Abstract
Background:
Chronic liver disease is traditionally conceived as a risk factor for cardiovascular surgery. Transcatheter aortic valve implantation (TAVI) has recently burgeoned to precede surgical aortic valve replacement (SAVR) in patients with severe aortic stenosis at intermediate to high surgical risk. The evidence regarding TAVI in the patients with chronic liver disease is currently scarce.
Methods:
This article aims to assess the application of TAVI technique in the patients with chronic liver disease.
Results:
TAVI in the patients with chronic liver disease produced acceptable postoperative results. The post-TAVI outcomes were comparable between the patients with or without chronic liver disease, except for a lower rate of pacemaker implantation in the patients with chronic liver disease (OR, 0.49[0.27–0.87], P = .02). In the patients with chronic liver disease, compared to SAVR, TAVI led to a decrease in the in-hospital mortality (OR, 0.43[0.22–0.86], P = .02) and need for transfusion (OR, 0.39[0.25–0.62], P < .0001). The rest outcomes were similar between the 2 groups.
Conclusions:
This systematic review and meta-analysis supported that TAVI is a reliable therapeutic option for treating severe aortic stenosis in the patients with chronic liver disease. Future large-scale randomized controlled trials investigating the mid-term and long-term prognosis are needed to further verify these results.
Keywords: chronic liver disease, cirrhosis, surgical aortic valve replacement, transcatheter aortic valve implantation
1. Introduction
Chronic liver disease, especially cirrhosis and end-stage liver disease, complicates the non-cardiac and cardiac operations and places this group of patients at a relatively higher risk of postoperative morbidity and mortality.[1,2] In the treatment of symptomatic severe aortic stenosis, transcatheter aortic valve implantation (TAVI) has recently emerged as a feasible therapeutic alternative of traditional surgical aortic valve replacement (SAVR) in the patients with moderate-to-high risk profiles.[3,4] This less invasive approach has even showed a line of promising results in the low-risk patients.[5,6] In the contemporary clinical settings, decision-making for cardiovascular surgery routinely relies on several well-documented risk scoring model including European System for Cardiac Operative Risk Evaluation (EuroSCORE) and Society of Thoracic Surgeons (STS) score. Those scores do not address the chronic liver disease as a prognostic factor at preoperative assessment.[7,8] However, chronic liver disease has been theorized to increase the likelihood of coagulopathy, multiorgan dysfunction, and serious infection postoperatively.[9,10] Thus TAVI might facilitate to reduce the perioperative complications by avoiding the invasive nature of SAVR and the use of cardiopulmonary bypass.[2] The clinical evidence is still lacking concerning the outcomes of TAVI in the patients with chronic liver disease. In this systematic review and meta-analysis, we sought to summarize the current literature to:
-
1)
quantify the postoperative outcomes after TAVI in the patients with chronic liver disease;
-
2)
compare the post-TAVI outcomes in the patients with or without chronic liver disease;
-
3)
compare the postprocedural results of TAVI versus SAVR in the patients with chronic liver disease.
2. Methods
This systematic review and meta-analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement and Meta-analysis of Observational studies in Epidemiology guidelines (MOOSE).[11,12] This study was conducted under the supervision of ethics committee of Shandong Provincial Hospital. And this study was a meta-analysis which only extracted and analyzed the data of existing literature, thus an ethical approval was not necessary.
2.1. Eligibility criteria
We digitally searched the MEDLINE, EMBASE and Cochrane databases for relevant literature which met the following criteria:
-
(A)
randomized controlled trials (RCTs) or observational studies published as full-text articles;
-
(B)
recruiting adult patients;
-
(C)
patients undergoing TAVI or SAVR due to aortic valve stenosis;
-
(D)
assessing the effects of chronic liver disease on the post-TAVI outcomes or comparing the outcomes of TAVI versus SAVR in the patients with chronic liver disease;
-
(E)
interested postoperative outcomes included in the studies: in-hospital mortality, stroke, major/life-threatening bleeding complications, (major) vascular complications, vascular complications requiring surgery, transfusion, requirement for pacemaker implantation and acute kidney injury (AKI);
-
(F)
data available for statistical analysis. Exclusion criteria were:
-
(a)
case reports, editorial comments, and review articles;
-
(b)
studies unpublished, published in duplicate, or with insufficient data.
-
(a)
2.2. Search strategy and data extraction
Two authors (Xiaochun Ma and Diming Zhao) independently searched the databases for eligible studies published from inception to July 2018. The searching keywords used either alone or in combination included: “transcatheter aortic valve implantation”, “transcatheter aortic valve replacement”, “TAVR”, “TAVI”, “surgical aortic valve replacement”, “SAVR”, “(liver/hepatic) cirrosis”, “(chronic) liver/hepatic disease”, and “end-stage liver disease”. At first, the abstracts of candidate articles were examined separately by Xiaochun Ma and Haizhou Zhang. Next, the full-text articles from primary selection were re-examined to finally determine the inclusion. The reference lists of retrieved articles were also manually checked for potential publications satisfying our criteria. Disagreements on inclusion or exclusion were solved by a further discussion among all the authors. Study quality evaluation for observational studies (cohort studies) was performed by the same 2 authors using the Newcastle-Ottawa scale (NOS), as previously described.[13] Two authors (Jinzhang Li and Diming Zhao) performed the data extraction dependently. Extracted data consisted mainly of the study design and quality, subject demographics, baseline characteristics, and outcomes of interest.
2.3. Data analysis
Statistical analyses were all performed using Stata 12.0. Random-effects model weighted by inverse variance was selected for pooling the extracted data. Categorical data are presented as frequencies and percentages and continuous variables are reported as mean ± SD or median and range. In-hospital mortality, stroke, major/life-threatening bleeding complications, major vascular complications, requirement for pacemaker implantation, and AKI were expressed as odds ratios (ORs) accompanied with 95% confidence intervals (CIs). A P value less than .05 was considered statistically significant. For individual studies with no events in one or both groups, a continuity correction of 0.5 was added to each cell for each effect measure. For measuring heterogeneity between the included study, a P value less than .1 was considered significant. Besides, I2 metric of 25%, 50%, and 75% suggests, respectively, mild, moderate, and severe heterogeneity. Publication bias was assessed with the Egger's regression test.
3. Results
3.1. Eligible studies
At first, 32 studies were identified for further review by digital search. After carefully checking the titles of these studies, 21 studies were classified as irrelevant, leaving 11 studies pending a formal examination. Next, 2 studies were removed after re-checking, leaving 9 observational studies for retrieval of full text. Finally, 2 studies were abandoned for not reporting our outcomes. A consensus among all the authors was reached regarding the inclusion or exclusion of full-text studies (Fig. 1).
Figure 1.
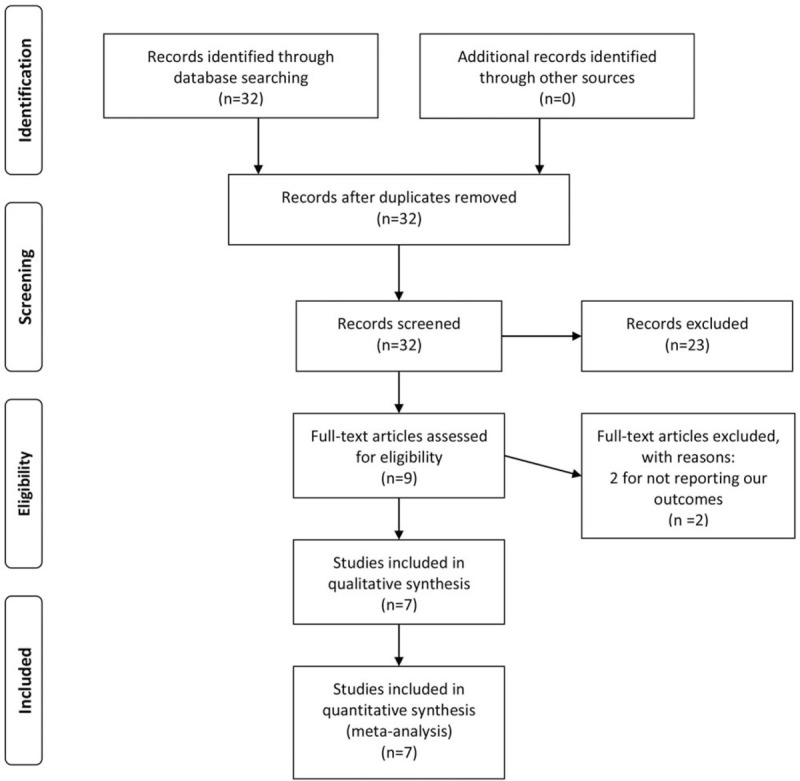
The PRISMA recommended flow-diagram depicting the methodology of article selection for this meta-analysis.
3.2. Study characteristics
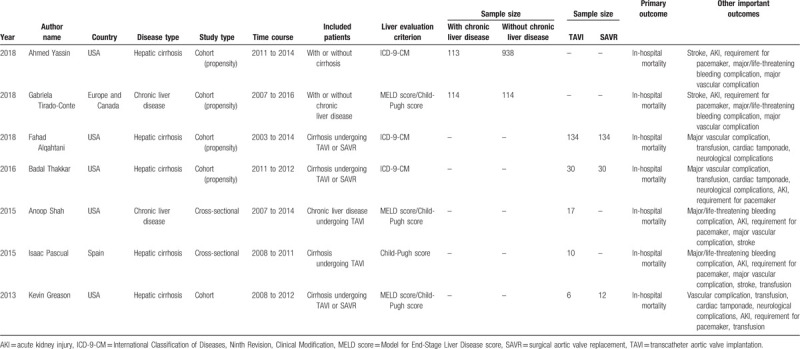
After a thorough digital search, 7 observational study containing 1652 patients ultimately satisfied our criteria and were included in this systematic review and meta-analysis.[14–20] No record of RCT was detected through the digital search. A sum of 1476 patients undergoing TAVI was recruited, among which 600 patients were afflicted with chronic liver disease. Characteristics of the included studies were detailed in Table 1. These studies were published from 2013 to 2018 and included the patients operated between 2003 and 2016. Five studies were conducted in the USA and 3 of them utilized the data from NIS database and another 2 were single-center studies.[14,16–18,20] One study was performed in Spain and 1 collecting the data from 12 institutions in Europe and Canada.[15,19] Five studies were cohort studies, among which 4 were propensity score-matched.[14–17,20] Another 2 studies were cross-sectional studies.[18,19] Five studies reported the outcomes of patients with hepatic cirrhosis and 2 describing the outcomes of ones with chronic liver disease. Specifically, Gabriela Tirado-Conte and his team recruited 114 patients with chronic liver disease and 73% of them were diagnosed with cirrhosis.[15] Anoop Shah and his colleagues included 17 patients with chronic liver disease and 82% of them were confirmed with cirrhosis.[18] For those 5 cohort studies, 2 compared the post-TAVI outcomes in the patients with or without chronic liver disease,[14,15] 3 addressed the issue comparing the postprocedural results of TAVI versus SAVR in the patients with chronic liver disease.[16,17,20] Two studies with cross-sectional design were single-arm studies and provided the descriptive data of patients with chronic liver disease who underwent TAVI.[18,19] Three studies selected the patients from NIS database using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM)[14,16,17] and the other 4 studies evaluated the hepatic function with Model for End-Stage Liver Disease score (MELD score) and/or Child-Pugh score.[15,18–20] All the 7 studies comprised of the outcomes of our interest including the primary outcome (in-hospital mortality) and secondary outcomes (postoperative complications) (Table 1).[14–20]
Table 1.
Study characteristics included in this mini-review and meta-analysis.
3.3. Patient demographics
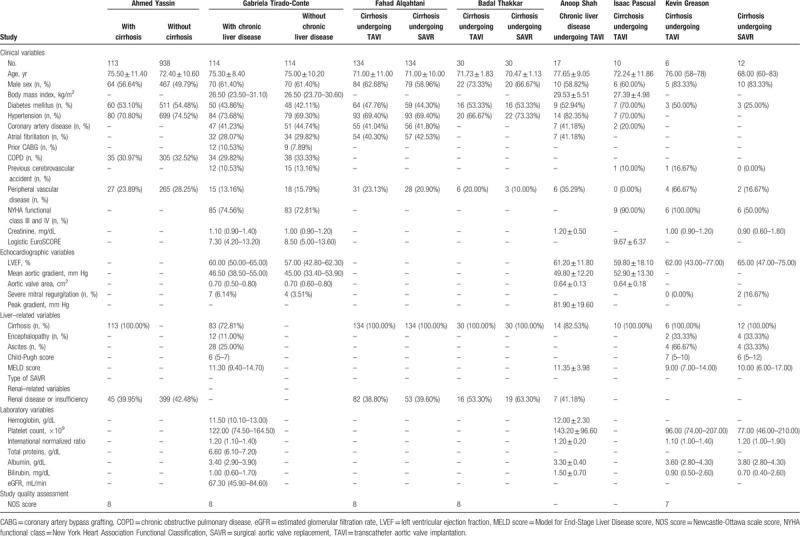
The detailed patient demographics were listed in Table 2. For each of the 4 propensity score-matched cohort studies, their 2 arms were comparable for the most variables.[14–17] For the cohort study by Kevin Greason, the TAVI group seemed to have poorer cardiac function than the SAVR group (New York Heart Association Functional Classification (NYHA functional class)) III and IV: 100% versus 50%).[20] For the 3 studies recruiting patients using ICD-9-CM, the detailed information of liver-related variables were not provided.[14,16,17] The mean or median MLED scores of the studies by Gabriela Tirado-Conte, Anoop Shah, and Kevin Greason were 11.3, 11.35, and 9 in the patients with chronic liver disease, respectively.[15,18,20] None of the included studies provided the detailed information of SAVR (e.g., mechanical or bioprosthetic valve). And neither of the included studies showed the detailed data of anticoagulant/antiplatelet strategy after TAVI and SAVR in the patients with chronic liver disease. NOS scores were provided for the included cohort studies among which 4 were assigned with 8 points and 1 with 7 points.
Table 2.
Patient demographics included in this mini-review and meta-analysis.
3.4. Pooled estimates of the post-TAVI outcomes in the patients with chronic liver disease
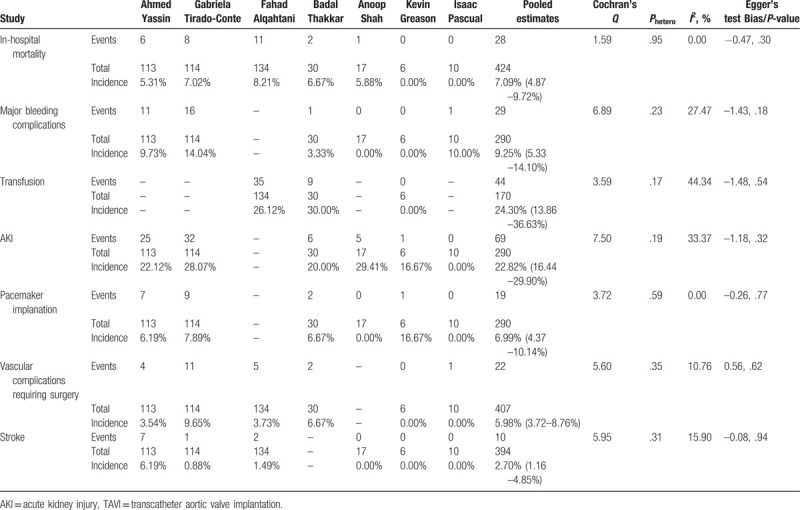
Seven observational studies (n = 424) were pooled to estimate the post-TAVI outcomes in the patients with chronic liver disease. The incidence of in-hospital mortality was 7.09% ([4.87–9.72%], I2 = 0%, Phetero = .95), that of major bleeding complications being 9.25% ([5.33–14.10%], I2 = 27.47%, Phetero = .23). The rate of transfusion was 24.30% ([13.86–36.63%], I2 = 44.34%, Phetero = .17). Postoperative AKI complicated 22.82% patients ([16.44–29.90%], I2 = 33.37%, Phetero = .19). Pacemaker was postoperatively implanted in 6.99% patients ([4.37–10.14%], I2 = 0%, Phetero = .59). There was an incidence of 5.98% (3.72–8.76%], I2 = 10.76%, Phetero = .35) for vascular complications requiring surgery (Fig. 2). The incidence of stroke was 2.70% ([1.16–4.85%], I2 = 15.90%, Phetero = .31). No obvious publication bias was observed for all the estimates above (Table 3).
Figure 2.
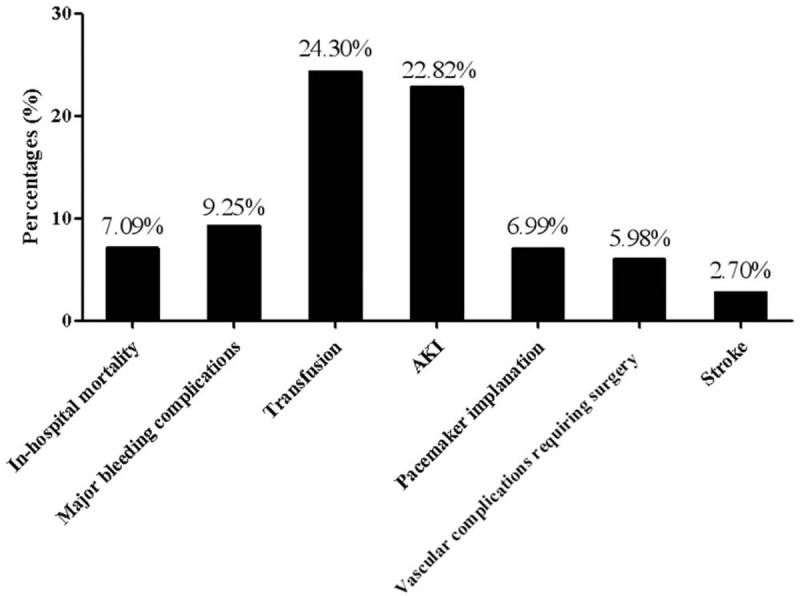
Post-TAVI outcomes in the patients with chronic liver disease estimated by this meta-analysis.
Table 3.
Pooled estimates of the post-TAVI outcomes in the patients with chronic liver disease.
3.5. Comparison of the post-procedural outcomes of patients with or without chronic liver disease
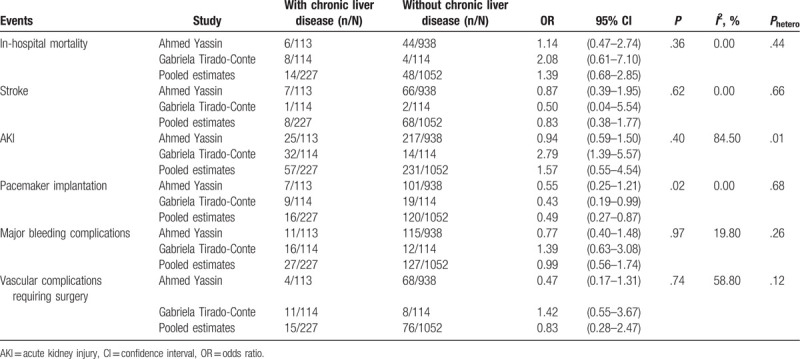
Two observational studies comparing the patients with (n = 227) or without (n = 1052) chronic liver disease were included. The in-hospital mortality was similar between the 2 groups (OR, 1.39[0.68–2.85], P = .36, I2 = 0%, Phetero = .44). The rate of stroke was also comparable between the groups (OR, 0.83[0.38–1.77], P = .62, I2 = 0%, Phetero = .66). Chronic liver disease did not increase the risk of postoperative AKI (OR, 1.57[0.55–4.54], P = .40, I2 = 84.5%, Phetero = .01). Interestingly, a lower rate of pacemaker implantation was noted in the patients with chronic liver disease (OR, 0.49[0.27–0.87], P = .02, I2 = 0%, Phetero = .68). Additionally, chronic liver disease was not associated with an increased incidence of major bleeding complications (OR, 0.99[0.56–1.74], P = .97, I2 = 19.80%, Phetero = .26). The incidence of vascular complications requiring surgery was not significantly different between the 2 groups (OR, 0.83[0.28–2.47], P = .74, I2 = 58.80%, Phetero = .12) (Table 4). Publication bias was inestimable using Egger's regression test when only 2 studies were pooled.
Table 4.
Comparison of the post-procedural outcomes of patients with or without chronic liver disease.
3.6. Comparison of the outcomes of patients with chronic liver disease undergoing TAVI versus SAVR
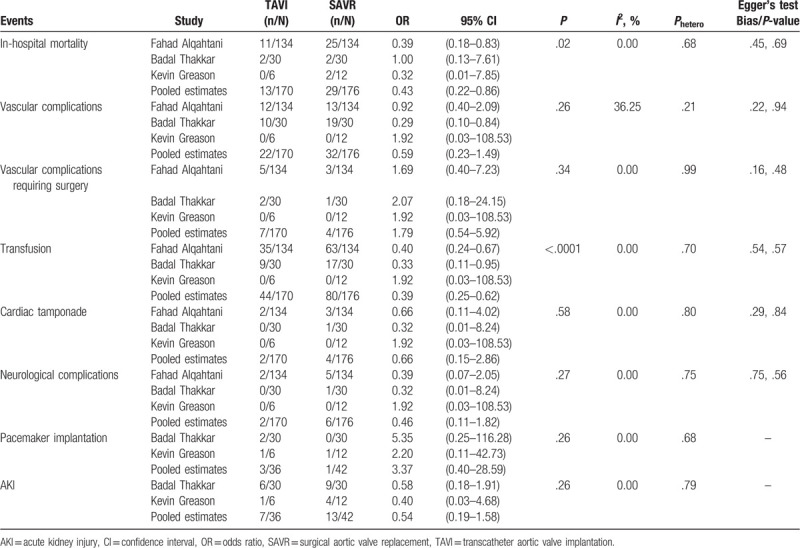
Three observational studies including 170 patients undergoing TAVI and 176 patients undergoing SAVR were pooled to estimate the relevant outcomes. SAVR increased the incidence of in-hospital mortality in the patients with chronic liver disease, compared to TAVI (OR, 0.43[0.22–0.86], P = .02, I2 = 0.00%, Phetero = .68). The 2 groups were comparable in the rates of vascular complication (OR, 0.59[0.23–1.49], P = .26, I2 = 36.25%, Phetero = .21) and vascular complication requiring surgery (OR, 1.79[0.54–5.92], P = .34, I2 = 0.00%, Phetero = .99). There was a lower rate of transfusion in the TAVI group in comparison with the SAVR group (OR, 0.39[0.25–0.62], P < .0001, I2 = 0.00%, Phetero = .70). Cardiac tamponade and neurological complications were similar between the 2 groups (cardiac tamponade: OR, 0.66[0.15–2.86], P = .58, I2 = 0.00%, Phetero = .80; neurological complications: OR, 0.46[0.11–1.82], P = .27, I2 = 0.00%, Phetero = .75). Additionally, the 2 groups did not differ in the rates of pacemaker implantation (OR, 3.37[0.40–28.59], P = .26, I2 = 0.00%, Phetero = 0.68) and AKI (OR, 0.54[0.19–1.58], P = .26, I2 = 0.00%, Phetero = .79). Publication bias was not obvious for all the estimatable results above (Table 5).
Table 5.
Comparison of the outcomes of patients with chronic liver disease undergoing TAVI versus SAVR.
4. Discussion
In this systematic review and meta-analysis, the relevant literature has been searched and reviewed regarding the application of TAVI in the patients with concomitant severe aortic stenosis and chronic liver disease. Statistical analysis has simultaneously been performed to produce pooled results of post-TAVI outcomes. Although evidence has been so far limited, 7 observational study containing 1476 patients undergoing TAVI met the inclusion criterion and were recruited, among which 600 patients were affected by chronic liver disease. It was concluded that TAVI is a feasible therapy for this special group of patients.
4.1. Summary of the included studies
Greason and his group firstly performed a study in 2013 comparing the outcomes of 18 patients with aortic valve stenosis and cirrhosis undergoing either TAVI (n = 6) or SAVR (n = 12). TAVI was successful in all the patients. There was no operative mortality in the TAVI group but 2 operation-related deaths occurred in the SAVR group. Compared to TAVI, the operative complications were higher in the SAVR group. Specifically, AKI occurred in 1 patient in the TAVI group and in 4 in the SAVR group and pacemaker implantation was present in 1 patient in both groups. TAVI patients all survived at last follow-up of 219 days (median, interquartile range, 29 to 723 days), while only 5 were alive in the SAVR group at 228 days (median, interquartile range, 36 to 719 days).[20]
In 2015 Pascual and his colleges conducted a cross-sectional study including 10 cases undergoing TAVI for severe aortic stenosis with accompanying liver cirrhosis. No death occurred during hospitalization or during 30 days after implantation. Four patients died during follow-up (median, 1031 days; interquartile range, 268–1737 days), with 1 due to refractory heart failure and the other 3 due to non-cardiac causes. An estimated median survival was 1374 days (95% CI, 823–1924 days), showing an overall survival of 60%.[19]
In the same year, Shah and his colleagues reported a series of 17 cases with chronic liver disease who underwent TAVI. Successful implantation was achieved in all the patients with 1 case of in-hospital mortality, 1 case of TIA, 5 cases of AKI, and 1 case of hepatic decompensation, but no bleeding, vascular complications, and pacemaker placement. 90-day mortality was 17.65% and median follow-up was 466 days (range, 12–1403 days) with 1 death from a proximate cardiac cause, 2 deaths from unknown factors, and 2 deaths from non-cardiac causes.[18]
In 2016, a team led by Thakkar evaluated the outcomes of propensity score-matched patients with cirrhosis undergoing either SAVR (n = 30) or TAVI (n = 30). In-hospital mortality rate was not different in the 2 groups (6.7% versus 6.7%, P = 1.000). Compared to the TAVI group, rate of transfusion of whole blood or blood products was higher (56.7% versus 30.0%, P = .037) and mean postprocedural length of stay was longer (14.26 versus 6.18, P = .006) in SAVR group. The team found no significant results regarding mean cost of hospitalization (79,263 versus 63,913, P = .20), rate of any common complications (80.0% versus 60%, P = .09) and liver complications (20% versus 10%, P = .500).[17]
Alqahtani et al reported their findings in which the outcomes of TAVI versus SAVR for aortic valve stenosis were compared in the propensity-matched patients (n = 174 for each group) with hepatic cirrhosis. Compared to TAVI, in-hospital mortality was higher in the SAVR group (18.7% versus 8.2%, P = .018). Nevertheless, the major adverse events were not significantly different between the 2 groups. Besides, there were a longer hospital length of stay and a higher rate of non-home disposition in the SAVR group (hospital length of stay: 16 versus 13, P = .005; non-home disposition: 31.3% versus 19.4%, P < .0001). A multivariate regression model revealed the strongest predictors of in-hospital mortality that include advanced age (OR, 1.03[1.01–1.04], P < .0001), male gender (OR, 1.76[1.31–2.36], P < .0001), advanced liver cirrhosis (OR, 2.34[1.73–3.16], P < .0001), SAVR (OR, 3.25[1.67–6.33], P = .001), IABP/LVAD (OR, 2.97[1.93–4.57], P < .0001), congestive heart failure (OR, 2.41[1.58–3.67], P < .0001), and chronic renal disease (OR, 1.71[1.23–2.38], P = .001) in all the subjects.[16]
A propensity score-matched study by Yassin and his colleagues have very recently presented the evidence comparing the outcomes of patients with (n = 113) or without liver cirrhosis (n = 938) who underwent TAVI. The primary outcome was in-hospital mortality and liver cirrhosis did not significantly increase the risk of in-hospital mortality (5.23% versus 4.71%, OR, 1.12[0.59–2.10], P = .734). For the secondary outcomes, the rates of most postprocedural complications were comparable between the groups. However, cirrhosis patients were at lower risk of vascular complications requiring surgery (3.56% versus 7.26%, OR, 0.47[0.23–0.98], P = .043), deep vein thrombosis (0.00% versus 1.82%, OR, <0.00[<0.001–<0.0001], P < .0001), and pacemaker implantation (6.23% versus 10.77%, OR, 0.55[0.31–0.99], P = .045), but with a higher likelihood of nonroutine hospital discharges (71.71% versus 62.75%, OR, 1.50[1.15–1.96], P = .003). Besides, the length of hospital stay and the cost of hospitalization were similar among 2 groups (the cost of hospitalization: 62,750 versus 67,698, MR, 0.93[0.84–1.02], P = .108; the length of hospital stay: 8.61 versus 9.07, MR, 0.95[0.79–1.14], P = .584).[14]
Gabriela Tirado-Conte and his colleagues have recently reported a multicenter propensity score-matched analysis in which short- and mid-term outcomes were compared among 114 matched pairs of patients undergoing TAVI from 2007 to 2016, with or without chronic liver disease. The results demonstrated no significant difference between the matched groups in the incidence of in-hospital mortality (7% versus 4%, P = .34), major vascular complications (10% versus 7%; P = .65), and life-threatening or major bleeding complications (14% versus 11%; P = .54). Nevertheless, chronic liver disease predisposed the postoperative AKI of all stages (31% versus 13%; P = .01). At 2-year follow-up, a higher rate of non-cardiac mortality was noted in the liver group (26% versus 15%; P = .03) while cardiovascular mortality and all-cause mortality was similar between the 2 groups (all-cause: 37% versus 25%; P = .07; cardiovascular: 9% versus 7%; P = .43). Another line of findings of this study was that the compromised estimated glomerular filtration rate (eGFR) (HR, 1.10[1.03–1.17], for each decrease of 5 mL/min in eGFR, P = .005) and Child-Pugh class B or C (HR, 3.11[1.47–6.56], P = .003) were the predictors of mortality in patients with chronic liver disease post-TAVI. And no more than 45% patients survived at 1-year follow-up and 83.2% patients carrying both the factors ended up with mortality at 2-year follow-up.[15]
4.2. Analysis of the pooled results
By including the above-mentioned studies and pooling their results, we noticed that firstly, the postoperative outcomes of patients with chronic liver disease undergoing TAVI were acceptable. The incidence of mortality was 7.09% and that of major bleeding complications was 9.25%. The rates of AKI, pacemaker implantation, and stroke were 22.82%, 6.99%, and 2.70%, respectively. Major bleeding complications and vascular complications requiring surgery occurred postoperatively among 9.25% and 5.98% of the patients, respectively. We did not observe obvious heterogeneity and publication bias for those pooled estimates.
Secondly, in order to testify the effects of chronic liver disease on the TAVI, the post-TAVI outcomes were compared between the groups with or without chronic liver disease. The 2 groups were similar in terms of in-hospital mortality and postoperative complications except for a decreased rate of pacemaker implantation in the patients with chronic liver disease. Interestingly, Gabriela Tirado-Conte and his colleagues demonstrated a similar trend that the patients with chronic liver disease appeared to have a lower incidence of pacemaker implantation.[15] This finding awaits further confirmation and elucidation in the future. No significant heterogeneity was noted except that a severe heterogeneity was found for AKI. It was due to a significantly higher risk of postoperative AKI in the patients with chronic liver disease in the study by Gabriela Tirado-Conte et al.[15] Nevertheless, the 2 groups were not different in the rate of AKI in the report by Ahmed Yassin and his team.[14] Although liver diseases at end-stage predispose to hepatorenal syndrome, TAVI might avoid the potential kidney injury because cardiopulmonary bypass is not utilized. Thus, it is yet unknown whether chronic liver disease indeed correlates with a higher possibility of postprocedural AKI after TAVI. Additionally, although our results showed that the chronic liver disease did not increase the in-hospital mortality after TAVI, Alqahtani and his group demonstrated that advanced cirrhosis predicted the in-hospital mortality.[16] Thus we recommended in the future the subgroup analysis according to Child-Pugh score, MELD score, or pathological evidence. To sum up, this line of evidence further supported that TAVI provides a safe and effective interventional option for the patients with concurrent chronic liver disease and severe aortic valve stenosis.
Thirdly, we further explored that whether TAVI displays advantages over SAVR in the patients with chronic liver disease. Despite that liver dysfunction is not routinely assessed by EuroSCORE and Society of Thoracic Surgeons (STS score), it still potentially places the patients at an excessive risk of coagulopathy, multiorgan dysfunction, and serious infection. In this group of patients, we demonstrated that compared to SAVR, TAVI associated with a decrease in in-hospital mortality, and need for transfusion. The 2 groups were comparable in the rest outcomes. Similarly, we did not detect any obvious heterogeneity and publication bias. TAVI has been recently attached importance in the patients with high surgical risk and our results again advocated the application of TAVI as a reliable alternative of SAVR in the patients with comorbidities of vital organs. Alqahtani et al also reported that SAVR was among the strongest predictors of in-hospital mortality in the patients with chronic liver disease.[16] In theory, the advantages of TAVI over SAVR on survival are mainly attributable to it nature of less invasive and avoidance of cardiopulmonary bypass. Nevertheless, it is still unclear in the patients with chronic liver disease that how TAVI exactly improves the short-term prognosis compared to SAVR.
Fourthly, liver transplantation offers the best chance for a cure in patients with end-stage liver disease (ESLD) and it is still considered to be one of the highest risk non-cardiac surgeries. Concomitant severe aortic valve stenosis in the patients with ESLD has been traditionally regarded as a contraindication for liver transplantation because it tremendously increases the risk of cardiovascular complications during the intraoperative and postoperative periods.[21] For this group of patients, surgical valve replacement represents a highly risky option and aortic balloon valvuloplasty also brings unpredictable complications like aortic regurgitation, infective endocarditis, and embolism.[22–25] Thus these patients will commonly be removed from the list of eligible candidates. To solve this clinical dilemma, TAVI has been attempted as a bridge therapy to liver transplantation. Several case reports or series have been published to share the successful and promising experiences of TAVI in the patients awaiting liver transplantation.[26,27] However, the case number was still very small and further investigation should be performed to validate the safety and efficacy of TAVI in this group of patients.
Fifthly, for the mid-term and long-term prognosis of patients with chronic liver disease undergoing TAVI, Gabriela Tirado-Conte and his colleagues demonstrated that the compromised eGFR and Child-Pugh class B or C could serve as prognostic factors of mortality following TAVI in the patients with chronic liver disease and no more than 20% of the patients with both factors survived at 2-year follow-up.[15] However, such evidence remains scarce for meta-analysis. Future exploration should be focused to further evaluate the effects of chronic liver disease on the prognosis of patients undergoing TAVI and the advantages of TAVI over SAVR at mid-term and long-term follow-up.
Sixthly, hemostasis in the patients with chronic liver disease is characteristic of deficits of procoagulants, anticoagulants, as well as platelets. Hence, hemostasis balance in those patients might rapidly shift from bleeding to thrombosis, depending on the leading situations. The 2014 American College of Cardiology Foundation (ACC)/American Heart Association (AHA) guidelines recommend that all patients receive daily aspirin and clopidogrel for 6 months after TAVI (Class IIb indication) and persist lifelong aspirin daily (Class IIb indication). And Canadian Cardiovascular Society Statement 2012 indicates that for TAVI. If oral anticoagulation indicated for atrial fibrillation (AF), avoid triple therapy unless definite indication exists. Other consensuses, such as the ACCF/AAATS/SCAI/STS Expert Consensus, have similar recommendations as above mentioned.[28] However, there has been controversy regarding the dual antiplatelet therapy (DAPT) following TAVI due to a potential increased risk of bleeding complications. And several studies revealed no additional benefit from DAPT as compared to single antiplatelet therapy (SAPT). Thus this empirical practise of DAPT requires the evidence-based support from future large-scale clinical trials.[29] For SAVR, oral anticoagulation (e.g., warfarin) is routinely administrated lifelong for mechanical valve and regular monitoring of coagulation indicators is suggested. And for bioprosthetic valve, anticoagulant (e.g., warfarin) or antiplatelet treatment (e.g., aspirin) is indicated for 3 to 6 months in the patients without AF.[30] However, no consensus has to date existed regarding the anticoagulant/antiplatelet strategy after TAVI or SAVR in the patients with chronic liver disease.
4.3. Limitations
Several limitations in our meta-analysis should be paid the attention and the results should be interpreted cautiously. Firstly, no RCTs have been found by our digital search and included in this systematic review and meta-analysis. Secondly, observational studies were potentially a source of bias because of their non-randomized, unblinded design. Thirdly, this meta-analysis contained only 7 studies with a relatively small sample size. Fourthly, differences between the included studies in the liver dysfunction, cardiac function, and other variables have been several possible confounders. Fifthly, it is elusive that whether severe liver disease or even end-stage liver disease could affect the outcomes of patients undergoing TAVI and whether TAVI still remains superior to SAVR in this subgroup of patients. Sixthly, evidence is missing regarding the mid-term and long-term prognosis of patients with chronic liver disease receiving TAVI or SAVR.
5. Conclusions
This systematic review and meta-analysis advocated that TAVI represents a feasible therapeutic alternative of traditional SAVR for the patients with chronic liver disease and concomitant severe aortic stenosis, which showed promising postoperative results. Large-scale randomized controlled trials with mid-term and long-term follow-up are required to further confirm the conclusions.
Author contributions
Data curation: Diming Zhao, Jinzhang Li, Peidong Yuan, Huibo Ma.
Formal analysis: Diming Zhao, Jianlin Zhang, Xiangqian Kong, Liangong Sun, Yuman Zhang.
Investigation: Xiaochun Ma.
Methodology: Xiaochun Ma, Dong Wei, Jiwei Ma, Qiqi Jiao, Zhengjun Wang.
Resources: Xiaochun Ma.
Software: Xiaochun Ma.
Supervision: Xiaochun Ma, Haizhou Zhang.
Writing – original draft: Xiaochun Ma.
Writing – review & editing: Xiaochun Ma, Haizhou Zhang.
Xiaochun Ma: 0000-0003-1055-4691.
Footnotes
Abbreviations: AKI = acute kidney injury, CABG = coronary artery bypass grafting, CIs = confidence intervals, COPD = chronic obstructive pulmonary disease, eGFR = estimated glomerular filtration rate, EuroSCORE = European System for Cardiac Operative Risk Evaluation, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, LVEF = Left ventricular ejection fraction, MELD score = Model for End-Stage Liver Disease score, MOOSE = Meta-analysis of Observational studies in Epidemiology guidelines, NOS = Newcastle-Ottawa scale, NYHA functional class = New York Heart Association Functional Classification, ORs = odds ratios, PRISMA Statement = Preferred Reporting Items for Systematic Reviews and Meta-analyses, RCTs = randomized controlled trials, SAVR = surgical aortic valve replacement, STS score = Society of Thoracic Surgeons, TAVI = transcatheter aortic valve implantation.
How to cite this article: Ma X, Zhao D, Li J, Wei D, Zhang J, Yuan P, Kong X, Ma J, Ma H, Sun L, Zhang Y, Jiao Q, Wang Z, Zhang H. Transcatheter aortic valve implantation in the patients with chronic liver disease: A mini-review and meta-analysis. Medicine. 2020;99:16(e19766).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
XM, DZ, and JL contributed equally to this work.
The study was supported by the National Key R&D Program of China (2017YFC1308000), National Natural Science Foundation of China (81500375 and 81800255), Natural Science Foundation of Shandong Province (ZR2018BH002 and ZR2015HQ001).
The authors have no conflicts of interest to disclose.
References
- [1].Arif R, Seppelt P, Schwill S, et al. Predictive risk factors for patients with cirrhosis undergoing heart surgery. Ann Thorac Surg 2012;94:1947–52. [DOI] [PubMed] [Google Scholar]
- [2].Hayashida N, Shoujima T, Teshima H, et al. Clinical outcome after cardiac operations in patients with cirrhosis. Ann Thorac Surg 2004;77:500–5. [DOI] [PubMed] [Google Scholar]
- [3].Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790–8. [DOI] [PubMed] [Google Scholar]
- [4].Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98. [DOI] [PubMed] [Google Scholar]
- [5].Witberg G, Lador A, Yahav D, et al. Transcatheter versus surgical aortic valve replacement in patients at low surgical risk: a meta-analysis of randomized trials and propensity score matched observational studies. Catheter Cardiovasc Interv: Official Journal of the Society for Cardiac Angiography & Interventions 2018;92:408–16. [DOI] [PubMed] [Google Scholar]
- [6].Tam DY, Vo TX, Wijeysundera HC, et al. Transcatheter vs surgical aortic valve replacement for aortic stenosis in low–intermediate risk patients: a meta-analysis. Can J Cardiol 2017;33:1171–9. [DOI] [PubMed] [Google Scholar]
- [7].Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg: Official Journal of the European Association for Cardio-thoracic Surgery 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- [8].Wendt D, Osswald BR, Kayser K, et al. Society of thoracic surgeons score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann Thorac Surg 2009;88:468–74. discussion 474-465. [DOI] [PubMed] [Google Scholar]
- [9].Gundling F, Seidl H, Gansera L, et al. Early and late outcomes of cardiac operations in patients with cirrhosis: a retrospective survival-rate analysis of 47 patients over 8 years. Eur J Gastroenterol Hepatol 2010;22:1466–73. [DOI] [PubMed] [Google Scholar]
- [10].Jacob KA, Hjortnaes J, Kranenburg G, et al. Mortality after cardiac surgery in patients with liver cirrhosis classified by the Child-Pugh score. Interact Cardiovasc Thorac Surg 2015;20:520–30. [DOI] [PubMed] [Google Scholar]
- [11].Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- [12].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [13].Ma X, Ma C, Yun Y, et al. Safety and efficacy outcomes of preoperative aspirin in patients undergoing coronary artery bypass grafting: a systematic review and meta-analysis. J Cardiovasc Pharmacol Ther 2014;19:97–113. [DOI] [PubMed] [Google Scholar]
- [14].Yassin AS, Subahi A, Abubakar H, et al. Outcomes and effects of hepatic cirrhosis in patients who underwent transcatheter aortic valve implantation. Am J Cardiol 2018;122:455–60. [DOI] [PubMed] [Google Scholar]
- [15].Tirado-Conte G, Rodes-Cabau J, Rodriguez-Olivares R, et al. Clinical outcomes and prognosis markers of patients with liver disease undergoing transcatheter aortic valve replacement: a propensity score-matched analysis. Circ Cardiovasc Interv 2018;11:e005727. [DOI] [PubMed] [Google Scholar]
- [16].Alqahtani F, Aljohani S, Ghabra A, et al. Outcomes of transcatheter versus surgical aortic valve implantation for aortic stenosis in patients with hepatic cirrhosis. Am J Cardiol 2017;120:1193–7. [DOI] [PubMed] [Google Scholar]
- [17].Thakkar B, Patel A, Mohamad B, et al. Transcatheter aortic valve replacement versus surgical aortic valve replacement in patients with cirrhosis. Catheter Cardiovasc Interv: Official Journal of the Society for Cardiac Angiography & Interventions 2016;87:955–62. [DOI] [PubMed] [Google Scholar]
- [18].Shah AM, Ogbara J, Herrmann HC, et al. Outcomes of transcatheter aortic valve replacement in patients with chronic liver disease. Catheter Cardiovasc Interv: Official Journal of the Society for Cardiac Angiography & Interventions 2015;86:888–94. [DOI] [PubMed] [Google Scholar]
- [19].Pascual I, Munoz-Garcia AJ, Lopez-Otero D, et al. Long-term outcome of cirrhotic patients with severe aortic stenosis treated with transcatheter aortic valve implantation. Rev Esp Cardiol (Engl Ed) 2015;68:353–4. [DOI] [PubMed] [Google Scholar]
- [20].Greason KL, Mathew V, Wiesner RH, et al. Transcatheter aortic valve replacement in patients with cirrhosis. J Card Surg 2013;28:492–5. [DOI] [PubMed] [Google Scholar]
- [21].Kertai MD, Bountioukos M, Boersma E, et al. Aortic stenosis: an underestimated risk factor for perioperative complications in patients undergoing noncardiac surgery. Am J Med 2004;116:8–13. [DOI] [PubMed] [Google Scholar]
- [22].Coverstone E, Korenblat K, Crippin JS, et al. Aortic balloon valvuloplasty prior to orthotopic liver transplantation: a novel approach to aortic stenosis and end-stage liver disease. Case Rep Cardiol 2014;2014:325136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Giakoustidis A, Cherian TP, Antoniadis N, et al. Combined cardiac surgery and liver transplantation: three decades of worldwide results. J Gastrointest Liver Dis 2011;20:415–21. [PubMed] [Google Scholar]
- [24].Modi A, Vohra HA, Barlow CW. Do patients with liver cirrhosis undergoing cardiac surgery have acceptable outcomes? Interact Cardiovasc Thorac Surg 2010;11:630–4. [DOI] [PubMed] [Google Scholar]
- [25].Kalarickal P, Liu Q, Rathor R, et al. Balloon aortic valvuloplasty as a bridge to liver transplantation in patients with severe aortic stenosis: a case series. Transplant Proc 2014;46:3492–5. [DOI] [PubMed] [Google Scholar]
- [26].Cabasa AS, Eleid MF, Suri RM. Transcatheter aortic valve replacement for native aortic valve regurgitation as a bridge to liver transplantation. Catheter Cardiovasc Interv: Official Journal of the Society for Cardiac Angiography & Interventions 2016;88:665–70. [DOI] [PubMed] [Google Scholar]
- [27].Silvestre OM, Bacal F, Ramos DS, et al. Transcatheter aortic valve implantation as rescue therapy for liver transplant candidates with aortic valve stenosis. Liver Transplant: Official Publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society 2014;20:1277–9. [DOI] [PubMed] [Google Scholar]
- [28].Geisler T, Droppa M, Muller K, et al. Antithrombotic therapy after TAVR. Curr Vasc Pharmacol 2018;16:437–45. [DOI] [PubMed] [Google Scholar]
- [29].Ma X, Xu Z, Li J, et al. Antiplatelet strategy after transcatheter aortic valve replacement: an updated meta-analysis. J Cardiovasc Surg 2019;60:624–32. [DOI] [PubMed] [Google Scholar]
- [30].Chakravarty T, Patel A, Kapadia S, et al. Anticoagulation after surgical or transcatheter bioprosthetic aortic valve replacement. J Am Coll Cardiol 2019;74:1190–200. [DOI] [PubMed] [Google Scholar]


