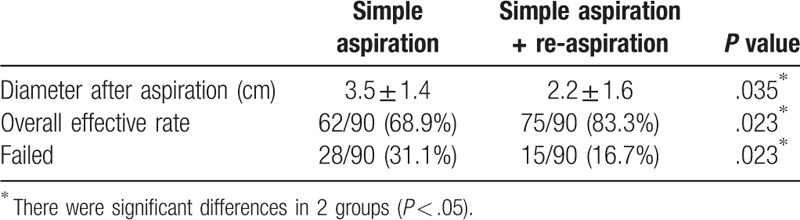
Immediate success was obtained in 62 out of the 90 patients with an attempt simple manual aspiration. The effective rate and failure rate were 68.9% (62/90) and 31.1% (28/90), respectively. Of the 28 patients in whom simple aspiration were unsuccessful at first attempt, a second attempt, which puncture site was away from the biopsy one, were performed to deal with the pneumothorax (Figs. 2 and 3). This procedure was succeeded in 13 patients with 15 patients undergoing a tube placement. The effective rate and failure rate were 46.4% (13/28) and 53.6% (15/28), respectively. Applying the modified procedure, total effective rate of aspiration elevated significantly from 68.9% (62/90) to 83.3% (75/90) (P < .05). Mean distance between parietal and visceral pleura of the 90 pneumothorax patients was (5.2 ± 1.6) cm. The corresponding value of simple aspiration and combination of the second attempt aspiration after changing puncture site were (3.5 ± 1.4) cm and (2.2 ± 1.6) cm, respectively (P < .05). Results of simple manual aspiration and combination of the modified re-aspiration are list in Table 2.
Figure 2.
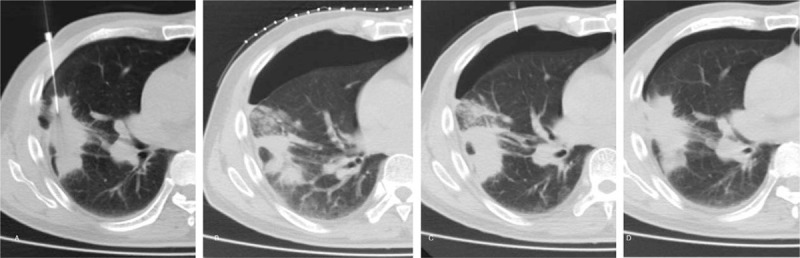
Second attempt aspiration approach in a 70-year-old male. (A) very small amount of pneumothorax has developed when the lung biopsy was performed. A large pneumothrax was detected in a while (B), and the aspiration was unsuccessful. (C) A second attempt aspiration treatment, which puncture site away from initial biopsy one, was conducted. (D) Final CT scan, obtained on completion of second attempt aspiration procedure, shows only a very small residual pneumothorax.
Figure 3.
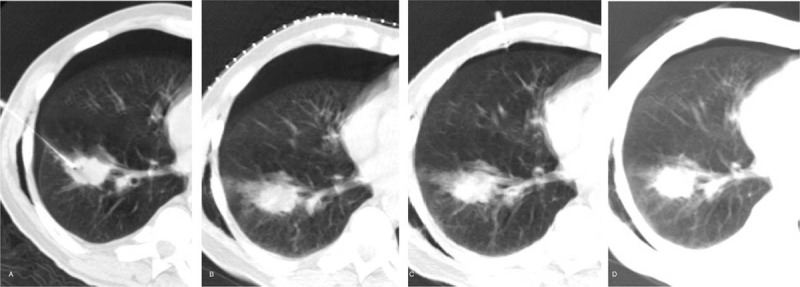
Second attempt aspiration approach in a 76-year-old male. (A) Pneumothorax was detected in the biopsy procedure. (B) Simple manual aspiration was ineffective and the pneumothorax increased. (C) Another puncture site, away from the biopsy one, was chosen for re-aspiration, and the pneumothorax decreased significantly. (D) Follow up CT shows that the pneumothorax has disappeared almost completely.
Table 2.
Results of simple manual aspiration and combination of the 2 approaches.
Chest tube placement were applied in the remaining 15 patients from the 236 pneumothorax cases (6.4%, 15/236). Ten patients had large tension pneumothorax with subcutaneous emphysema and developed diminishing oxygen saturation. The re-aspiration after changing puncture site in those cases were ineffective, chest tube placement was performed when they suffered from severe dyspnea and hypoxemia. In another 5 patients, pneumothorax decreased when re-aspiration performed, but recurrent in a short period followed up by CT scan. Therefore, chest tube placement was performed. No serious side effects were detected in the period of aspiration procedure and no tumor metastasis were reported in the follow-up.
4. Discussion
Percutaneous CT-guided transthoracic biopsy of lung nodules and masses is now frequently required in thoracic practices to provide safe and accurate diagnosis of lung lesions.[10] Pneumothorax is the most frequent complication of percutaneous transthoracic biopsy, occurring in 12% to 45% of procedures according to previous studies.1,3 The rate of pneumothorax necessitating insertion of a drainage catheter ranges from 5% to 22.8%.3,4 Development of a pneumothorax evaluates not only costs but also the need of hospitalization to manage the patient's symptoms such as chest pain, respiratory distress or shortness of breath. Various maneuvers for minimizing post biopsy pneumothorax and chest drain insertions have been trailed in lung biopsies including rapid rollover, deep expiration and breath-hold on needle extraction, autologous blood patch, tract plug and normal saline tract sealant.5,7,11,12,13 There is no consensus on the most effective of these techniques.
The manual aspiration of a pneumothorax was considered to be an effective method of preventing an increased pneumothorax that would otherwise require chest tube placement. But simple manual aspiration may be unsuccessful if the parenchymal tear is large, and have a risk of short-period recurrence for the reason
-
(1)
the alveolar-to-pleural pressure gradient may increase when the aspiration was performed near the region surrounding the leak
-
(2)
and the procedure does not promote pleural symphysis.
Zidulka et al demonstrated the effect of puncture site down positioning, on the rate of pneumothorax formation in a dog model. The animal experiments hypothesized that placing the site of pleural air leak in a dependent position causes reduction in alveolar size and alveolar to pleural pressure gradient, causing decreased rate of pneumothorax formation[14]. But biopsy site down technique has been cause for some debate. Although some studies report no difference in pneumothorax rate when repositioning the patient after the procedure, others have demonstrated a significant reduction in pneumothorax and chest tube placement rate in case of patient repositioning.3,9,15,16,17
Our previous studies demonstrated that aspiration in puncture-site down position was safety and efficacy in treating pneumothorax after CT-guided lung biopsy, and reduce the rate of pneumothorax requiring drainage catheter placement significantly.[18] This minimally invasive technique also have favorable results in dealing with delayed pneumothorax following lung biopsy.[8] The puncture-site down aspiration technique has been widely adopted and get encouraging results. But in our clinical practice, we cannot turn the patient to the puncture-site down position immediately after needle removal because we have to check the adequacy of the sample tissue and a second puncture may be required. On the other hand, some elderly patients have difficulty turning over because of pain and some other reasons. Based on the existing experience of aspiration in puncture-site down position, we designed the current technique, a second attempt, which puncture site was away from the biopsy one, to deal with the pneumothorax when first attempt was unsatisfied. We noticed that some second attempt aspiration results were encouraging, which decrease the incidence of pneumothorax require chest tube placement significantly.
Ninety patients (38.1%, 90/236) were treated with attempt simple manual aspiration in our study, which was succeed in 62 (68.9%, 62/90), similar to other reports. In the other 28 patients with failed first attempt simple manual aspiration, another puncture site, away from the biopsy one, was selected for re-aspiration. The re-aspiration succeeds in 13 patients who would have otherwise required a tube thoracostomy. There were 15 patients needed drainage catheter insertions. The overall success rate of aspiration elevated from 68.9% (62/90) to 83.3% (75/90), significantly higher compared to other studies. Chest tube thoracostomy was applied only in 6.4% (15/236) of all pneumothorax patients, which significantly lower than many other international reports.
The postbiopsy pneumothorax was mainly caused by visceral pleural rupture. It is reported that transfissural needle path represents a potentially risk factor for developing pneumothorax and increasing chest tube requirement.4,19 The results suggest that transfissural needle path may predispose a patient to air leak after lung biopsy, with the hypothesis behind this phenomenon being that multiple pleural punctures leave multiple opportunities for air leak. The second attempt aspiration after changing puncture site had a favorable result, partially because it was the parietal pleural rupture while not visceral pleural rupture occurred in the aspiration, which did not increase the likelihood of an air leak.[8]
In the first attempt aspiration, the needle from the lesion was retracted back into the pleural space after biopsy and then aspiration was performed. This procedure may increase alveolar-to-pleural pressure gradient surrounding the needle track, causing further air to enter between the parietal and visceral pleural layers, resulting in the enlargement of an already established pneumothorax.[9] While the second attempt aspiration, with puncture site away from initial biopsy one, demonstrated an encouraging efficacy. The pathophysiologic basis for this is likely due to reduction in alveolar-to-pleural pressure gradient at the puncture site, which decrease the likelihood of an air leak through the needle tract. and we believe this is the most important contributing factor to the result. We believe patients who do not benefit from secondary intervention and required tube placement may mainly related to larger parenchymal tear and parietal pleural rupture was occurred.
These findings should be interpreted in view of certain limitations. First, this study was a retrospective with an unknown bias. Second, the size of our study population was limited, and further larger patient population studies are required to confirm our results.
5. Conclusion
In general, when pneumothorax is revealed after CT-guided lung biopsy, needle from the lesion can be retracted back into the pleural space for aspiration. If the first attempt aspiration was failed, immediate percutaneous aspiration, which puncture site away from initial biopsy one, is worth trying before chest tube placement. This modified procedure improved the efficiency of treatment significantly, and reduced the rate of pneumothorax requiring chest tube placement after CT-guided lung biopsy.
Author contributions
Formal analysis: Lichuan Zeng.
Investigation: Lichuan Zeng, Huaqiang Liao, Wen-bin Wu, Yudong Zhang, Fengchun Ren.
Methodology: Lichuan Zeng, Huaqiang Liao, Wen-bin Wu, Yudong Zhang.
Resources: Qu Wang.
Software: Lichuan Zeng.
Supervision: Mingguo Xie.
Writing – original draft: Lichuan Zeng, Qu Wang, Mingguo Xie.
Writing – review & editing: Lichuan Zeng, Mingguo Xie.
Footnotes
Abbreviation: CT = computed tomography.
How to cite this article: Zeng LC, Liao HQ, Wu WB, Zhang YD, Ren FC, Wang Q, Xie MG. Effect of puncture sites on pneumothorax after lung CT-guided biopsy. Medicine. 2020;99:15(e19656).
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The authors have no conflicts of interests to disclose.
References
- [1]. Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Yoon SH, Park CM, Lee KH, et al. Analysis of complications of percutaneous transthoracic needle biopsy using CT-guidance modalities in a multicenter cohort of 10568 biopsies. Korean J Radiol 2019;20:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Huo YR, Chan MV, Habib AR, et al. Post-biopsy manoeuvres to reduce pneumothorax incidence in CT-guided transthoracic lung biopsies: a systematic review and meta-analysis. Cardiovasc Intervent Radiol 2019;42:1062–72. [DOI] [PubMed] [Google Scholar]
- [4]. Ozturk K, Soylu E, Gokalp G, et al. Risk factors of pneumothorax and chest tube placement after computed tomography-guided core needle biopsy of lung lesions: a single-centre experience with 822 biopsies. Pol J Radiol 2018;83:e407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Schnell J, Beer M, Eggeling S, et al. Management of spontaneous pneumothorax and post-interventional pneumothorax: German S3 guideline. Respiration 2018;1–33. [DOI] [PubMed] [Google Scholar]
- [6]. Khorochkov E, Garvin GJ, Potoczny S, et al. Injection of saline into the biopsy tract and rapid patient rollover decreases pneumothorax size following computed tomography-guided transthoracic needle biopsy. Can Assoc Radiol J 2018;69:489–92. [DOI] [PubMed] [Google Scholar]
- [7]. Nishiuma T, Ohnishi H, Katsurada N, et al. Evaluation of simple aspiration therapy in the initial treatment for primary spontaneous pneumothorax. Intern Med 2012;51:1329–33. [DOI] [PubMed] [Google Scholar]
- [8]. Zeng LC, Yang HF, Xu XX, et al. Manual aspiration in the biopsy-side down position to deal with delayed pneumothorax after lung biopsy. J Thorac Dis 2018;10:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Leger T, Jerjir N, Gregory J, et al. Does ipsilateral-dependent positioning during percutaneous lung biopsy decrease the risk of pneumothorax? AJR Am J Roentgenol 2019;212:461–6. [DOI] [PubMed] [Google Scholar]
- [10]. César DN, Torres US, D’Ippolito G, et al. CT-guided transthoracic core-needle biopsies of mediastinal and lung lesions in 235 consecutive patients: factors affecting the risks of complications and occurrence of a final diagnosis of malignancy. Arch Bronconeumol 2019;55:297–305. [DOI] [PubMed] [Google Scholar]
- [11]. Graffy P, Loomis SB, Pickhardt PJ, et al. Pulmonary intraparenchymal blood patching decreases the rate of pneumothorax-related complications following percutaneous CT-guided needle biopsy. J Vasc Interv Radiol 2017;28:608–13. [DOI] [PubMed] [Google Scholar]
- [12]. De Filippo M, Milanese G. Curved needles in CT-guided fine needle biopsies of pulmonary lesions: an ability to reduce the incidence of pneumothorax. Cardiovasc Intervent Radiol 2016;39:1525–7. [DOI] [PubMed] [Google Scholar]
- [13]. Neyaz Z, Mohindra N. Is the rapid needle-out patient-rollover approach after CT-guided lung biopsy really effective for pneumothorax prevention? J Thorac Dis 2015;7:E350–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Zidulka A, Braidy TF, Rizzi MC, et al. Position may stop pneumothorax progression in dogs. Am Rev Respir Dis 1982;126:51–3. [DOI] [PubMed] [Google Scholar]
- [15]. Kim JI, Park CM, Lee SM, et al. Rapid needle-out patient-rollover approach after cone beam CT-guided lung biopsy: effect on pneumothorax rate in 1,191 consecutive patients. Eur Radiol 2015;25:1845–53. [DOI] [PubMed] [Google Scholar]
- [16]. O’Neill AC, McCarthy C, Ridge CA, et al. Rapid needle-out patient-rollover time after percutaneous CT-guided transthoracic biopsy of lung nodules: effect on pneumothorax rate. Radiology 2012;262:314–9. [DOI] [PubMed] [Google Scholar]
- [17]. Collings CL, Westcott JL, Banson NL, et al. Pneumothorax and dependent versus nondependent patient position after needle biopsy of the lung. Radiology 1999;210:59–64. [DOI] [PubMed] [Google Scholar]
- [18]. Zeng LC, Du Y, Yang HF, et al. Efficacy of an opposite position aspiration on resolution of pneumothorax following CT-guided lung biopsy. Br J Radiol 1051;88:20150227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Lee HY, Lee IJ. Assessment of independent risk factors of developing pneumothorax during percutaneous core needle lung biopsy: focus on lesion depth. Iran J Radiol 2016;13:e30929. [DOI] [PMC free article] [PubMed] [Google Scholar]


