Abstract
High on-treatment platelet reactivity (HTPR) was suggested to be better correlated with recurrent ischemic events as compared with gene polymorphism, whereas most of the results were from white populations with acute coronary disease. The evidence is relatively limited regarding HTPR and its genetic determinants in predicting clinical outcomes of stroke among Chinese-Han patients.
A prospective study including 131 Chinese-Han stroke patients treated with clopidogrel was analyzed. Platelet function was assessed by light transmission aggregometry (LTA)- adenosine diphosphate (ADP) method. HTPR was defined as 5 μM ADP induced platelet aggregation > 46%. CYP2C19 and P2Y12 genotype were detected using the PCR-RFLP method. The difference in the occurrence of the primary endpoint was analyzed according to platelet function and genetic status.
Sixty-three (48.1%) subjects displayed HTPR after administering clopidogrel for 1 week. The prevalence of HTPR was significantly higher in CYP2C19 loss-of-function (LOF) alleles (∗2, ∗3) carriers vs wild-type homozygotes (71.7% vs 32.1%, P < .01), and logistic regression analysis showed that carriers of CYP2C19 LOF alleles were an independent risk factor of HTPR. Survival analysis indicated that patients with HTPR had an increased risk of primary endpoints (20.6% vs 7.3%, P = .04), whereas the presence of CYP2C19 LOF alleles or P2Y12 H2 haplotype did not increase the incidence of ischemic events. Cox regression analysis demonstrated that HTPR was an independent predictor of the primary composite endpoint (HR, 3.1; 95% CI, 1.07–8.99; P = .04).
We identified a high prevalence of clopidogrel-HTPR in a cohort of Chinese-Han patients with acute ischemic stroke, and patients with HTPR may have an increased risk of recurrent ischemic stroke events. CYP2C19 LOF alleles are associated with HTPR but not with stroke prognosis. Further clinical trials with large samples are needed to confirm these findings.
Keywords: CYP2C19, genotype, high on-treatment reactivity, ischemic stroke, P2Y12
1. Introduction
Clopidogrel and other antiplatelet agents are a mainstay in the secondary prevention of ischemic stroke.[1] However, the recurrence of ischemic events during clopidogrel maintenance therapy remains a major concern. Both genetic testing and platelet function testing to optimize the efficacy of clopidogrel in preventing the occurrence of ischemic events have been extensively investigated. In particular, gene polymorphism within CYP2C19 and P2Y12, as well as high on-treatment reactivity (HTPR) to adenosine diphosphate (ADP) are indicated to have prognostic values in selected patients.2,3,4
However, the vast majority of the evidence comes from studies concerning subjects with coronary artery disease or undergoing percutaneous coronary intervention (PCI) among white populations. In consideration of the higher stroke incidence and higher frequency of CYP2C19 loss-of-function (LOF) alleles (∗2/∗3) in Chinese compared to that in Caucasians,5,6 as well as the heterogeneity of the ischemic cerebrovascular disease, more efforts are needed to test the hypothesis. Thus, we performed an observational study, to evaluate the relation of common variants within CYP2C19 & P2Y12 gene with HTPR defined by ADP-specific platelet function assay, as well as the role of genotype and phenotype test in predicting clinical outcomes, in a cohort of ischemic stroke patients, consist of northeastern Chinese- Han people.
2. Methods
2.1. Study populations
Patients diagnosed with acute ischemic stroke and treated with clopidogrel were consecutively enrolled in this study. Inclusion criteria were as follows: age older than 18 years, with computed tomography (CT) or magnetic resonance imaging (MRI) evidence of stroke symptoms, large artery atherosclerosis or small arteries occlusion subtype according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST),[7] baseline National Institute of Health stroke scale (NIHSS) score ≤22. Patients who met the following criteria were excluded: recent cerebral or gastrointestinal hemorrhage, any bleeding disorder or significant coagulopathy, with a tumor or other terminal medical comorbidities, allergic or intolerant to clopidogrel, platelet count < 100 × 1012/L or > 450 × 1012/L. All patients were treated with clopidogrel 75 mg/d without loading dose for at least 7 days before platelet function assay. The study was approved by the ethics committee of The First Affiliated Hospital of China Medical University. Informed consent was obtained from all patients.
2.2. Data collection
Demographic and laboratory data of each patient were collected, including age, gender, ethnicity, and traditional risk factors of cerebral vascular disease, such as a history of hypertension, diabetes, smoking status, cholesterol, and triglyceride profile, concomitant medications, height and weight of patients. A 6-months follow-up was completed via a telephone call or by outpatient clinic visits.
2.3. Blood sample and platelet function test
Blood samples for platelet function test were collected after 7 days of clopidogrel administering. Briefly, fasting peripheral venous blood samples were collected in vacutainer tubes containing 3.8% trisodium citrate, platelet-rich plasma was prepared by centrifugation at 725 rpm for 10 minutes at ambient temperature and platelet-poor plasma (PPP) was prepared after subsequent centrifugation at 3000 rpm for 10 minutes. Platelet aggregation was assessed using routine aggregometer (Helena Laboratory, USA). Platelets were stimulated with ADP with a final concentration of 5 μM, aggregation was expressed as the maximal percent change in light transmission from baseline with PPP as a reference.
2.4. Genotyping test
Genomic DNA was extracted from peripheral blood using a DNA extraction kit according to the manufacturer's instruction (WanleiBio, co., Ltd., Shenyang, China). Genotyping for CYP2C19∗2 (681G>A, rs4244285), ∗3(636G>A, rs4986893), and P2Y12 T744C (rs2046934) was performed by using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) as previously described with few modifications.[8]
Briefly, the PCR amplification for CYP2C19∗2 and ∗3 consisted of an initial denaturation at 94°C for 3 minutes, followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 52°C for 30 seconds, and extension at 72°C for 45 seconds, and a final extension at 72°C for 2 minutes. The following primers were used for CYP2C9∗2 and ∗3 PCR amplification: forward 5′-AATTACAACCAGAGCTTGGC-3′ and reverse 5′-CTTTTCCTATCCTGACATCC-3′ for CYP2C19∗2 and forward 5′-TAGCTTCACCCTGTGATCCC-3′ and reverse 5′-GAAGACTCCAAAGTGCCT-GG-3′ for CYP2C19∗3. The Fast-digest restriction enzyme Eco88I (Thermo Scientific, USA) for CYP2C19∗2 and BamHI (Thermo Scientific, USA) for CYP2C19∗3 were used to digest the PCR product with 1 hour of incubation at 37°C, followed by electrophoresis of digested fragments.
Regarding the P2Y12 gene polymorphism, T744C (rs2046934) was found in total linkage disequilibrium with 3 other SNPs, thus was used as a tag-SNP for the H1/ H2 haplotype.[9] Carriers of the variant C allele were regarded as the minor haplotype H2. The PCR was carried out in same condition as above, with forward primer 5′-CCACACAGCAGTAGCAGGAAAG-3′ and reverse primer 5′-GCATTCCTCAAAACAGGGCAT-3′. The amplification product was digested using TaiI restriction enzyme (Thermo Scientific, USA). The 10% of the results were confirmed by randomly selected individuals of each genotype (CYP2C19 and P2Y12) by DNA sequencing.
2.5. Definition of HTPR and outcomes
Clopidogrel HTPR (phenotype) was defined as maximal platelet aggregation (MPA) > 46% in response to 5 μM ADP by light transmittance aggregometry (LTA) method,[2] and patients with MPA less than or equal to the cut-point were considered as having normal on-treatment platelet reactivity.
The primary clinical efficacy endpoint was a composite of ischemic stroke (IS), transient ischemic attack (TIA), myocardial infarct (MI), or death during a 6-month follow up after the first admission.
2.6. Statistical analysis
Statistical analysis was performed using SPSS 10.1 (SPSS Inc., Chicago, IL). The characteristics of the subjects are presented as mean ± SD for continuous variables and as counts with percentages for categorical variables. The difference between HTPR and non-HTPR groups were analyzed with t test for continuous variables and the chi-square test or Fisher's exact test as needed for categorical variables respectively. Multiple logistic regression was used to identify factors potentially associated with HTPR. Event-free survival curves were constructed using the Kaplan-Meier method, and the difference between groups was compared using the log-rank test. Multivariate Cox regression analysis was used to identify independent correlates of the primary clinical endpoint. For all statistical analyses, a value of P < .05 was considered significant. Analyses were performed using the software package SPSS version 19 (SPSS, Inc.) and GraphPad Prism 7 (GraphPad Software, Inc.)
3. Results
3.1. Patient characteristics
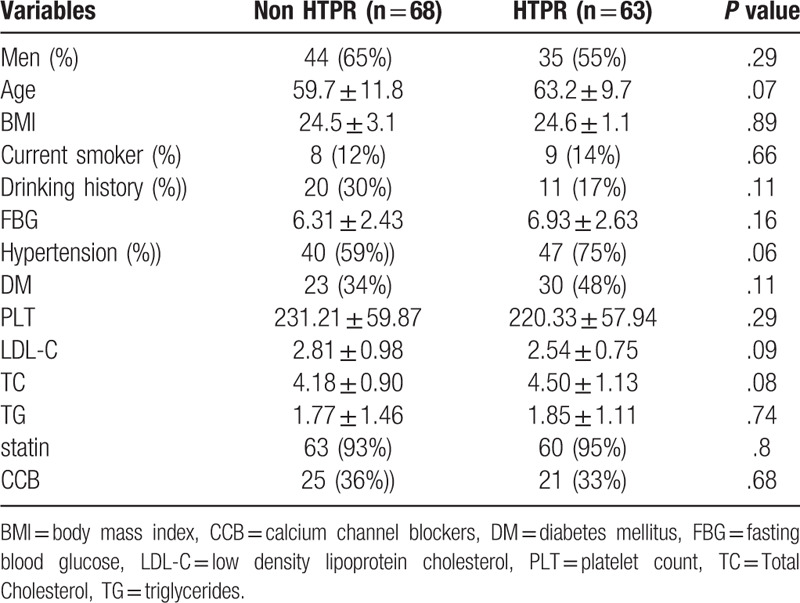
Among the 175 patients who were initially enrolled, 21 subjects switched to aspirin, 14 subjects rejected blood test for genotype or platelet function and 9 subjects refused to continue antiplatelet therapy after discharge from hospital thus were excluded, the remaining 131 patients who completed all the genotyping and platelet function test and the 6-months follow-up were analyzed in this study. All the patients are Chinese-Han origins, with 83 (79%) males and a mean age of 61.4 ± 10.9 years. A total of 79 (60%) subjects were recognized as large artery atherosclerosis stroke, 52 (40%) subjects were recognized as small artery occlusion, according to the TOAST classification system. Traditional risk factors of cerebral vascular disease are common, 66.4% and 42.7% of the patients had hypertension and diabetes mellitus respectively. Of note, 34% of the patients had a history of stroke, and 93.9% were taking lipid-lowering drugs. The prevalence of HTPR was 48.1%. Demographic and clinical characteristics of the patients are summarized in Table 1.
Table 1.
Baseline characteristics of patients according to HTPR.
3.2. CYP2C19 and P2Y12 genetic polymorphism
Genetic polymorphism of each allele was identified and 10% of the results were confirmed using DNA sequencing method (Table 2 and Fig. 1). Concerning polymorphism of CYP2C19, 90 subjects of 131 (68.7%) were wild-type homozygotes (∗1/∗1), 35 (26.7%) were heterozygotes (∗1/∗2), 6 (4.6%) were homozygous ∗2 carriers (∗2/∗2). Regarding the ∗3 allele, 115 subjects (87.8%) were wild-type homozygous (∗1/∗1), 16 (12.2%) were heterozygous ∗3 carriers (∗1/∗3), no subjects were homozygous for CYP2C19∗3 (∗3/∗3). Thus, carriers of the loss-of-function allele were common, 31.3% of the patients were ∗2 carriers (harboring at least one ∗2 allele), and 12.2% were ∗3 carriers. Accordingly, the ∗2 and ∗3 allele were slightly less frequent as compared to other reports among Chinese population.[6] As for P2Y12 polymorphism, 89 subjects of 131 (67.9%) were wild-type homozygotes (H1/H1), 33 (25.2%) were heterozygous H2 carriers (H1/H2), 9 (6.9%) were homozygous H2 carriers (H2/H2). All the SNPs investigated in the study were in Hardy-Weinberg equilibrium (P > .05). Due to the relatively small sample size and low frequency of the CYP2C19 LOF allele & P2Y12 H2 haplotype, most of the statistical analysis was based on the comparison between wild type and LOF carriers, as well as the wild type and H2 haplotype carriers.
Table 2.
Genotype distribution in HTPR and non-HTPR patients.
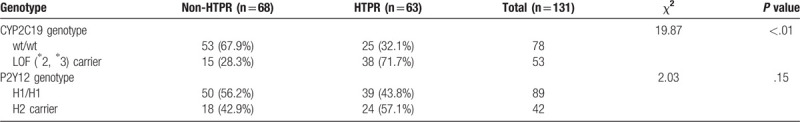
Figure 1.
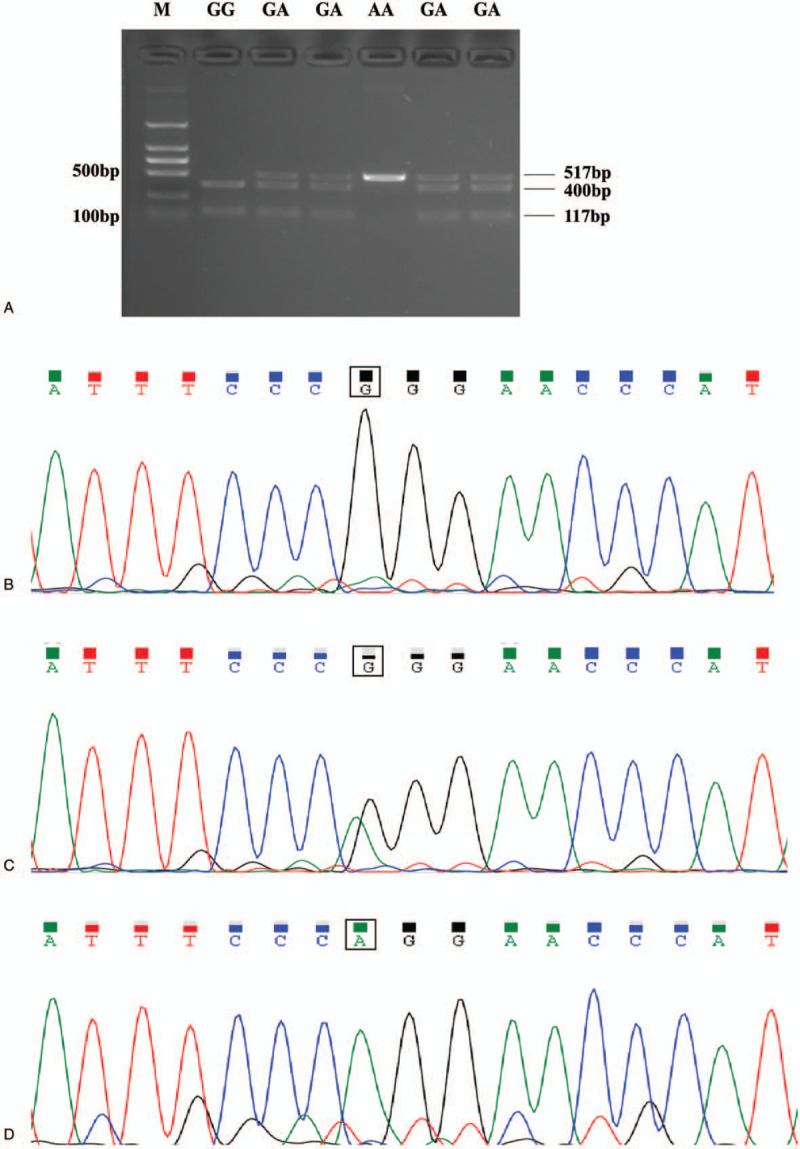
Representative PCR-RFLP results and DNA sequencing maps of CYP2C19∗2 (681G>A) polymorphism. (A) Electrophoretic patterns following Eco88I digestion. The wild-type G/G genotype (∗1/∗1) yielded two bands (400-bp and 117-bp), the mutant homozygote A/A genotype (∗2/∗2) without restriction site yielded a single 517-bp band, the heterozygote G/A genotype (∗1/∗2) yielded three bands (517-bp, 400-bp and 117-bp); Corresponding DNA sequencing confirmed the results: (B) G/G genotype, (C) G/A genotype, and (D) A/A genotype. M: DNA marker.
3.3. Genotype and platelet function
Platelet function was determined by LTA method as outlined above. The MPA induced by 5 μM ADP varied from 11% to 81%, with a median value of 45% (IQR, 34%–59%). Carrier of CYP2C19 LOF allele was associated with higher maximal aggregation in response to ADP, and MPA was significantly greater in the LOF carriers than that in non-carriers (P < .01), whereas no significant difference was seen between with and without H2 haplotype (P = .55, Fig. 2).
Figure 2.
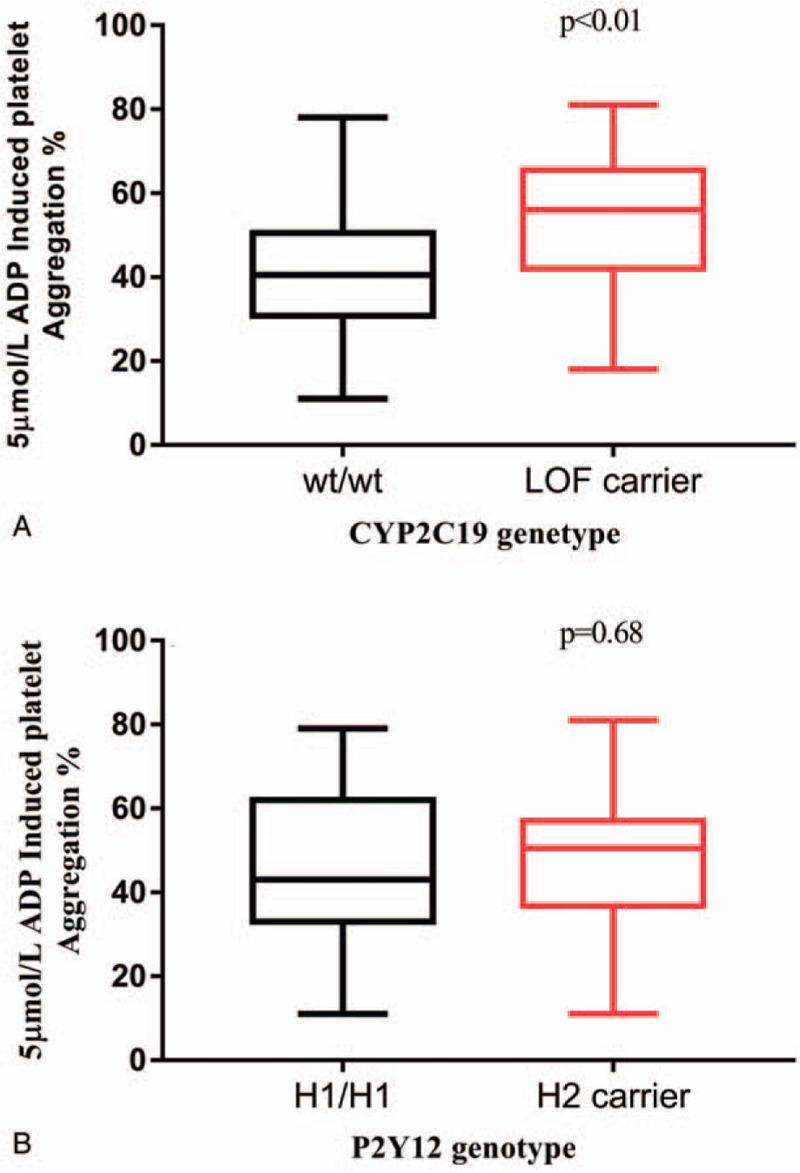
Association of genetic variants with 5 μM ADP induced maximal platelet aggregation after clopidogrel treatment for 7 days. The comparison was carried out between wild type and carrier of genetic variants (Mann–Whitney U test was used to determine the difference).
3.4. HTPR and genotype
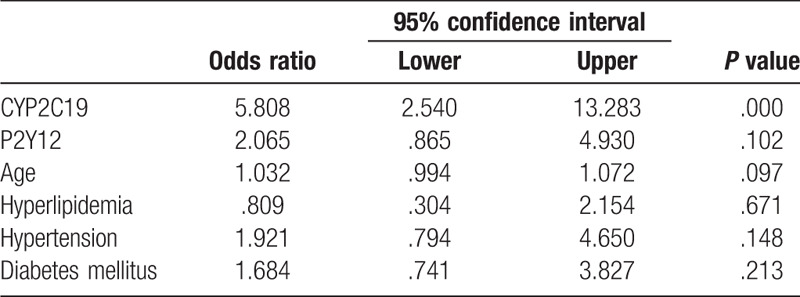
According to the predefined cut-off value of HTPR, 63 (48.1%) were HTPR after taking clopidogrel 75 mg/d for 1 week. The prevalence of HTPR was significantly higher in CYP2C19 LOF carriers vs wild-type homozygotes (71.7% vs 32.1%, P < .01), and there was a trend of higher frequency of HTPR in P2Y12 H2 haplotype carriers compared with non-carriers, whereas no significant difference was found (43.8% vs 57.1%, P = .15). Multivariate logistic regression analysis was performed to identify genetic determents of HTPR. After adjustment for gender, age, body mass index, platelet count, current smoking, hypertension, hyperlipidemia, diabetes mellitus, comedication and other variables considered to be related with platelet aggregation, CYP2C19 LOF allele was demonstrated to be the only independent risk factor to HTPR, odds ratio for HTPR was 5.81 (95% CI, 3.54–13.28; P < .001) in carriers of at least one LOF allele as compared with wild-type homozygotes. However, no significant association between P2Y12 H2 haplotype and the HTPR was found (OR 2.07, 95%CI 0.87–4.93, P = .10). (Table 3).
Table 3.
Logistic regression analysis of independent predictor for HTPR.
3.5. Clinical endpoints during follow-up
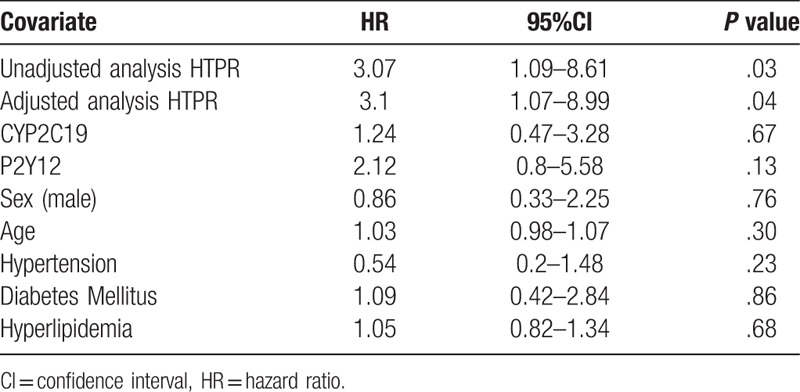
The primary clinical endpoint was a composite of recurrent IS, TIA, MI, or death within 6 months. During follow-up, a total of 17 ischemic events (6 of TIA+11 of IS) and one death were recorded, with 13 (20.6%) of that occurred in HTPR group and 5 (7.3%) occurred in non-HTPR group. In addition, 2 patients in the non-HTPR group experienced cerebral hemorrhage during the follow-up. The Kaplan–Meier curve was constructed to assess the effect of HTPR on the time to primary clinical endpoints, and the risks of ischemic events were higher in the HTPR group (log-rank P = .04, Fig. 3A), whereas no significant difference of ischemic events incidence was shown between different genotype groups (with and without CYP2C19 LOF allele, 15.09% vs 11.54%, P = .26; with and without P2Y12 H2 haplotype, 21.43% vs 8.99%, P = .18; Fig. 3B, C). In the subsequent multivariable Cox regression analysis, HTPR was found to be associated with a worse clinical outcome (HR, 3.07; 95% CI, 1.09–8.61; P = .03). After adjusting for covariates (age, gender, hypertension, hyperlipidemia, diabetes, etc.), the presence of HTPR was shown to be an independent risk factor of the primary composite clinical endpoint (HR, 3.1; 95% CI, 1.07–8.99; P = .04), whereas genotype of CYP2C19 and P2Y12 was not (P = .67, P = .13, respectively) (Table 4).
Figure 3.
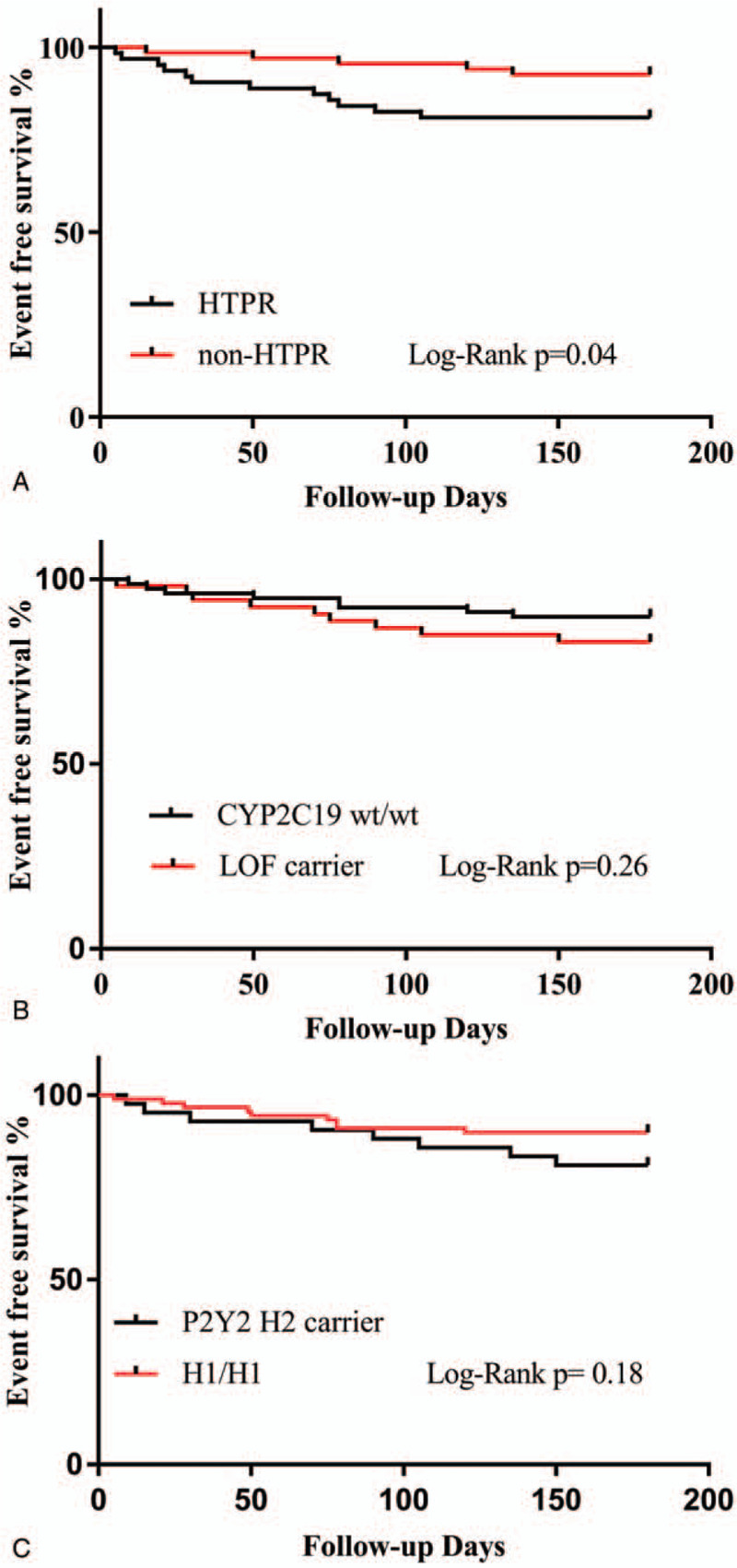
Kaplan–Meier Curves for Event-free Survival According to HTPR (Fig. 2A and genetic status (Fig. 2B, C). Fig. 2A shows a significant decrease in the primary endpoint in the HTPR group. Fig. 2B, C shows that there is no difference in the primary endpoint associated with the genotype of CYP2C19 or P2Y12.
Table 4.
Unadjusted and adjusted effects of HTPR on clinical outcomes using Cox regression analysis.
4. Discussion
Suboptimal response to clopidogrel and the corresponding recurrence of ischemic events during clopidogrel therapy had been a major concern for many years. Numerous previous studies underlined the role of genetic polymorphism, mainly the CYP2C19 LOF allele (∗2, ∗3), in low response to clopidogrel and as a predictor of worse clinical outcome, among white populations with acute coronary disease.[10] In the setting of cerebral vascular disease, the previous studies had also linked the CYP2C19 ∗2/∗3 LOF alleles with a decreased pharmacodynamic response and adverse clinical outcomes. Jia et al[11] demonstrated that CYP2C19 genotype was not only associated with pharmacodynamic response to clopidogrel but also with the prognosis of stroke, in a Chinese stroke population. In the genetic analysis of Clopidogrel in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) trial,[12] which was conducted in 2933 patients with minor stroke or TIA, the combination of clopidogrel and aspirin reduced the rate of new stroke by 33% compared with aspirin alone (HR,0.51;95%CI, 0.35–0.75), only in the CYP2C19 noncarriers group, supporting a role of CYP2C19 genotype in the efficacy of dual antiplatelet therapy. In contrast to these findings, the CHARISMA genetics study[13] and some other studies[14] failed to demonstrate that carriers of CYP2C19 loss-of-function alleles were at increased risk of ischemic events. Likewise, a meta-analysis by Holmes[15] demonstrated that CYP2C19 genotype was associated with clopidogrel responsiveness but not with cardiovascular events. Similarly, in our current research, CYP2C19 LOF alleles was found to be correlated with clopidogrel on-treatment platelet reactivity and was the only independent risk factor of HTPR, whereas no significant correlation was observed between CYP2C19 LOF alleles and the primary composite endpoint.
Some factors may contribute to the difference between our study and the previous study by Jia et al[11], in which the CYP2C19 LOF allele was demonstrated to significantly impact both the clopidogrel pharmacodynamic response and the prognosis of stroke in Chinese patients. First, it was proposed that the oxidation of clopidogrel to an active metabolite mainly depends on CYP2C19, whereas CYP3A4 was now recognized as the major pathway for clopidogrel activation,[16] and the contribution of CYP2C19∗2 loss-of-function polymorphism to interindividual variability in on-treatment platelet reactivity is modest.17,18 Furthermore, a genome-wide association study conducted in 513 healthy participants identified novel genetic variants which contributed to clopidogrel response other than CYP2C19 polymorphism,[19] indicating that the clopidogrel resistance could not be attributed to each gene polymorphism alone. In addition, the endpoint was defined by changes in NIHSS and modified Rankin scale scores at 3 and 6 months after treatment in Jia study, whereas a composite of recurrent IS, MI, TIA, or death was applied in our study, which was more often used in other similar studies and should be a better indicator of antiplatelet treatment failure.
In terms of the P2Y12 gene polymorphism, H2 Haplotype represented by T744C SNP was indicated to play a role in ADP-induced platelet aggregation among healthy volunteers and was subsequently confirmed to be associated with peripheral arterial disease by Fontana et al.[9] In a recent meta-analysis by Cui et al,[20] P2Y12 receptor gene C34T and G52T polymorphism were found to be a risk factor for the poor response to clopidogrel therapy, whereas no such association was found between T744C polymorphism and clopidogrel resistance. Another study from Lee et al,[21] discovered 20 SNPs and 6 haplotypes in an Asian population through DNA direct sequencing, and demonstrated that the H3(742 T > C) but not H2 haplotype was correlated with the ADP-induced platelet aggregation response variation. Our result was in line with the meta-analysis, there was no significant difference between carriers and noncarriers of P2Y12 H2 haplotype (T744C) in platelet aggregation by LTA-ADP (P = .55). Accordingly, P2Y12 H2 haplotype was not related with HTPR nor composite clinical outcome.
Compared with genetic polymorphism, HTPR was suggested to be a better option in predicting the adverse clinical outcome.[22] The study of PEGASUS-PCI,[23] which investigated different platelet function assay compared with polymorphisms of CYP2C19∗2 and CYP2C19∗17 in predicting the efficacy and safety of clopidogrel, concluded that phenotyping vs genotyping was more related with stent thrombosis. In a systemic review regarding the platelet function test in ischemic cerebrovascular disease population, the prevalence of HTPR was 8% to 61% in clopidogrel monotherapy group, depending on the different methods and cut-off value used.[24] A recent study,[25] which focuses on the role of HTPR in predicting ischemic events, among 265 patients with minor ischemic stroke or high-risk TIA, a prevalence of 20.1% of HTPR was identified by TEG-ADP method, and HTPR group had a higher risk of recurrent ischemic events compared with the non-HTPR group. Our study was in line with the above research, the prevalence of HTPR by LTA-ADP was 48.1% in the cohort of our study, and HTPR was the only independent predictor of a composite clinical endpoint after adjusting for covariates. Though CYP2C19 LOF allele was identified as an independent risk factor for HTPR, whereas no association was shown between CYP2C19 genotype and the occurrence of the primary clinical endpoint.
Certainly, we are fully aware that the present study is just an observational one with relatively small sample size and short follow-up duration. Besides, the phenotype of HTPR was only determined at a single time point via a single method, which may also increase the risk of bias in consideration of the time and test method dependency of clopidogrel response,24,26 therefore does not allow for a solid conclusion. Nevertheless, we identified a high prevalence of HTPR (>48%) in a specific stroke population with high risk of recurrent ischemic events, furthermore, HTPR was the risk factor of the clinical outcome, which was in line with results of other previous studies. However, the minor significant statistic difference between HTPR and non-HTPR group by Kaplan-Meier analysis (P = .04) and Cox regression analysis (P = .04) needs confirmation with a lager sample size.
5. Conclusion
In conclusion, our study demonstrated a high prevalence of HTPR in clopidogrel-treated patients with recent ischemic stroke among a northeastern Chinese-Han population. Gene polymorphism of CYP2C19 (∗2, ∗3) but not P2Y12 (T744C) was associated with HTPR, whereas no direct correlation was found between CYP2C19 genetic status and the adverse clinical outcomes. Patients with HTPR may have an increased risk of recurrent ischemic stroke events. Further randomized controlled clinical trials with large samples are needed to confirm these findings.
Author contributions
Conceptualization: Hefei Fu, Zhiyi He.
Data curation: Hefei Fu, Pan Hu, Fei Peng.
Formal analysis: Hefei Fu.
Funding acquisition: Zhiyi He.
Investigation: Hefei Fu, Pan Hu, Chunmei Ma, Fei Peng.
Methodology: Chunmei Ma, Zhiyi He.
Project administration: Zhiyi He.
Resources: Hefei Fu, Pan Hu, Chunmei Ma.
Software: Hefei Fu, Pan Hu, Fei Peng.
Validation: Hefei Fu.
Visualization: Hefei Fu.
Writing – original draft: Hefei Fu.
Writing – review & editing: Zhiyi He.
Footnotes
Abbreviations: ADP = adenosine diphosphate, CT = computed tomography, HTPR = high on-treatment platelet reactivity, IS = ischemic stroke, LOF = loss-of-function, LTA = light transmittance aggregometry, MI = myocardial infarct, MPA = maximal platelet aggregation, MRI = magnetic resonance imaging, NIHSS = National Institute of Health stroke scale, PCI = percutaneous coronary intervention, PPP = platelet-poor plasma, TIA = transient ischemic attack.
How to cite this article: Fu H, Hu P, Ma C, Peng F, He Z. Association of clopidogrel high on-treatment reactivity with clinical outcomes and gene polymorphism in acute ischemic stroke patients: An observational study. Medicine. 2020;99:15(e19472).
This study was supported by a grant (81070913) from the National Natural Science Foundation of China.
The authors report no conflicts of interest.
References
- [1]. Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:3754–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Bonello L, Tantry US, Marcucci R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010;56:919–33. [DOI] [PubMed] [Google Scholar]
- [3]. Angiolillo DJ, Ferreiro JL, Price MJ, et al. Platelet function and genetic testing. J Am Coll Cardiol 2013;62:S21–31. [DOI] [PubMed] [Google Scholar]
- [4]. Topcuoglu MA, Arsava EM, Ay H. Antiplatelet resistance in stroke. Expert Rev Neurother 2011;11:251–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Tsai CF, Thomas B, Sudlow CL. Epidemiology of stroke and its subtypes in Chinese vs white populations: a systematic review. Neurology 2013;81:264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Zhou Y, Ingelman-Sundberg M, Lauschke VM. Worldwide distribution of cytochrome P450 alleles: a meta-analysis of population-scale sequencing projects. Clin Pharmacol Ther 2017;102:688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- [8]. de Morais SM, Wilkinson GR, Blaisdell J, et al. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 1994;269:15419–22. [PubMed] [Google Scholar]
- [9]. Fontana P, Dupont A, Gandrille S, et al. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation 2003;108:989–95. [DOI] [PubMed] [Google Scholar]
- [10]. Mega JL, Simon T, Collet J-P, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel Predominantly for PCI: a meta-analysis. JAMA 2010;304:1821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Jia DM, Chen ZB, Zhang MJ, et al. CYP2C19 polymorphisms and antiplatelet effects of clopidogrel in acute ischemic stroke in China. Stroke 2013;44:1717–9. [DOI] [PubMed] [Google Scholar]
- [12]. Wang Y, Zhao X, Lin J, et al. Association Between CYP2C19 Loss-of-Function Allele Status and Efficacy of Clopidogrel for Risk Reduction Among Patients With Minor Stroke or Transient Ischemic Attack. JAMA 2016;316:70–8. [DOI] [PubMed] [Google Scholar]
- [13]. Bhatt DL, Paré G, Eikelboom JW, et al. The relationship between CYP2C19 polymorphisms and ischaemic and bleeding outcomes in stable outpatients: the CHARISMA genetics study. Eur Heart J 2012;33:2143–50. [DOI] [PubMed] [Google Scholar]
- [14]. Guillaume P, Mehta SR, Salim Y, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med 2010;363:1704–14. [DOI] [PubMed] [Google Scholar]
- [15]. Michael VH, Pablo P, Tina S, et al. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA 2011;306:2704–14. [DOI] [PubMed] [Google Scholar]
- [16]. Ford NF. The Metabolism of Clopidogrel: CYP2C19 is a minor pathway. J Clin Pharmacol 2016;56:1474–83. [DOI] [PubMed] [Google Scholar]
- [17]. Bouman HJ, Harmsze AM, van Werkum JW, et al. Variability in on-treatment platelet reactivity explained by CYP2C19∗2 genotype is modest in clopidogrel pretreated patients undergoing coronary stenting. Heart 2011;97:1239–44. [DOI] [PubMed] [Google Scholar]
- [18]. Reny J-L, Combescure C, Daali Y, et al. for the PON1 Meta-Analysis Group. Influence of the paraoxonase-1 Q192R genetic variant on clopidogrel responsiveness and recurrent cardiovascular events: a systematic review and meta-analysis. J Thromb Haemost 2012;10:1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Backman JD, O’Connell JR, Tanner K, et al. Genome-wide analysis of clopidogrel active metabolite levels identifies novel variants that influence antiplatelet response. Pharmacogenet Genomics 2017;27:159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Cui G, Zhang S, Zou J, et al. P2Y12 receptor gene polymorphism and the risk of resistance to clopidogrel: A meta-analysis and review of the literature. Adv Clin Exp Med 2017;26:343–9. [DOI] [PubMed] [Google Scholar]
- [21]. Lee S-J, Jung I-S, Jung E-J, et al. Identification of P2Y12 single-nucleotide polymorphisms and their influences on the variation in ADP-induced platelet aggregation. Thromb Res 2011;127:220–7. [DOI] [PubMed] [Google Scholar]
- [22]. Bennett D, Yan B. Suboptimal response to clopidogrel: A genetic risk factor for recurrent ischaemic stroke. J Clin Neurosci 2013;20:767–70. [DOI] [PubMed] [Google Scholar]
- [23]. Siller-Matula JM, Delle-Karth G, Lang IM, et al. Phenotyping vs genotyping for prediction of clopidogrel efficacy and safety: the PEGASUS-PCI study. J Thromb Haemost 2012;10:529–42. [DOI] [PubMed] [Google Scholar]
- [24]. Lim ST, Coughlan CA, Murphy SJ, et al. Platelet function testing in transient ischaemic attack and ischaemic stroke: A comprehensive systematic review of the literature. Platelets 2015;26:402–12. [DOI] [PubMed] [Google Scholar]
- [25]. Rao Z, Zheng H, Wang F, et al. High on-treatment platelet reactivity to adenosine diphosphate predicts ischemic events of minor stroke and transient ischemic attack. J Stroke Cerebrovasc Dis 2017;26:2074–81. [DOI] [PubMed] [Google Scholar]
- [26]. Meves SH, Schroder KD, Endres HG, et al. Clopidogrel high-on-treatment platelet reactivity in acute ischemic stroke patients. Thromb Res 2014;133:396–401. [DOI] [PubMed] [Google Scholar]


