Abstract
We assessed the predictive value of serum ammonia level on admission for the 28-day mortality of patients with sepsis.
We retrospectively included septic patients admitted to the emergency department of West China Hospital, Sichuan University and The Fourth People's Hospital of Zigong city from June 2017 to May 2018. Patients were divided into 2 groups according to 28-day survival. Comparisons of serum ammonia level and sequential organ failure assessment (SOFA) score were made between 2 groups. Multivariate logistic regression models were employed to determine independent risk factors affecting 28-day mortality rate, and receiver operating characteristic (ROC) curve was also used to evaluate the efficacy of risk factors.
Total of 316 patients were included into the study, 221 survived to 28 days and 95 were died before 28 days. The 28-day mortality rate was 30.06%. Multivariate logistic regression analyses revealed that the ammonia level, C reactive protein, SOFA score, and the leukocyte were independent risk factors for the 28-day mortality rate. In predicting the 28-day mortality rate, the SOFA score presented an area under the ROC curve (AUC) of 0.815, and the ammonia levels presented the AUC of 0.813.
The ammonia level, C reactive protein, SOFA score, and the leukocyte are independent risk factors for 28-day mortality rate in septic patients. Moreover, the serum ammonia and SOFA score have similar predictive values. The serum ammonia level is also a suitable early indicator for prognostic evaluation of patients with sepsis as well.
Keywords: mortality rate, ROC curve, sepsis, serum ammonia, SOFA score
1. Introduction
Sepsis is an acute and critical disease in emergency departments. Its acute onset, rapid progress, and high mortality rate make timely assessment of the disease and its prognosis particularly important for physicians specializing in severe acute diseases.[1–3] Systemic multiple organ dysfunction is an essential feature of sepsis,[4,5] and the liver is commonly involved in the multiple organ dysfunction syndrome caused by sepsis. Liver dysfunction can lead to coagulation dysfunction and ammonia metabolism disorder, lead to abnormal blood coagulation and elevated serum ammonia level.[6,7] In recent years, studies proved that sepsis may cause swelling of intestinal mucosa, leading to defective barrier function and intestinal bacterial translocation, which aggravate systemic infection and cause additional ammonia in the intestine to be absorbed into the blood.[8,9] In addition, patients with sepsis are generally undergoing the decomposition of massive amount of amino acids while ammonia production appears to increase, due to the highly active metabolic rates and negative nitrogen balance. Numan et al[3] suggested that the elevated ammonia was related to longer hospital stay for patients with sepsis. However, whether serum ammonia levels can be used to evaluate prognosis among septic patients is yet to be determined. In this study, we investigated the role of serum ammonia level on the 28-day mortality evaluation of patients with sepsis through a retrospective analysis of septic patients. We hypothesized that the serum ammonia level on admission was associated with the 28-day mortality of the patients with sepsis.
2. Methods
2.1. Patient enrolment
This was a 2-center retrospective study. The definition of sepsis was based on the third international consensus definitions for sepsis and septic shock.[10] Septic patients admitted to the Emergency Department of the West China Hospital, Sichuan University and The Fourth People's Hospital of Zigong city from June 1, 2017 to May 30, 2018 were enrolled and divided into the survival group and death group according to the 28-day survival.
2.2. Inclusion and exclusion criteria
Inclusion criteria for the study were:
-
(1)
patients who visited the emergency department of the West China Hospital, Sichuan University and Zigong Fourth People's Hospital with the diagnosis of sepsis and age ≥18 years;
-
(2)
patients with essential data for this study.
Exclusion criteria were:
-
(1)
patients with acute or chronic liver disease;
-
(2)
gastrointestinal bleeding;
-
(3)
malignant tumor(s);
-
(4)
recent administration of immunosuppressive agents or high-dose hormones;
-
(5)
human immunodeficiency virus infection;
-
(6)
pregnancy;
-
(7)
missing follow-up.
2.3. Methods
The study was in compliance with the Declaration of Helsinki and was approved by the Human Ethical Committee of West China Hospital of Sichuan University (No. 2017 (279)). Vital signs (blood pressure, heart rate, body temperature, SPO2) and Glasgow Coma scale (GCS) score were measured in the emergency department. The patients were examined for blood routine, biochemical indicators, and other clinical factors immediately after admission to the emergency department. All patient data were collected and analyzed using the electronic medical record system of the hospital. Survival was determined by 28 days follow-up after admission.
2.4. Quality control
The data were double-checked, and the recording of the data was supervised by the administrative team. When the data verification was observed to be inconsistent, the medical team leader verified the data to ensure its authenticity and reliability. Follow-up was carried out by a dedicated person. Wrong telephone numbers, 3 unanswered calls at different time or not being amenable to follow-up was defined as lost to follow-up.
2.5. Statistical analysis
The sample size was calculated based the serum ammonia. A 2-sided P-value was set as .05 and power of the test was set as 80%. We estimated that at least 5 variables were tested simultaneously in multivariate regression models. According to the variable number was required for at least 10 times of sample size of the outcome events, the minimal number of patients with 28-day mortality was 50. The former study in our hospital showed the 28-day mortality of the septic patients of approximately 30%. Therefore, a minimal sample size was about 167. Considering the number of excluded and lost follow up patients, we finally determined a necessary sample size of at least 250.
Continuous data were expressed as mean ± standard deviation. Categorical data were expressed as counts and proportions. Assessment of normality of continuous variables was conducted using the Skewness–Kurtosis test. Logistic regression analysis was used to estimate the risk factors. The receiver operating characteristic (ROC) curves were used to evaluate the predictive value of serum ammonia level for prognosis of septic patients. Pairwise comparison of ROC curve was used to compare the difference of areas under the curve (AUC) among different groups. The area under the curve (AUC) >0.75 was defined as good predictive value. A 2-sided P-value < .05 was considered statistically significant for all tests. All analyses were performed using SPSS statistical software version 24.0 (IBM Corporation, Armonk, NY) and Medcalc version 18.2.
3. Results
3.1. Baseline information
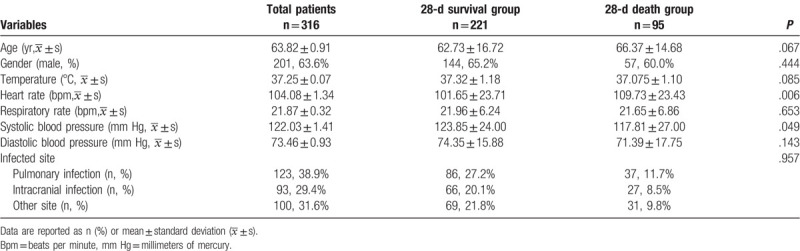
A total of 316 septic patients were enrolled in this study, including 201males (63.6%), with an average age of 63.82 ± 0.91 years. Total of 95 patients died before 28 days. Baseline information such as age, sex, body temperature, respiratory rate, and blood pressure did not significantly differ between the 2 groups (P > .05). However, the heart rate in the survival group was significantly lower than that in the death group (P < .05) (Table 1).
Table 1.
General clinical data of 28-d surviving group and death group in patients with sepsis.
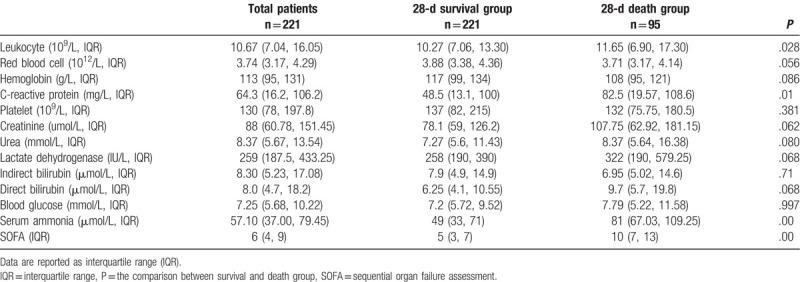
The leukocyte, C-reactive protein (CRP), total bile acid, serum ammonia and SOFA score were significantly lower in the 28-day survival group than those in the death group (P < .05), while the GCS score were significantly higher in the survival group than those in the death group (P < .05) (Table 2).
Table 2.
Comparison of laboratory indicators and related scores between 28-d surviving group and death group in sepsis.
3.2. Univariate logistic regression analyses of 28-day mortality rate in patients with sepsis
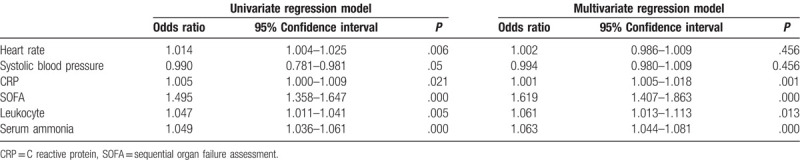
Univariate logistic regression analyses were employed to analyze the risk factors that featured statistical differences between the 28-day survival group and the death group. It was observed that heart rate, systemic blood pressure, CRP, SOFA score, leukocyte, and serum ammonia level are the risk factors affecting the prognosis of septic patients, as shown in Table 3.
Table 3.
Univariate and multivariate logistic regression analysis of 28-d mortality in patients with sepsis.
3.3. Multivariate logistic regression analyses of factors affecting 28-day mortality in patients with sepsis
The risk factors in univariate logistic regression analyses were further performed multivariate logistic regression analysis. After the confounder adjustment, the CRP (odds ratio [OR] 1.001, 95% confidence interval [CI] 1.005–1.018, P = .001), SOFA score (OR 1.619, 95% CI 1.407–1.863, P < .001), leukocyte (OR 1.061, 95% CI 1.013–1.113, P = .013), and serum ammonia level (OR 1.063, 95% CI 1.044–1.081, P < .001), were independent risk factors affecting the 28-day mortality rate of septic patients, as shown in Table 3.
3.4. Predictive value of the independent risk factors
The predictive value of CRP, SOFA score, leukocyte, and ammonia level in predicting 28-day mortality risk of septic patients was analyzed using the ROC curve. Both the SOFA score and the serum ammonia had the ideal predictive value, with the area under the ROC curve (AUC) of 0.815, and 0.813. The best cut-off value for serum ammonia was 61.1 μmol/L, with an estimated sensitivity and specificity of 0.77 and the 0.70, respectively (Table 4 and Fig. 1).
Table 4.
ROC curve analysis of prediction for 28-d mortality in patients with sepsis.
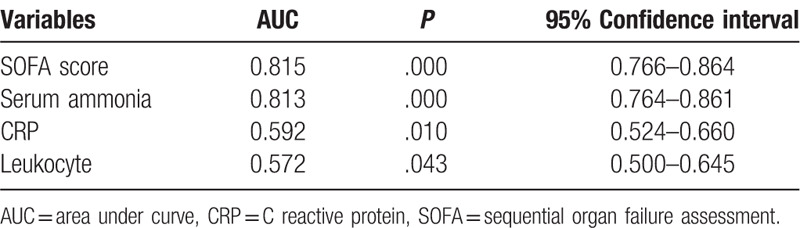
Figure 1.
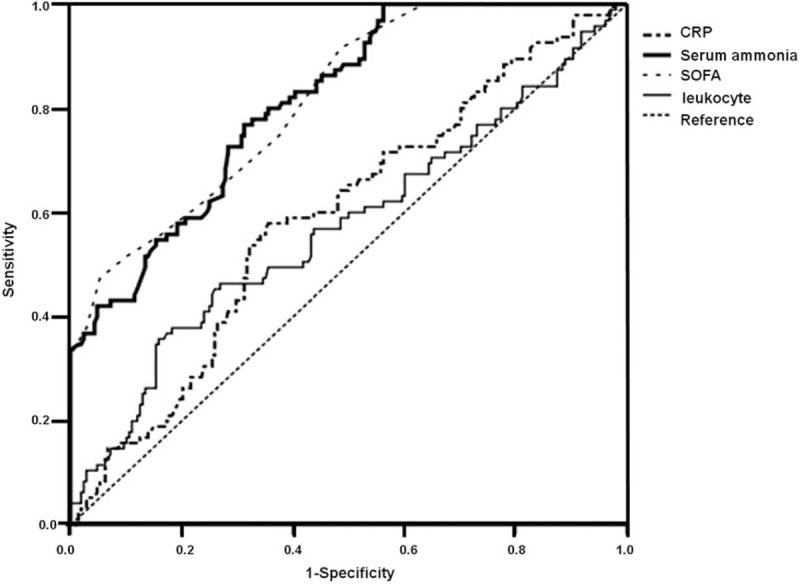
ROC curve of serum ammonia, CRP, leukocyte and SOFA score to predict 28-d mortality in patients with sepsis. CRP = C-reactive protein, ROC = receiver operating characteristic, SOFA = sequential organ failure assessment.
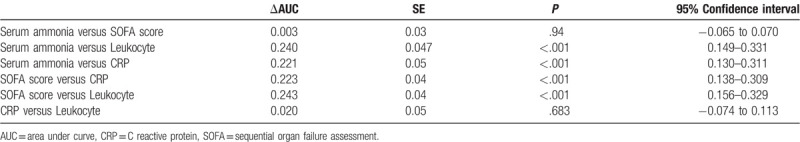
Further analysis revealed that the AUC of ammonia is significantly higher than the leukocyte (P < .001) and CRP (P < .001). The AUC of ammonia is significantly higher than the leukocyte (P < .001) and CRP (P < .001) as well (Table 5).
Table 5.
Comparison of efficacy of different risk factors in evaluating the 28-d mortality in patients with sepsis.
4. Discussion
In this 2-center retrospective study, we demonstrated that elevated ammonia level on admission was significantly higher in septic patients with 28-day mortality. Using multivariate regression analyses, we founded that the heart rate, CRP, SOFA score, and the serum ammonia levels were independent risk factors for the prognosis of septic patients. Fever and systemic hypermetabolism can lead to increased heart rates in septic patients.
The elevated serum ammonia level on admission in patients with sepsis was found to be an independent risk factor that affects their prognosis. The possible explanations are as follows: first, sepsis may lead to hepatic dysfunction, which impairs the capacity of ammonia metabolism and cause the serum ammonia level elevation. That is to say, serum ammonia cannot be metabolized normally with impaired liver function, and the accumulation of ammonia in the body leads to increased concentrations of ammonia in the blood. Recent studies have found that abnormal liver function occurs at an early stage of sepsis, which leads to the liver metabolism dysfunction and metabolic waste accumulation in the body.[11,12] It has also been shown that sepsis leads to a marked deterioration of prognosis and a significant increase in mortality rate in patients with abnormal liver function.[13] The pathophysiological mechanisms that may underlie this are associated with the activation of macrophages in liver, abnormal distribution of liver blood flow, ischemia-and hypoxia-activated oxidative stress in liver tissues, and the injury of vascular endothelial cells.[14–20] Second, sepsis also results in gastrointestinal mucosa swelling, defective barrier function, and intestinal bacterial translocation. Furthermore, enhanced ammonia absorption into the blood is another possible explanation. In recent years, it has been confirmed by a series of studies that acute gastrointestinal injury caused by sepsis is a cause leading to the aggravation and poor prognosis of septic patients.[21–23] Therefore, ammonia levels are associated with abnormal liver function and acute injury to the gastrointestinal tract in septic patients; thus, this is an independent risk factor for poor prognosis and increased mortality rate in sepsis.
This study also indicated that the CRP, leukocyte and SOFA score is an independent risk factor for 28-day mortality in patients with sepsis. The CRP and leukocyte level reflect the extent of infection and body systemic inflammation response in some extent, which was quite common in patients with critical infections.[24–26] The results reminded us that the systemic inflammation had great effects on the prognosis of septic patients. Timely and effective interventions to alleviate the inflammation, such as the early antibiotics treatment, were quite important for improving outcomes.[27] Similar to other studies, we also demonstrated the association between the SOFA score and the outcome of patients with sepsis. SOFA score reflects the degree of systemic multiple organ dysfunction, and it is widely accepted as a prognostic assessment for septic patients.[28–31]
4.1. Clinical implications
In the present study, we found that both the ammonia level and the SOFA score had similar predictive value for the prognosis of patients with sepsis. However, given the complexity of calculating the SOFA score and its requirement for the assessment of numerous indicators, in contrast, the ammonia level in serum is a single parameter, and it is easy to obtain in the course of clinical practice. It has unique advantages for the early prognostic assessment of septic patients, especially in the work of emergency physicians performing rapid assessments of patients with sepsis. Furthermore, based on our findings, we suggested that early interventions to protect the liver function and reduce the serum ammonia level might be helpful to improve the prognosis of patients with sepsis.
5. Limitations
This study has some limitations. First, as a retrospective study, some unmeasured confounders might influence the final results. But we conducted the study in the 2 large medical centers with adequate sample size to minimize the underlying effects. Second, certain biochemical indicators and clinical symptoms are missing for some septic patients included in this study and could not be further confirmed, which may have led to bias in research findings. Finally, because our hospital is a regional critical care center, several sepsis patients here are already critically ill patients referred from other hospitals. Therefore, if the septic patients included in this study had more severe systemic syndromes, including multiple organ dysfunction, higher SOFA score, and serum ammonia level, than the average population of septic patients, this may be one a main reason that ammonia level was an independent risk factor for prognosis.
Nevertheless, it remains necessary to compare these results with those of other studies, and caution must be urged with regard to the effectiveness of serum ammonia in the prognostic evaluation of all patients with sepsis.
6. Conclusion
Serum ammonia and SOFA score are independent risk factors for 28-day mortality rate in septic patients. But serum ammonia might be a more suitable early prognostic indicator for evaluation in patients with sepsis.
Acknowledgments
Thanks to all the participants for their work of the data collection and analysis.
Author contributions
Conceptualization: Yu Cao, JunZhao Liu.
Data curation: Jie Zhao, Ping Xu.
Formal analysis: Sheng Ye.
Investigation: Jie Zhao.
Methodology: JunZhao Liu, Ping Xu.
Project administration: Yu Cao.
Resources: Ping Xu.
Software: YaRong He.
Supervision: YaRong He.
Writing – original draft: Jie Zhao.
Yu Cao orcid: 0000-0003-4715-7331.
Footnotes
Abbreviations: AUC = area under the curve, CI = confidence interval, CRP = C-reactive protein, GCS = Glasgow Coma scale, OR = odds ratio, ROC = receiver operating characteristic, SOFA = sequential organ failure assessment.
How to cite this article: Zhao J, He Y, Xu P, Liu J, Ye S, Cao Y. Serum ammonia levels on admission for predicting sepsis patient mortality at D28 in the emergency department: A 2-center retrospective study. Medicine. 2020;99:11(e19477).
The present work was supported by the National Natural Science Foundation of China (Grant Nos. 81471836 and 81772037 to YC, No. 81801883 to YRH), the Discipline Excellence Development 1•3•5 Project of West China Hospital, Sichuan University (Grant No. ZYJC18019) to YC, and the Chengdu Science and Technology Huimin Project (Grant No. 2016-HM02-00099-SF) to YC.
The authors have no conflicts of interest to disclose.
References
- [1].Churpek MM, Zadravecz FJ, Winslow C, et al. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med 2015;192:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Prucha M, Bellingan G, Zazula R. Sepsis biomarkers. Clin Chim Acta 2015;440:97–103. [DOI] [PubMed] [Google Scholar]
- [3].Numan Y, Jawaid Y, Hirzallah H, et al. Ammonia vs. lactic acid in predicting positivity of icrobial culture in sepsis: the ALPS pilot study. J Clin Med 2018;7:E182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jawad I, Luksic I, Rafnsson SB. Assessing available information on the burden of sepsis: global estimates of incidence, prevalence and mortality. J Glob Health 2012;2:010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Galic S, Csuka D, Prohászka Z, et al. A case report of a child with sepsis induced multiorgan failure and massive complement consumption treated with a short course of Eculizumab: a case of crosstalk between coagulation and complement? Medicine (Baltimore) 2019;98:e14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Woźnica EA, Inglot M, Woźnica RK, et al. Liver dysfunction in sepsis. Adv Clin Exp Med 2018;27:547–51. [DOI] [PubMed] [Google Scholar]
- [7].Wang D, Yin Y, Yao Y. Advances in sepsis-associated liver dysfunction. Burns Trauma 2014;2:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fay KT, Ford ML, Coopersmith CM. The intestinal microenvironment in sepsis. Biochim Biophys Acta Mol Basis Dis 2017;1863:2574–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Reintam A, Parm P, Kitus R, et al. Gastrointestinal failure score in critically ill patients: a prospective observational study. Crit Care 2008;12:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Squires JE, McKiernan P, Squires RH. Acute liver failure: an update. Clin Liver Dis 2018;22:773–805. [DOI] [PubMed] [Google Scholar]
- [12].Rajaram P, Subramanian R. Management of acute liver failure in the intensive care unit setting. Clin Liver Dis 2018;22:403–8. [DOI] [PubMed] [Google Scholar]
- [13].La Mura V, Pasarín M, Meireles CZ, et al. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction. Hepatology 2013;57:1172–81. [DOI] [PubMed] [Google Scholar]
- [14].Cai J, Zhang M, Han T, et al. Characteristics of infection and its impact on short-term outcome in patients with acute-on-chronic liver failure. Medicine (Baltimore) 2017;96:e8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marshall JC. New translational research provides insights into liver dysfunction in sepsis. PLoS Med 2012;9:e1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Norris EJ, Larion S, Culberson CR, et al. Hydrogen sulfide differentially affects the hepatic vasculature in response to phenylephrine and endothelin 1 during endotoxemia. Shock 2013;39:168–75. [DOI] [PubMed] [Google Scholar]
- [17].Eum HA, Park SW, Lee SM. Role of nitric oxide in the expression of hepatic vascular stress genes in response to sepsis. Nitric Oxide 2007;17:126–33. [DOI] [PubMed] [Google Scholar]
- [18].Traeger T, Mikulcak M, Eipel C, et al. Kupffer cell depletion reduces hepatic inflammation and apoptosis but decreases survival in abdominal sepsis. Eur J Gastroenterol Hepatol 2010;22:1039–49. [DOI] [PubMed] [Google Scholar]
- [19].Tamandl D, Jørgensen P, Gundersen Y, et al. Nitric oxide administration restores the hepatic artery buffer response during porcine endotoxemia. J Invest Surg 2008;21:183–94. [DOI] [PubMed] [Google Scholar]
- [20].Semeraro F, Colucci M, Caironi P, et al. Platelet drop and fbrinolytic shutdown in patients with sepsis. Crit Care Med 2018;46:e221–8. [DOI] [PubMed] [Google Scholar]
- [21].Piton G, Belon F, Cypriani B, et al. Enterocyte damage in critically ill patients is associated with shock condition and 28-day mortality. Crit Care Med 2013;41:2169–76. [DOI] [PubMed] [Google Scholar]
- [22].Reintam Blaser A, Malbrain ML, Starkopf J, et al. Gastrointestinal function in intensive care patients: terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med 2012;38:384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang D, Fu R, Li Y, et al. Comparison of the clinical characteristics and prognosis of primary versus secondary acute gastrointestinal injury in critically ill patients. J Intensive Care 2017;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Djordjevic D, Rondovic G, Surbatovic M, et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia? Mediators Inflamm 2018;2018:3758068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li W, Ai X, Ni Y, et al. The association between the neutrophil-to-lymphocyte ratio and mortality in patients with acute respiratory distress syndrome: a retrospective cohort study. Shock 2019;51:161–7. [DOI] [PubMed] [Google Scholar]
- [26].Basile-Filho A, Lago AF, Menegueti MG, et al. The use of APACHE II, SOFA, SAPS 3, C-reactive protein/albumin ratio, and lactate to predict mortality of surgical critically ill patients: a retrospective cohort study. Medicine (Baltimore) 2019;98:e16204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen CH, Chen YM, Chang YJ, et al. Continuous versus intermittent infusions of antibiotics for the treatment of infectious diseases: meta-analysis and systematic review. Medicine (Baltimore) 2019;98:e14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Desai S, Lakhani JD. Utility of SOFA and APACHE II score in sepsis in rural set up MICU. J Assoc Physicians India 2013;61:608–11. [PubMed] [Google Scholar]
- [29].Song JU, Sin CK, Park HK, et al. Performance of the quick sequential (sepsis-related) organ failure assessment score as a prognostic tool in infected patients outside the intensive care unit: a systematic review and meta-analysis. Crit Care 2018;22:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gursel G, Demirtas S. Value of APACHE II, SOFA and CPIS scores in predicting prognosis in patients with ventilator-associated pneumonia. Respiration 2006;73:503–8. [DOI] [PubMed] [Google Scholar]
- [31].Yousef AA, Suliman GA. The predictive prognostic values of serum TNF-( in comparison to SOFA score monitoring in critically ill patients. Biomed Res Int 2013;2013:258029. [DOI] [PMC free article] [PubMed] [Google Scholar]


