Supplemental Digital Content is available in the text
Keywords: interstitial lung disease, krebs von den Lungen-6, meta-analysis, systematic review
Abstract
Background:
Past investigations showed inconsistent results for diagnostic and prognostic predictive values of Krebs von den Lungen-6 (KL-6) for interstitial lung disease (ILD).
Methods:
Web of Science and PubMed were systematically searched on for articles exploring the association of KL-6 and ILDs published between September 1993 and March 2019. For comparisons between-groups, the standard mean difference and 95% confidence intervals (CIs) were computed as the effect sizes. For diagnostic studies, a summary of sensitivity, specificity, positive likelihood ratios, negative likelihood ratios, and diagnostic odds ratio, which indicated the accuracy of KL-6 in the differentiation of ILDs and no ILDs, were calculated from the true positive, true negative, false positive, and false negative of each study. In addition, the summary receive-operating characteristics curve was constructed to summarize the TP and FP rates. For follow-up study, we computed hazard ratios (HRs) and 95% CIs for mortality. ILD patients showed elevated concentrations of KL-6, compared to healthy controls and patients without ILD.
Results:
The meta-analysis showed a sensitivity (0.85 [95% CI: 0.77–0.91]) and specificity (0.97 [95% CI: 0.90–0.99]) of KL-6 for ILDs. In addition, it showed elevated baseline circulating levels of KL-6 in subsequent active ILD, compared to subsequent inactive ILD. Moreover, there was a significant association between baseline levels of circulating KL-6 and mortality of ILD (HR 2.95, 95% CI 2.45–3.55, I2 = 65.9%, P = .032).
Conclusion:
In conclusion, the study suggested that circulating KL-6 showed diagnostic and prognostic predictive values for ILDs.
1. Introduction
Interstitial lung disease (ILD) is a group of diffuse parenchymal lung diseases affecting the alveolar epithelium, pulmonary capillary endothelium, basement membrane, perivascular and perilymphatic tissues.[1] ILDs concern idiopathic pulmonary fibrosis (IPF), connective tissue disease-associated ILDs (CTD-ILDs), and hypersensitive pneumonia (HP). The global incidence of ILDs is between 10.7 and 27.14 per 100,000 people.[2] In 2013, ILDs affected 595,000 people worldwide and resulted in 471,000 deaths.[3]
Krebs von den Lungen-6 (KL-6), a high-molecular-weight glycoprotein encoded by the MUC1 gene and distributed mainly on the cell surface of type II alveolar epithelial cells (AECs), was firstly identified by Kohno in 1994.[4] KL-6 splits off at the S-S bond near the membrane surface of damaged typeIIAECs. After that, soluble KL-6 is released into pulmonary epithelial lining fluid and blood flow.[5] Therefore, serum KL-6 is usually identified as a biomarker for pulmonary damage.[5] Previous studies supported that serum KL-6 levels might be a diagnostic biomarker of ILDs and could evaluate disease activity of ILDs. However, different studies showed incompatible results regarding the associations between circulating KL-6 levels and ILDs. Hamai et al[6] reported that circulating KL-6 levels showed high sensitivity and high specificity in the diagnosis of ILDs, whereas Hu et al[7] found that circulating KL-6 levels showed low sensitivity and high specificity in the diagnosis of ILDs. To enhance the strength of evidence, meta-analysis was conducted to summarize results of studies evaluating diagnostic and prognostic predictive values of circulating KL-6 levels for ILDs.
2. Materials and methods
This study was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[8] Ethical approval was not applicable in the study.
2.1. Search strategy and selection criteria
Articles published between September 1993 and March 2019 were searched in Web of Science and PubMed databases. Search terms showed as follows: (“Interstitial lung disease” OR “ILD”) AND (“Krebs von den Lungen-6” OR “KL-6”). After removing duplicates, 381 articles detecting association of circulating KL-6 and ILD were screened in this study. In addition, only literatures with English language were included. Moreover, we excluded reviews, meta-analysis, and case studies.
2.2. Data collection and meta-analysis
Titles and abstracts of articles were looked through by 2 individuals. Full texts of 23 articles were read after excluding some articles. Following data were extracted from these articles: author and publication years, country, sample size, specimen, detection methods, time of circulating sampling follow-up periods, and clinical outcomes. For cross-sectional studies, we extracted mean and standard deviation (SD) of concentrations of circulating KL-6 in ILD patients, patients without ILD and healthy controls (HC). For diagnostic studies, true positive (TP), true negative (TN), false positive (FP), and false negative (FN) were collected or calculated from selected studies. For follow-up studies, the hazard ratios (HRs) and 95% confidence intervals (CIs) for clinical outcomes were extracted from included studies.
These data were conducted with STATA 12.0 software and Meta-Disc Version 1.4. For cross-sectional studies, the standard mean difference (SMD) and 95% CI were computed as the effect size of following comparisons (comparison between patients with and without ILD, comparison between ILD patients and HC, comparison between patients with active ILD and inactive ILD). For diagnostic studies, after a summary of sensitivity, specificity, positive likelihood ratios (PLR), negative likelihood ratios (NLR), and diagnostic odds ratio (DOR), the receive-operating characteristics (SROC) curve was performed to summarize TP and FP rates. For follow-up studies, HRs and 95% CIs were computed for individual clinical outcomes. Heterogeneity between studies and the amount of variation derived from heterogeneity were evaluated with Q test and computed I2, respectively. With high heterogeneity (P value for Q test ≤ 0.05, I2 ≥ 50%), random effects models were performed as pooling methods. Otherwise, fixed-effects models were conducted as pooling methods.
3. Results
3.1. Search results
Figure 1 showed the processing flow. Supplementary Table 1 showed some important elements of the included 23 articles. Mean and standard deviation (SD) of concentrations of circulating KL-6 in ILD and HC were extracted from 14 studies[7,9–21] (ILD patients: n = 1120, HC: n = 625). Mean and SD of concentrations of circulating KL-6 for patients with and without ILD were collected from 11 studies[7,9,16,18,19,21–26] (ILD patients: n = 767, patients without ILD: n = 1132). Data were collected from 6 studies[6,7,11,12,16,20] for the diagnostic studies with circulating KL-6 for ILD (ILD patients: n = 634, HC: n = 365). Baseline circulating levels of KL-6 between patients with active ILD and inactive ILD were collected from 3 studies[10,17,25] to explore the predictive effect of KL-6 for acute exacerbation (AE) of ILD. Moreover, data were collected from 4 studies[6,11,13,27] for follow-up studies with clinical outcome of mortality.
Figure 1.
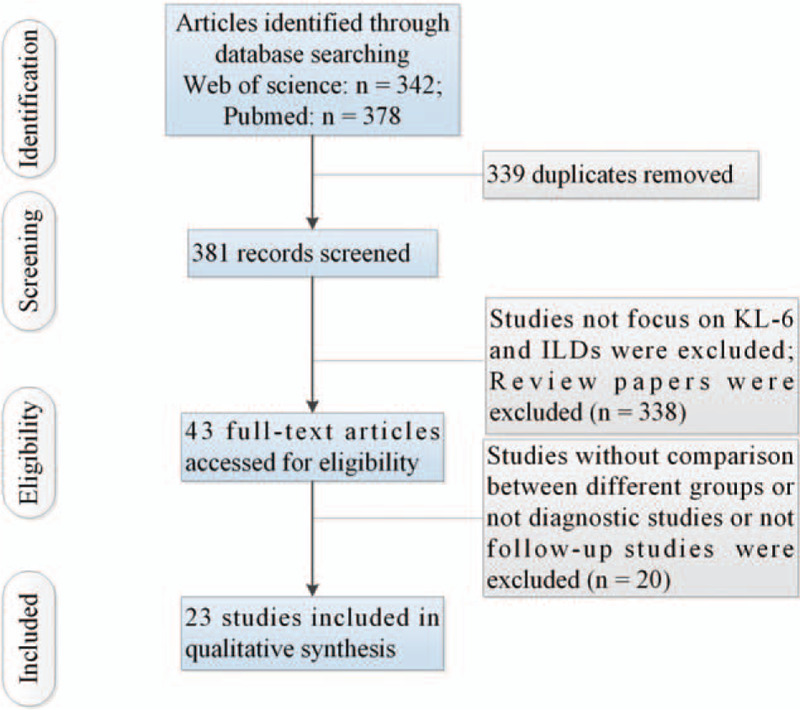
Flow of information through the different phases of a systematic review.
3.2. Meta-analysis results
Circulating KL-6 showed a diagnostic value for ILD. ILD patients showed elevated concentrations of KL-6, compared to HC and patients without ILD (comparison between ILD patients and HC: I2 = 92.3%, P < .001; mean value of KL-6 level [ILD patients vs HC]: 1096 vs 224 U/mL; comparison between patients with and without ILD: I2 = 97.6%, P < .001; mean value of KL-6 level [ILD patients vs patients without ILD]: 1284 vs 329 U/mL; see Figs. 2 and 3). Additionally, the pooled parameters calculated were as follows: sensitivity, 0.85 (95% CI: 0.77–0.91); specificity, 0.97 (95% CI: 0.90–0.99); PLR, 24.4 (95% CI: 8.6–69.3); NLR, 0.15 (95% CI: 0.10–0.24); and DOR, 159 (95% CI: 46–551). The analysis showed a significant heterogeneity (sensitivity, I2 = 80.30%, P < .01; specificity, I2 = 87.59%, P < .01, see Fig. 4A). Figure 4B showed the SROC curve, with an area under curve (AUC) of 0.96 (95% CI: 0.93–0.97).
Figure 2.
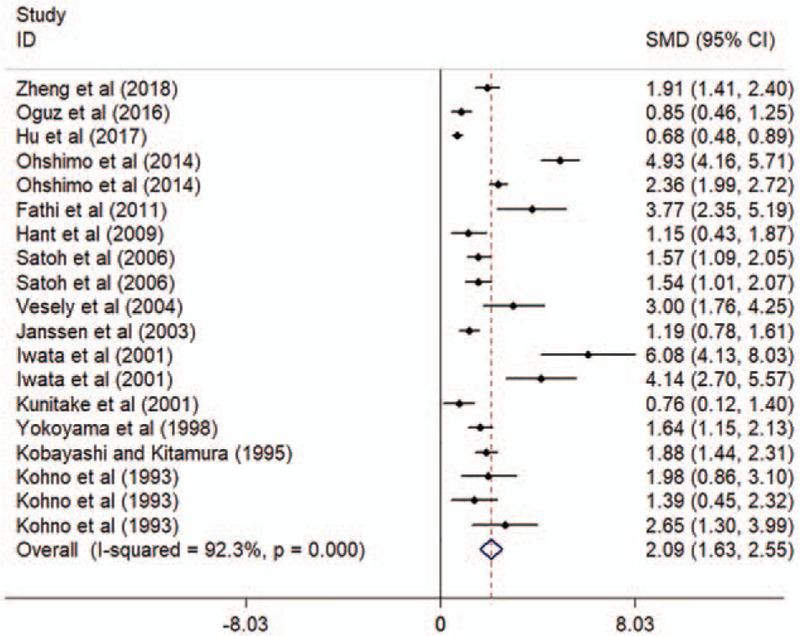
Forest plot of the circulating KL-6 levels in ILD patients and HC. CI = confidence interval, HC = healthy controls, ILD = interstitial lung disease, KL-6 = Krebs von den Lungen-6, SMD = standard mean difference.
Figure 3.
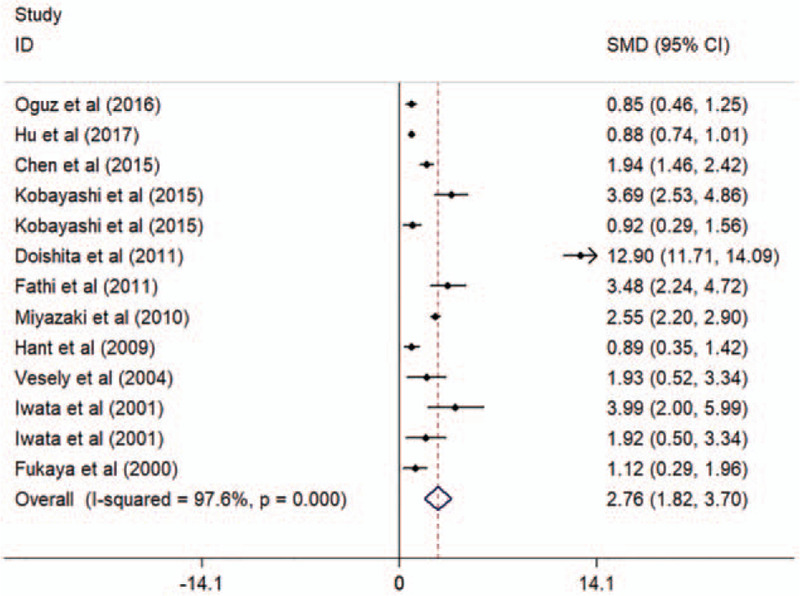
Forest plot of the circulating KL-6 levels in patients with and without ILDs. CI = confidence interval, ILD = interstitial lung disease, KL-6 = Krebs von den Lungen-6, SMD = standard mean difference.
Figure 4.
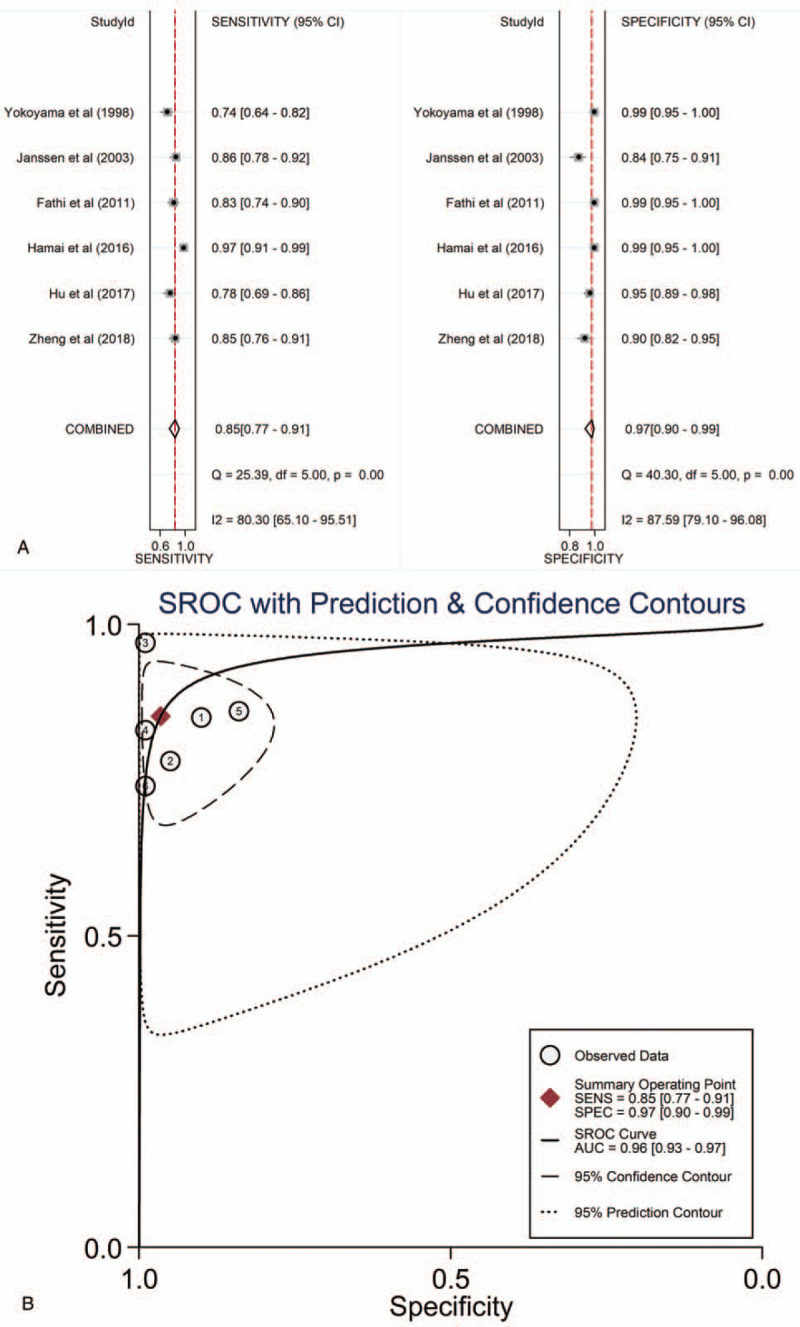
The sensitivity, specificity, DOR, SROC curve with AUC of circulating KL-6 in the diagnosis of ILDs. (A) Sensitivity and specificity. (B) SROC curve with AUC. AUC = area under the curve, DOR = diagnostic odds ratio, ILDs = interstitial lung diseases, KL-6 = Krebs von den Lungen-6, SROC = summary receiver operator characteristic.
Circulating KL-6 showed a predictive value of AE and mortality for ILD. The study showed elevated baseline circulating levels of KL-6 in subsequent active ILD, compared to subsequent inactive ILD (mean value of KL-6 level [active ILD vs inactive ILD]: 1977 vs 917 U/mL; Figure 5A). In addition, there was a significant association between baseline levels of circulating KL-6 and mortality of ILD (HR 2.95, 95% CI 2.45–3.55, I2 = 65.9%, P = .032, Fig. 5B).
Figure 5.
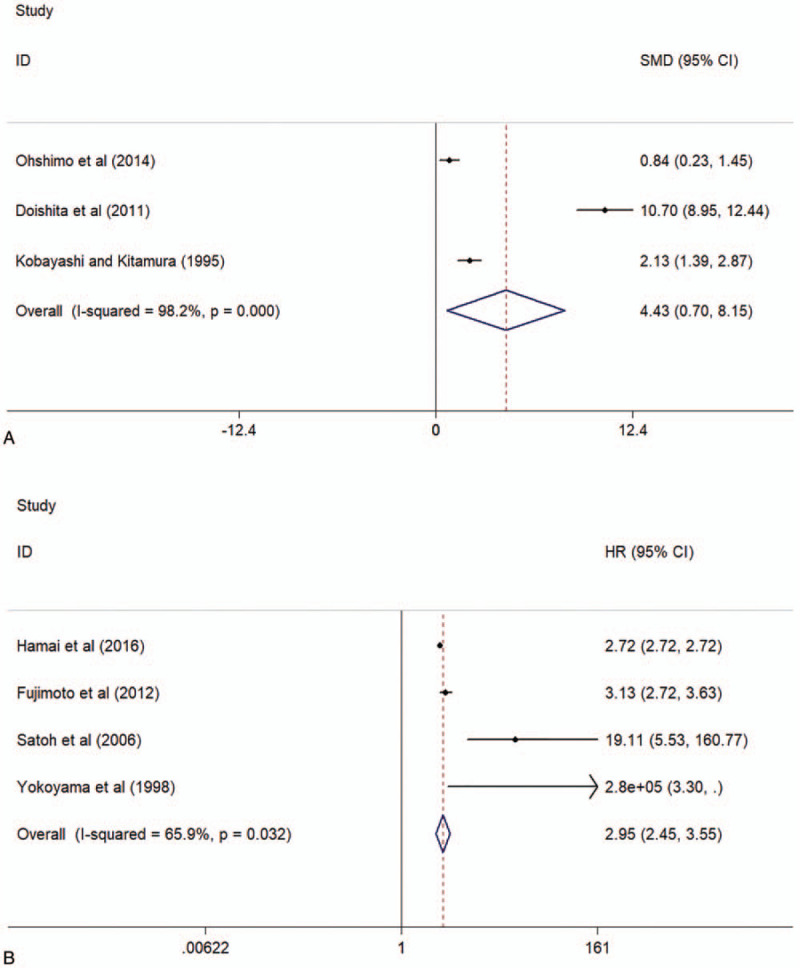
Forest plot of the baseline circulating KL-6 levels in patients with active ILDs and inactive ILDs (A) and the associations between baseline circulating KL-6 and mortality after ILDs (B). ILDs = interstitial lung diseases, KL-6 = Krebs von den Lungen-6.
4. Discussion
The study showed that circulating KL-6 showed a diagnostic value and a predictive value of AE and mortality for ILD.
Recently, a lot of studies have supported that elevated levels of circulating KL-6 were detected in different kinds of ILDs patients (including IPF, CTD-ILD, and HP), compared to HC.[9,28,29] The present study showed increased levels of circulating KL-6 in ILD patient, compared to HC and patients with following diseases (CTD, pulmonary diseases and lung cancer) and without ILDs.[9,25,26] These changes might be caused by increased KL-6 production by regenerating typeII AECs and/or increased permeability caused by destroyed air-blood barrier in ILDs.[28] Additionally, the study showed a high sensitivity (0.85 [95% CI: 0.77–0.91]) and high specificity (0.97 [95% CI: 0.90–0.99]) of circulating KL-6 levels for ILDs. In Chinese participants, Zheng et al[12] reported that at a cutoff value of 500 U/mL, the sensitivity and specificity of serum KL-6 levels as a diagnostic biomarker of ILDs was 77.75% and 94.51%, respectively. In Sweden, Fathi et al[16] indicated that at a cutoff value of 549 U/mL, the sensitivity and specificity of KL-6 for diagnosis of ILD were 83% and 100%, respectively. Moreover, previous studies indicated that serum KL-6 levels were associated with disease severity of ILDs. A study showed that serum KL-6 levels were significantly negatively correlated with the percentage values of forced expiratory volume in the first second (FEV1) and forced vital capacity.[16] The present study supported that circulating KL-6 levels were highly valuable for the diagnosis of ILDs. The study was the first meta-analysis to summarize results of studies evaluating diagnostic and prognostic predictive values of circulating KL-6 levels for ILDs.
The study indicated that baseline circulating levels of KL-6 were higher in subsequent active ILD, compared to subsequent inactive ILD. In addition, a significant association was indicated between baseline levels of circulating KL-6 and mortality of ILD. AE of ILDs is a condition characterized by an acute worsening of dyspnea resulting in hypoxemic respiratory failure. The process of AE-ILDs usually was accompanied by new infiltrates on radiologic images. In addition, AE is the major cause of ILDs-related mortality.[30] These studies supported that circulating KL-6 levels were highly valuable for the prognostic prediction of ILDs.
Most of these meta-analyses showed significant heterogeneities between studies. Previous studies demonstrated ethnic difference in development and progression of ILDs.[31] Additionally, serum KL-6 levels were significantly affected by MUC1 genetic polymorphism. Moreover, the study included studies for different kinds of ILDs (IPF, CTD-ILD, and HP).
The present study had some limitations. Most importantly, the number of included studies was limited to explore the sources of heterogeneities.
In conclusion, the present study suggested that circulating KL-6 showed diagnostic and prognostic predictive values for ILDs.
Author contributions
Data curation: Hongying Zhang, Lizhou Chen, Xinhang Wang, Heng Weng.
Investigation: Luling Wu, Jinbao Huang, Hongyan Li.
Methodology: Hongying Zhang, Lizhou Chen, Jinbao Huang, Hongyan Li, Heng Weng.
Project administration: Luling Wu.
Supervision: Heng Weng.
Validation: Xinhang Wang.
Writing – original draft: Hongying Zhang, Heng Weng.
Writing – review & editing: Heng Weng.
Supplementary Material
Footnotes
Abbreviations: AE, acute exacerbation, AECs, alveolar epithelial cells, ALDH, aldehyde dehydrogenase, AUC, area under curve, CIs, confidence intervals, CTD-ILDs, connective tissue disease-associated ILDs, DOR, diagnostic odds ratio, FEV1, forced expiratory volume in the first second, FN, false negative, FP, false positive, FVC, forced vital capacity, HP, hypersensitive pneumonia, HRs, hazard ratios, ILD, interstitial lung disease, IPF, idiopathic pulmonary fibrosis, KL-6, Krebs von den Lungen-6, NLR, negative likelihood ratios, PLR, positive likelihood ratios, PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses, SMD, standard mean difference, SROC, summary receive-operating characteristics, TN, true negative, TP, true positive.
How to cite this article: Zhang H, Chen L, Wu L, Huang J, Li H, Wang X, Weng H. Diagnostic and prognostic predictive values of circulating KL-6 for interstitial lung disease: A PRISMA-compliant systematic review and meta-analysis. Medicine. 2020;99:16(e19493).
This study was supported by the Clinical key specialty construction project of Fujian Province for respiratory department (NO 2018-145).
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].King TE., Jr Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med 2005;172:268–79. [DOI] [PubMed] [Google Scholar]
- [2].Karakatsani A, Papakosta D, Rapti A, et al. Epidemiology of interstitial lung diseases in Greece. Respir Med 2009;103:1122–9. [DOI] [PubMed] [Google Scholar]
- [3].Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2015;385:117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kohno N, Inoue Y, Hamada H, et al. Difference in sero-diagnostic values among KL-6-associated mucins classified as cluster 9. Int J Cancer 1994;8:81–3. [DOI] [PubMed] [Google Scholar]
- [5].Inoue Y, Barker E, Daniloff E, et al. Pulmonary epithelial cell injury and alveolar-capillary permeability in berylliosis. Am J Respir Crit Care Med 1997;156:109–15. [DOI] [PubMed] [Google Scholar]
- [6].Hamai K, Iwamoto H, Ishikawa N, et al. Comparative study of circulating MMP-7, CCL18, KL-6, SP-A, and SP-D as disease markers of idiopathic pulmonary fibrosis. Disease markers 2016;2016:4759040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu Y, Wang LS, Jin YP, et al. Serum Krebs von den Lungen-6 level as a diagnostic biomarker for interstitial lung disease in Chinese patients. The Clin Respir J 2017;11:337–45. [DOI] [PubMed] [Google Scholar]
- [8].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Oguz EO, Kucuksahin O, Turgay M, et al. Association of serum KL-6 levels with interstitial lung disease in patients with connective tissue disease: a cross-sectional study. Clin Rheumatol 2016;35:663–6. [DOI] [PubMed] [Google Scholar]
- [10].Ohshimo S, Ishikawa N, Horimasu Y, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med 2014;108:1031–9. [DOI] [PubMed] [Google Scholar]
- [11].Yokoyama A, Kohno N, Kondo K, et al. Comparative evaluation of sialylated carbohydrate antigens, KL-6, CA19-9 and SLX as serum markers for interstitial pneumonia. Respirology (Carlton, Vic) 1998;3:199–202. [DOI] [PubMed] [Google Scholar]
- [12].Zheng P, Liu X, Huang H, et al. Diagnostic value of KL-6 in idiopathic interstitial pneumonia. J Thorac Dis 2018;10:4724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Satoh H, Kurishima K, Ishikawa H, et al. Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med 2006;260:429–34. [DOI] [PubMed] [Google Scholar]
- [14].Kohno N, Awaya Y, Oyama T, et al. KL-6, a mucin-like glycoprotein, in bronchoalveolar lavage fluid from patients with interstitial lung disease. Am Rev Respir Dis 1993;148:637–42. [DOI] [PubMed] [Google Scholar]
- [15].Kunitake R, Kuwano K, Yoshida K, et al. KL-6, surfactant protein A and D in bronchoalveolar lavage fluid from patients with pulmonary sarcoidosis. Respiration 2001;68:488–95. [DOI] [PubMed] [Google Scholar]
- [16].Fathi M, Barbasso Helmers S, Lundberg IE. KL-6: a serological biomarker for interstitial lung disease in patients with polymyositis and dermatomyositis. J Intern Med 2012;271:589–97. [DOI] [PubMed] [Google Scholar]
- [17].Kobayashi J, Kitamura S. KL-6: a serum marker for interstitial pneumonia. Chest 1995;108:311–5. [DOI] [PubMed] [Google Scholar]
- [18].Vesely R, Vargova V, Ravelli A, et al. Serum level of KL-6 as a marker of interstitial lung disease in patients with juvenile systemic sclerosis. J Rheumatol 2004;31:795–800. [PubMed] [Google Scholar]
- [19].Iwata Y, Wada T, Furuichi K, et al. Serum levels of KL-6 reflect disease activity of interstitial pneumonia associated with ANCA-related vasculitis. Intern Med (Tokyo, Japan) 2001;40:1093–7. [DOI] [PubMed] [Google Scholar]
- [20].Janssen R, Sato H, Grutters JC, et al. Study of Clara cell 16, KL-6, and surfactant protein-D in serum as disease markers in pulmonary sarcoidosis. Chest 2003;124:2119–25. [DOI] [PubMed] [Google Scholar]
- [21].Hant FN, Ludwicka-Bradley A, Wang HJ, et al. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol 2009;36:773–80. [DOI] [PubMed] [Google Scholar]
- [22].Kobayashi N, Takezaki S, Kobayashi I, et al. Clinical and laboratory features of fatal rapidly progressive interstitial lung disease associated with juvenile dermatomyositis. Rheumatology (Oxford, England) 2015;54:784–91. [DOI] [PubMed] [Google Scholar]
- [23].Fukaya S, Oshima H, Kato K, et al. KL-6 as a novel marker for activities of interstitial pneumonia in connective tissue diseases. Rheum Int 2000;19:223–5. [DOI] [PubMed] [Google Scholar]
- [24].Chen F, Lu X, Shu X, et al. Predictive value of serum markers for the development of interstitial lung disease in patients with polymyositis and dermatomyositis: a comparative and prospective study. Intern Med J 2015;45:641–7. [DOI] [PubMed] [Google Scholar]
- [25].Doishita S, Inokuma S, Asashima H, et al. Serum KL-6 level as an indicator of active or inactive interstitial pneumonitis associated with connective tissue diseases. Intern Med (Tokyo, Japan) 2011;50:2889–92. [DOI] [PubMed] [Google Scholar]
- [26].Miyazaki K, Kurishima K, Kagohashi K, et al. Serum KL-6 levels in lung cancer patients with or without interstitial lung disease. J Clin Lab Anal 2010;24:295–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fujimoto K, Taniguchi H, Johkoh T, et al. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur Radiol 2012;22:83–92. [DOI] [PubMed] [Google Scholar]
- [28].Ohnishi H, Yokoyama A, Kondo K, et al. Comparative study of KL-6, surfactant protein-A, surfactant protein-D, and monocyte chemoattractant protein-1 as serum markers for interstitial lung diseases. Am J Respir Crit Care Med 2002;165:378–81. [DOI] [PubMed] [Google Scholar]
- [29].Kohno N. Serum marker KL-6/MUC1 for the diagnosis and management of interstitial pneumonitis. J Med Invest 1999;46:151–8. [PubMed] [Google Scholar]
- [30].Collard HR, Calfee CS, Wolters PJ, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Lung Cell Mol Physiol 2010;299:L3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ishikawa N, Hattori N, Yokoyama A, et al. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Invest 2012;50:3–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


