Abstract
Findings on the association between cotton dust exposure and lung cancer risk in epidemiologic studies have been inconsistent. Therefore, we conducted a meta-analysis of data from observational studies to quantify this association.
PubMed, EMBASE, and the Cochrane library databases were searched for observational studies with data on cotton dust exposure and lung cancer risk. Studies that reported adjusted relative risks (RRs) with 95% confidence intervals (CIs) of lung cancer associated with cotton dust exposure were included. Subgroup analyses were conducted according to key characteristics.
Fifteen studies involving a total of 73,812 individuals were included in the meta-analysis. Combining estimates from all the 15 observational studies, cotton dust exposure was associated with a decreased risk of lung cancer (combined RR, 0.78; 95% CI, 0.66–0.91; P = .002). Pooled estimates of multivariate RRs by gender were 0.71 (95% CI, 0.58–0.88; P = .001) among males, based on 7 studies, and 0.77 (95% CI, 0.67–0.89; P < .001) among females, based on 9 studies. Further analyses examining the influence of a single study on the results by omitting a study at each turn yielded a range of RR from 0.74 to 0.82.
Our meta-analysis indicates that cotton dust exposure is associated with a decreased risk of lung cancer.
Keywords: cotton, lung cancer, meta-analysis, risk factor
1. Introduction
Lung cancer is the most common cause of cancer death worldwide, accounting for over 20% of all cancer deaths.[1] It consists of 2 subtypes, small cell lung cancer (SCLC) and nonsmall cell lung cancer (NSCLC) and standard anticancer therapy includes chemotherapy, radiotherapy, and/or surgery.[2] Due to a lack of effective screening, most cases of lung cancer are diagnosed at a relatively advanced stage, and consequently survival is very low (15% five-year survival rate).[3] Identifying additional strategies to reduce lung cancer risk would be of particular relevance for high risk groups such as former smokers, and could have a substantial public health impact.
During the past 50 years, concern has mounted regarding the environmental factors associated with lung cancer, with growing interest in altering factors and reversing this global epidemic. Textile and clothing manufacturing is one of the world's largest industries, with women comprising approximately 40% of the workforce.[4] Textile industry exposures that have been investigated in relation to risk of lung cancer include cotton and wool dusts.[5]
The relationship between cotton dust exposure and lung cancer has been investigated in many epidemiologic studies, but findings remain inconsistent.[6–11] Lenters et al[12] combined the results of 11 observational studies in a meta-analysis published in 2010 and found that occupational exposure to endotoxin in cotton textile production was protective against lung cancer [relative risk (RR) = 0.72, 95% confidence interval (CI): 0.57, 0.90)]. However, the relatively small number of studies limited the statistical power of the analysis. Since 2008, 4 additional studies on the association between cotton dust exposure and lung cancer have been published.[8–11]
In order to provide a more definitive answer to the role of cotton dust exposure and risk of lung cancer, we carried out a meta-analysis to explore the magnitude and shape of the association between cotton dust exposure and lung cancer risk.
2. Materials and methods
We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) checklist to write this systematic review and report the results.[13] All analyses were based on previous published studies, so ethical approval and patient consent were not required.
2.1. Literature search
We performed a systematic search of PubMed, EMBASE, and the Cochrane library databases for potential observational studies published before April 2018. The search terms used for the PubMed search included cancer, carcinoma, neoplasms; dust, cotton dust, cotton fiber, and lung. Our search was restricted to studies conducted in humans, and no restriction was imposed with respect to the language of the publications. We also screened the reference lists of relevant reviews and included relevant articles in these lists.
2.2. Study selection
Studies that satisfied the following criteria were included in our meta-analysis: the study of adult patients had a cohort, case-cohort, or case-control design; the exposure of interest was cotton dust; the outcome of interest was lung cancer; and reported estimates of relative risk (RR) or hazard ratio (HR) and the corresponding 95% CI of lung cancer. Studies were excluded if: they were cross-sectional or a clinical trial; if the RR was not adjusted for both age and sex; and the study was duplicated.
2.3. Data abstraction
Two reviewers independently reviewed the full text of selected eligible studies, and extracted the following information: the first author's name, publication year, study type, country of origin, study population, exposure assessment, duration of follow-up, source of controls, number of lung cancer cases, RR and 95% CI, and adjustment variables. Any discrepancy was resolved through discussion to reach consensus between the 2 authors. When necessary, the original authors were contacted for supplementary information.
2.4. Assessment of study quality
Quality assessment was performed in accordance with the Newcastle-Ottawa scale (NOS) for nonrandomized studies.[14] A score of up to 9 stars was assigned to each study: participant selection (up to 4 stars), comparability of study groups (up to 2 stars), and assessment of outcome or exposure in the cohorts (up to 3 stars). A higher score represented better methodological quality. We regarded scores of 0–3, 4–6, and 7–9 as reflecting low, moderate, and high quality, respectively.
2.5. Statistical analysis
Multivariate-adjusted outcome data were used for analysis, and HRs and incidence rate ratios were considered to approximate RRs. We converted these values in every study by using their natural logarithms and calculated the standard errors from these logarithmic numbers and their corresponding 95% CIs. Results were expressed as RRs with 95% CIs (a fixed-effect approach was used, unless there was significant heterogeneity, in which case a random-effects statistical model was used).[15] Study heterogeneity was explored using tau2 and the amount of variance in the summary effect due to between-study heterogeneity, which was defined by I2. This was considered statistically significant at the P < .10 level, as determined by Cochran's Q statistical test.[16] To explore possible explanations for heterogeneity and to test the robustness of the association, we conducted sensitivity analyses and stratified analysis by sex.[17] We also performed the Egger test and constructed funnel plots to visualize a possible asymmetry.[18] In the case of publication bias, the “nonparametric trim-and-fill” method was used to compute for risk estimates corrected for this bias.[19] All statistical analyses were performed using the Stata version 11 (Stata Corporation, College Station, TX). Except where noted otherwise, differences with a P value < .05 were considered significant.
3. Results
3.1. Literature search and characteristics
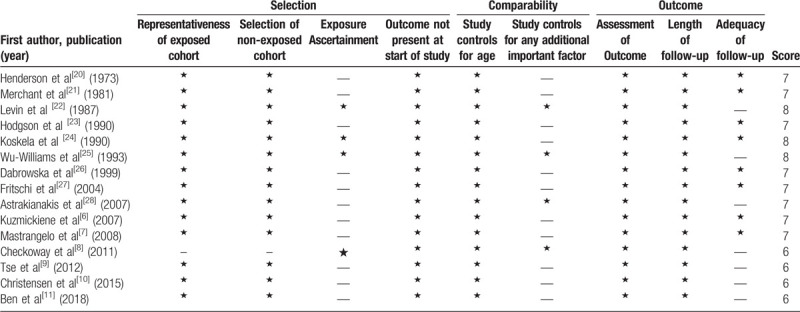
Our search strategy identified 127 potentially relevant articles, the titles and abstracts of which were screened for inclusion. The full texts of 22 articles were retrieved, of which 15[6–11,20–28] met the inclusion criteria (Fig. 1). Reasons for exclusion of the remaining articles included: the outcome was not lung cancer (n = 1), review or meta-analysis (n = 3), or without available data (n = 3). Finally, the present meta-analysis included results from 15 observational studies (5 case-control studies, 8 retrospective studies, and 2 case-cohort study).[6–11,20–28] Fifteen studies on the relationship between cotton dust exposure and lung cancer incidence (73,812 individuals) were published between 1973 and 2018 (Table 1). Five studies were conducted in China,[8,9,22,25,28] 7 in Europe,[6,7,11,23,24,26,27] and 3 in North America.[10,20,21] Of the 15 studies, 6 studies reported outcomes for males and females separately,[6,8,9,22,23,26] while 5 provided data for both sexes combined.[6,7,10,11,22] All articles were graded as moderate or high quality according to the NOS (the online-only Table 2).
Figure 1.
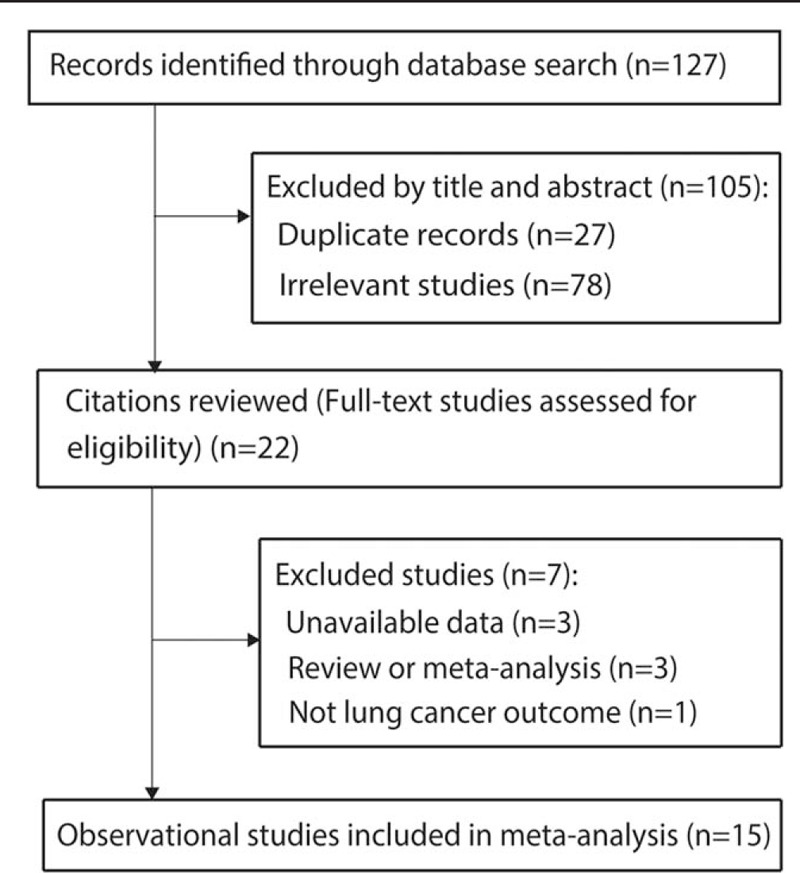
Process of literature search and study selection.
Table 1.
Study characteristics.
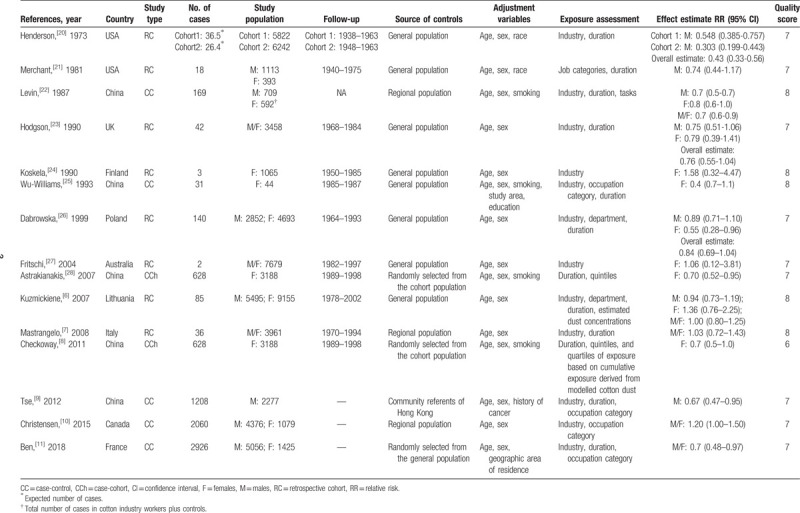
Table 2.
Quality assessment of included studies using the Newcastle-Ottawa scale.
3.2. Cotton dust exposure and risk of lung cancer
The multivariable adjusted RRs of lung cancer incidence in relation to cotton dust exposure from individual studies and the combined RR are presented in Figure 2. Combining estimates from all the 15 observational studies, cotton dust exposure was associated with a decreased risk of lung cancer (combined RR, 0.78; 95% CI, 0.66–0.91; P = .002). Substantial heterogeneity was observed (P < .001; I2 = 71.3%). Pooled estimates of multivariate RRs by gender were 0.71 (95% CI, 0.58–0.88; P = .001) among males, based on 7 studies, and 0.77 (95% CI, 0.67–0.89; P < .001) among females, based on 9 studies. The forest plots of multivariate RRs and 95% CI for lung cancer incidence and cotton dust exposure were shown in Figure 3.
Figure 2.
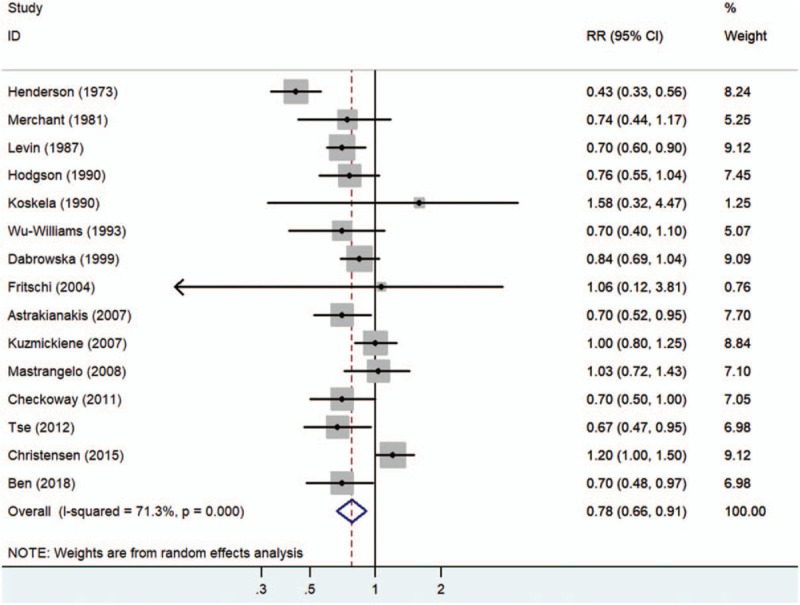
Forest plot for summary risk ratios with 95% CIs for lung cancer risk associated with working in the cotton textile industry.
Figure 3.
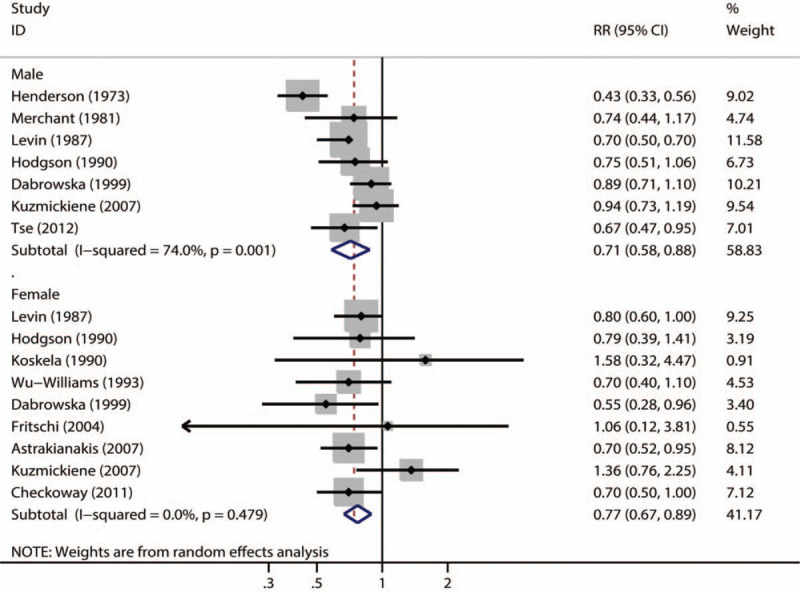
Forest plot for sex-specific summary risk ratios with 95% CIs for lung cancer risk associated with working in the cotton textile industry.
3.3. Sensitivity analysis and publication bias
The robustness of our results was evaluated by sensitivity analysis. Examining the influence of a single study on the results by omitting a study at each turn yielded a range of RR from 0.74 to 0.82 (Fig. 4). There was no evidence of publication bias among studies for lung cancer incidence using Egger test (P = .731) or Begg test (P = .882) (Fig. 5).
Figure 4.
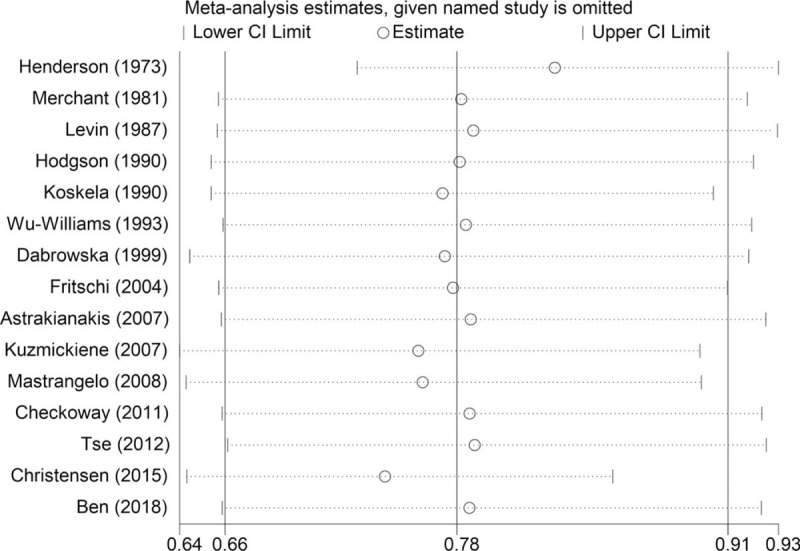
Sensitivity analysis: examining the influence of individual studies to pooled results.
Figure 5.
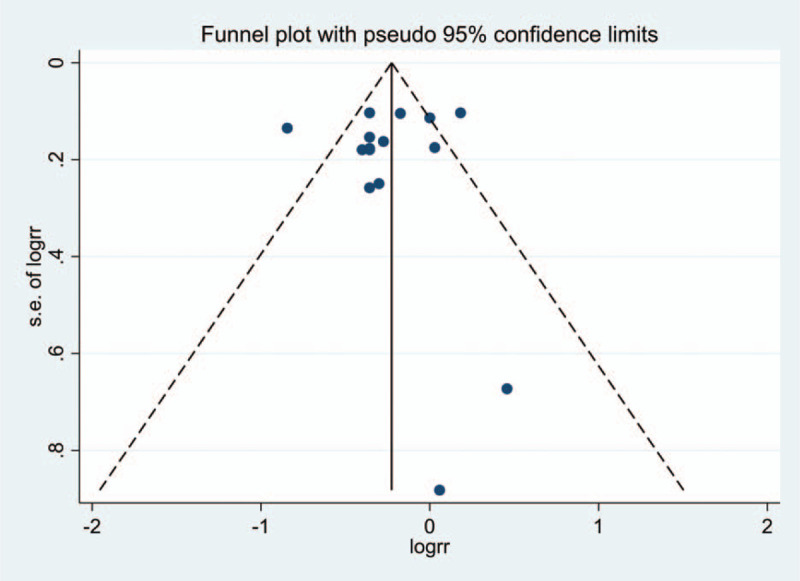
Funnel plot for publication bias test about lung cancer incidence. Each point represents a separate study for the indicated association.
3.4. Meta-regression
Since there was significant heterogeneity among individual studies, a univariable regression was conducted to explore the predefined possible source of heterogeneity under lung cancer incidence. The results of regression suggested that sex, study type, and quality score were not significant sources of heterogeneity (data not shown).
4. Discussion
The present meta-analysis provided additional evidence about the association of cotton dust exposure and risk of lung cancer. The current analysis indicated that cotton dust exposure was associated with a decreased risk of lung cancer.
We used NOS score to assess the study quality and all studies had a moderate or high-quality. As the sample size of each primary single study was relatively small, our meta-analysis increased the statistical power to detect a possible association between cotton dust exposure and lung cancer risk and further determined a precise risk estimation. However, potential limitations should be considered. First, one limitation of our meta-analysis was the observational design of the included retrospective cohort and case-control studies, which have the potential of selection bias. Second, the potential biases were not excluded and the dose–response analysis were not conducted owing to the different methods used to assess cotton dust exposure[6–9,11,21–23,28] and restricted cubic splines with 3 knots at fixed percentiles of 10%, 50%, and 90% of the distribution of cotton dust exposure were not available.[10,20,24–27] Third, in spite of some important confounders seeming unlikely to alter the role of cotton dust exposure on the decrement of lung cancer risk based on our sensitivity analysis of adjustments, other factors potentially accounted for the observed association cannot be ruled out (e.g., diet, smoking habits, physical activity, or other occupational exposures).[29] Fourth, cotton dust exposure time has different lung cancer incidence overall, while there are not enough data to perform work time subgroup analysis. Finally, though the meta-analysis is based on 15 studies with 73,812 people, the populations considered are only in Lithuania, Italy, China, Canada, and France. Further epidemiological studies are required to evaluate the cotton dust exposure and risk of lung cancer in more countries worldwide.
Few epidemiological studies have focused on the association between occupational exposure to textile dust and lung cancer risk.[6–11] A published meta-analysis including 11 observational studies reported a significant inverse association between occupational exposure to endotoxin in cotton textile production and the lung cancer risk.[12] However, they found substantial heterogeneity between studies, possibly due to inadequate adjustment for smoking and other occupational exposures that could lead to residual confounding.
Since the 1970s, epidemiologic studies have reported a reduced risk of lung cancer among textile workers.[20,21] One of the largest studies on textile workers has been conducted by Checkoway et al[8] in a cohort of women in Shanghai. They found an inverse exposure-response relation between cumulative exposure to endotoxin and lung cancer.[8] Similar to the case-cohort study conducted by Checkoway et al, a population-based case-referent study found that long-term exposure to cotton dust seemed to be protective lung cancer.[9] Additionally, in a large retrospective cohort, Mastrangelo et al[7] found that a high and prolonged exposure to cotton dust and other endotoxin-containing organic dusts was related to a lower risk of lung cancer. Consistent with the results of the above observational studies, our meta-analysis also found that cotton dust exposure was associated with a decreased risk of lung cancer. Most previous studies of cotton exposed workers had no or little information available on smoking habits.[10] The validity of the smoking data is questionable since the RR estimates for smoking and lung cancer were quite low compared with other studies that have estimated relative risks among female smokers. However, very low cumulative smoking might explain this weak association.
Despite extensive research, it remains unclear whether the association between cotton dust and subsequent lung cancer incidence is causal. Several points that may help further our understanding of this association merit considerations. An argument supporting a role for endotoxin in decreasing lung cancer risk is that lung cancer deficits have not been consistently observed in other (noncotton) types of mills, in which exposure to endotoxin is lower.[12] Additionally, endotoxin levels are minimal in synthetic textile dusts, although levels slightly elevated compared to background levels have been documented in some mills due to contamination of humidification and lubricant mist systems.[30] Endotoxin exposure is possibly beneficial with respect to reducing lung cancer risk. However, it should be noted that studies indicate that acute exposure to cotton dust can cause chest tightness, organic dust toxic syndrome and byssinosis, and long-term exposure is associated with accelerated decline in lung function and chronic respiratory disease.[31–33] The lipid-A portion of endotoxin has been found to suppress tumor growth in animal models.[34] Explanations and evidence for plausible mechanistic pathways are limited. It seems that removing exposure—when farmers quit farming, or switch to a farming type with purportedly lower endotoxin exposures—causes deficits in lung cancer risk to disappear over time.[35,36]
Some limitations should be acknowledged. Some of the included studies were older, making data extraction more complicated. For instance, in Table 1, the “No. of cases” from the Henderson study is not the number of cases but the expected number of cases. The “Study population” of the Levin study is the total number of cases in cotton industry workers plus controls. Therefore, the results of the present meta-analysis should be taken with some caution in light of those limitations.
5. Conclusions
Our meta-analysis, based on 15 observational studies, indicated that cotton dust exposure is associated with a decreased risk of lung cancer. Future research should investigate the dose–response relationship between cotton dust exposure and risk of lung cancer and focus on possible sources of heterogeneity in this relation.
Acknowledgments
We thank Hong Chen for the general supervision of a research group and the technical editing of the manuscript.
Author contributions
Conceptualization: Xinru Huang.
Data curation: Xinru Huang.
Formal analysis: Xinru Huang.
Investigation: Xinru Huang.
Methodology: Xuzho.
Supervision: Xinru Huang.
Validation: Xinru Huang.
Visualization: Xinru Huang.
Writing – original draft: Xinru Huang.
Writing – review & editing: Xinru Huang.
Footnotes
Abbreviations: CIs = confidence intervals, HR = hazard ratio, NOS = Newcastle-Ottawa scale, NSCLC = nonsmall cell lung cancer, RR = relative risk, RRs = relative risks, SCLC = small cell lung cancer.
How to cite this article: Huang X. Cotton dust exposure and risk of lung cancer: A meta-analysis of observational studies. Medicine. 2020;99:14(e19565).
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
The data set supporting the results of this article are included within the article.
This work was financially supported by the National Natural Science Foundation of China (Nos. 71673271, 71473248, 71473247), the Major Project of the National Social Science Foundation of China (No. 16ZDA056), the 333 High-level Talents Project of Jiangsu Province (2016), the Social Science Foundation Base Project of Jiangsu Province (No. 14JD026), the Research and Practice on the Graduate Educational Teaching Reform in Jiangsu Province (No. JGZZ16_078), Jiangsu Philosophy and Social Sciences Excellent Innovation Cultivation Team (2017), and the Program of Innovation Team Supported by China University of Mining and Technology (No. 2015ZY003).
The authors have no conflicts of interest to disclose.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Blandin Knight S, Crosbie PA, Balata H, et al. Progress and prospects of early detection in lung cancer. Open Biol 2017;7: 170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Shannon HS, Walters V, Lewchuck W, et al. Workplace organizational correlates of lost-time accident rates in manufacturing. Am J Ind Med 1996;29:258–68. [DOI] [PubMed] [Google Scholar]
- [5].Cole SR, Richardson DB, Chu H, et al. Analysis of occupational asbestos exposure and lung cancer mortality using the g formula. Am J Epidemiol 2013;177:989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kuzmickiene I, Stukonis M. Lung cancer risk among textile workers in Lithuania. J Occup Med Toxicol 2007;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mastrangelo G, Fadda E, Rylander R, et al. Lung and other cancer site mortality in a cohort of Italian cotton mill workers. Occup Environ Med 2008;65:697–700. [DOI] [PubMed] [Google Scholar]
- [8].Checkoway H, Ray RM, Lundin JI, et al. Lung cancer and occupational exposures other than cotton dust and endotoxin among women textile workers in Shanghai, China. Occup Environ Med 2011;68:425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tse LA, Yu IT, Qiu H, et al. Occupational risks and lung cancer burden for Chinese men: a population-based case-referent study. Cancer Causes Control 2012;23:121–31. [DOI] [PubMed] [Google Scholar]
- [10].Christensen KY, Lavoue J, Rousseau MC, et al. Lack of a protective effect of cotton dust on risk of lung cancer: evidence from two population-based case-control studies. BMC Cancer 2015;15:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ben Khedher S, Neri M, Guida F, et al. Occupational exposure to textile dust and lung cancer risk: results from the ICARE Study. Am J Ind Med 2018;61:216–28. [DOI] [PubMed] [Google Scholar]
- [12].Lenters V, Basinas I, Beane-Freeman L, et al. Endotoxin exposure and lung cancer risk: a systematic review and meta-analysis of the published literature on agriculture and cotton textile workers. Cancer Causes Control 2010;21:523–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [14].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [15].Moher D, Pham B, Jones A, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet 1998;352:609–13. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [20].Henderson V, Enterline PE. An unusual mortality experience in cotton textile workers. J Occup Med 1973;15:717–9. [PubMed] [Google Scholar]
- [21].Merchant JA, Ortmeyer C. Mortality of employees of two cotton mills in North Carolina. Chest 1981;79:6s–11s. [DOI] [PubMed] [Google Scholar]
- [22].Levin LI, Gao YT, Blot WJ, et al. Decreased risk of lung cancer in the cotton textile industry of Shanghai. Cancer Res 1987;47:5777–81. [PubMed] [Google Scholar]
- [23].Hodgson JT, Jones RD. Mortality of workers in the British cotton industry in 1968-1984. Scand J Work Environ Health 1990;16:113–20. [DOI] [PubMed] [Google Scholar]
- [24].Koskela R, Klockars M, Järvinen E. Mortality and disability among cotton mill workers. Br J Ind Med 1990;47:384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wu-Williams AH, Xu Z, Blot WJ, et al. Occupation and lung cancer risk among women in northern China. Am J Ind Med 1993;24:67–79. [DOI] [PubMed] [Google Scholar]
- [26].Szeszenia-Dabrowska N, Wilczynska U, Strzelecka A, et al. Mortality in the cotton industry workers: results of a cohort study. Int J Occup Med Environ Health 1999;12:143–58. [PubMed] [Google Scholar]
- [27].Fritschi L, Lakhani R, Nadon L. Cancer incidence in textile manufacturing workers in Australia. J Occup Health 2004;46:493–6. [DOI] [PubMed] [Google Scholar]
- [28].Astrakianakis G, Seixas NS, Ray R, et al. Lung cancer risk among female textile workers exposed to endotoxin. J Natl Cancer Instit 2007;99:357–64. [DOI] [PubMed] [Google Scholar]
- [29].Blair A, Stewart P, Lubin JH, et al. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am J Ind Med 2007;50:199–207. [DOI] [PubMed] [Google Scholar]
- [30].Kateman E, Heederik D, Pal TM, et al. Relationship of airborne microorganisms with the lung function and leucocyte levels of workers with a history of humidifier fever. Scand J Work Environ Health 1990;16:428–33. [DOI] [PubMed] [Google Scholar]
- [31].Christiani DC, Wegman DH, Eisen EA, et al. Cotton dust and gram-negative bacterial endotoxin correlations in two cotton textile mills. Am J Ind Med 1993;23:333–42. [DOI] [PubMed] [Google Scholar]
- [32].Wang XR, Zhang HX, Sun BX, et al. A 20-year follow-up study on chronic respiratory effects of exposure to cotton dust. Eur Respir J 2005;26:881–6. [DOI] [PubMed] [Google Scholar]
- [33].Schilling RS, Hughes JP, Dingwall-Fordyce I, et al. An epidemiological study of byssinosis among Lancashire cotton workers. Br J Ind Med 1955;12:217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pance A, Reisser D, Jeannin JF. Antitumoral effects of lipid A: preclinical and clinical studies. J Investig Med 2002;50:173–8. [DOI] [PubMed] [Google Scholar]
- [35].Laakkonen A, Pukkala E. Cancer incidence among Finnish farmers, 1995-2005. Scand J Work Environ Health 2008;34:73–9. [DOI] [PubMed] [Google Scholar]
- [36].Mastrangelo G, Grange JM, Fadda E, et al. Lung cancer risk: effect of dairy farming and the consequence of removing that occupational exposure. Am J Epidemiol 2005;161:1037–46. [DOI] [PubMed] [Google Scholar]


