Abstract
Background:
MRI findings of carotid plaque components have been studied recently as a tool to predict recurrent ischemic events. We performed a systematic review and meta-analysis to summarize the association of MRI-determined intraplaque hemorrhage, lipid-rich necrotic core, and thinning/rupture of the fibrous cap with recurrent ischemic events.
Methods:
Electronic search was performed in PUBMED, EMBASE, Cochrane Controlled Register of Trials (CENTRAL) from inception to Oct 30, 2018. We included cohort studies with an average follow-up time of more than 1 month in which intraplaque hemorrhage, lipid-rich necrotic core, or thinning/rupture of the fibrous cap were associated with recurrent ipsilateral stroke or ischemic events. We performed heterogeneity assessment before carrying out meta-analysis. According to the heterogeneity, we selected fixed-effect model for meta-analysis of the included cohort studies.
Results:
Using a prespecified search strategy, of the 2128 articles, 6 studies with a total number of 621 participants met eligibility for systematic review and meta-analysis. The hazard ratios of intra-plaque hemorrhage, thinning/rupture of the fibrous cap and lipid rich necrotic core as recurrent Stroke/Transient ischemic attack (TIA) were 7.14(95% confidence interval, 4.32 to 11.82), 5.68(95% confidence interval, 2.40 to 13.47), and 2.73(95% confidence interval, 1.04 to 7.16), respectively. No significant heterogeneity was found in the 3 meta-analyses.
Conclusions:
The presence of intraplaque hemorrhage, lipid-rich necrotic core, and thinning/rupture of the fibrous cap on MRI of carotid plaque are strong predictors of recurrent stroke events. However, due to the lack of original studies, larger cohort studies are warranted.
Keywords: carotid plaque, magnetic resonance imaging, recurrent stroke, systematic review
1. Introduction
Stroke is the second most important cause of death in the world.[1] As one of the primary contributors to ischemic stroke, large-artery atherosclerosis has been commonly found in patients who suffered from ischemic stroke.[2] Previous studies have shown that the degree of carotid artery stenosis is a biological indicator to evaluate the occurrence of stroke events.[3] However, some studies have shown that carotid artery high-risk plaques are significantly correlated with cerebrovascular events.[4,5] The high-risk plaques are defined as a plaque with a large lipid necrotic core, intraplaque hemorrhage, or rupture of the plaque surface. Subsequent studies have demonstrated that the components of carotid atherosclerotic plaques that contribute to carotid artery stenosis might be well detected by magnetic resonance imaging and also used as a tool for predicting and assessing stroke risk.[6,7] MRI description of specific plaque components of IPH, LRNC, and TRFC could provide additional assessment and measurement for assessing the occurrence of stroke events.[6–17] Moreover, whether they could continue to be used as tools for predicting and evaluating the risk of recurrence of stroke events needs to be further confirmed. Meanwhile, recent studies have shown that IPH, TRFC, and LRNC can be used as biological indicators to predict recurrent cerebral ischemic events.[9–11,14,15,18,19] However, MRI-determined plaque components and the study of recurrent ischemic events are relatively new projects. Individual studies are small, and the differences among various studies were unclear. In addition, whether there is a difference in the risk distribution of specific plaque components still remain unclear, such as intra-plaque hemorrhage (IPH), lipid rich necrotic core (LRNC), and thinning/rupture of the fibrous cap (TRFC). Therefore, we conducted a systematic review and meta-analysis to further confirm whether the components of carotid artery plaque can be used as an independent biological indicator to predict and evaluate the risk of stroke recurrence.
2. Materials and methods
Study design and implementation were based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.[20]
2.1. Ethical review
Ethical or board review approval was not required because this study did not include any human participants or animals.
2.2. Study registration
The protocol of this systematic review and meta-analysis has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42019124043 on February 19, 2019.
2.3. Study eligibility criteria
We included cohort studies, with language limited to English. Inclusion criteria were as follows:
-
1.
English language articles;
-
2.
MRI of carotid vessel plaque composition;
-
3.
mean follow-up >1 month;
-
4.
assessment for development of recurrent ipsilateral stroke or recurrent TIA;
-
5.
designed as prospective cohort study;
-
6.
measuring any of the following plaque elements: IPH, LRNC, and TRFC. Eligible studies were up to all the inclusion standard. Case reports and reviews were excluded.
In cases where outcome data was not clear, we attempted to contact the corresponding author for more specific details.
2.4. Information search and data collection
Electronic search were performed in PUBMED, EMBASE, Cochrane Controlled Register of Trials (CENTRAL) from inception to Oct 30, 2018. The reference list of previously published systematic reviews focusing on predicting the risk of stroke and eligible studies were searched. We manually retrieved conference proceedings and academic exchange summaries. We used the Mesh terms and keywords in combination: carotid artery, carotid plaque, atherosclerosis, stroke, recurrent, recurrence, ischemic events. The search strategy for PubMed is shown in Table 1 and this strategy also were used on other data databases.
Table 1.
Search strategy used in PubMed.

2.5. Data extraction
The 2 reviewers independently extracted data for each eligible study: first author's last name, year of publication, country of origin, number of male and female patients, baseline risk factors, and adjusted HR results. Any differences were resolved by consensus.
2.6. Risk of bias assessment
Two reviewers independently assessed the methodological quality of the included studies with the Newcastle–Ottawa Scale (NOS) (Wells et al., 2009) for cohort studies, which consists of 3 parameters of quality: selection, comparability, and outcome assessment. The NOS assigns a maximum score of 4 for selection, 2 for comparability, and 3 for outcome. Hence, a score of 9 is the highest and reflects the highest quality. Discrepancies were addressed in consultation with the third one.
2.7. Statistical analyses
All studies that reported HR results or provided appropriate data which were obtained by Univariate Cox Regression analysis for HR calculation were selected for meta-analyses. Due to differences in study size, follow-up time, magnetic resonance imaging technique, and patient characteristics, we used a relatively conservative fixed-effect model to incorporate HR. Heterogeneity was measured using the I2 statistic, and the I2 statistic, this statistic yields results ranged from 0 to 100% (I2 = 0%–25%, no heterogeneity; I2 = 25%–50%, moderate heterogeneity; I2 = 50%–75%, large heterogeneity; and I2 = 75%–100%, extreme heterogeneity. If there was very large heterogeneity between studies, subgroup analysis would be conducted according to magnetic resonance sequence. If heterogeneity existed, the random-effects model was used, otherwise, the fixed-effects model was used. In addition, funnel plots were also conducted to find a potential publication bias. We performed subgroup analyses of the IPH group and stratified them according to whether the multi-sequence carotid coil magnetic resonance technique was used. At the same time, the amount of studies reporting HR results of LRNC and TRFC was not enough for subgroup analyses, so we decided to use words to describe them. The number of studies included in the meta-analysis was insufficient for publication bias analysis. All data analyses were conducted by RevMan 5.3 (Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) software provided by Cochrane collaboration (www.cochrane.org)
3. Results
3.1. Study selection
A total of 2128 studies were retrieved through retrieval methods and related literatures, among which 14 literatures that met the inclusion criteria were selected through reading abstracts (OA_ Guidelines Flow Diagram). Among the 14 studies, 1 study was excluded due to the plaque components were obtained by histopathological analysis from endarterectomy and 3 studies were excluded due to the absence of specific effect size indicators that could not meet the requirements. In addition, 4 literatures were excluded due to the case-control studies did not meet the inclusion criteria. The remaining 6 studies met eligibility for the systematic review.
3.2. Characteristics of included studies
All 6 articles included in the selection criteria are prospective cohort studies (Table 2). The plaque characteristics of the 4 studies, the diagnostic criteria of IPH were consistent: the signal intensity of the plaque exceeded that of adjacent skeletal muscle by more than 50%.[9–11,14] Plaques in 2 studies were evaluated by specialized vascular wall analysis software.[15,18] All plaque analyses used Kaplan–Meier analysis and univariate Cox regression analysis to explore the effect of plaque characteristics on the recurrence rate of cerebral ischemia (stroke, TIA or AmF). The results of the 6 studies (recurrent ischemic events) were recorded by clinical evaluators during follow-up, while all the recurrent strokes in 5 studies were confirmed by neuroimaging,[9–11,14,15] and 1 study was prospectively assessed by clinical investigators before follow-up.[11] All results were recorded by follow-up, which lasted more than 1 month. Two studies were followed up to clinical endpoint events (carotid endarterectomy or death).[11,14] Six studies included IPH in their results,[9–11,14,15,18] while 2 reported TRFC[15,18] and also 2 focused on LRNC.[15,18] For the 6 original studies, 4 studies performed in United Kingdom,[5–7,10] 1 in China[18] and 1 in Netherlands.[15] The age of the population concerned in all the studies was 66 to 73, and male accounted for a large proportion in all the 7 articles (range, 64%–75%). The proportion of hypertension in the study population ranged from75% to 79% in 6 articles,[9–11,14,15,18] only 3 articles collected the proportion of heart disease patients[9,10,14] and the proportion of smokers in 6 articles ranged from 38% to 70%.[9–11,14,15,18] There were considerable differences in the degree of carotid artery stenosis collected in 6 articles: 3 studies focused on moderate to severe (≥50%) stenosis[10,11,14]; 2 studies focused on mild to moderate (40% 70%) stenosis[9,15]; 1 study did not give information on the extent of carotid artery stenosis.[18] Three studies were from the same research group.[9–11] The time range and characteristics of the 3 studies were different, and there were no 2 or 3 studies covered by the same population.
Table 2.
Overview of patient characteristics in studies evaluating the risk of recurrent stroke in patients with carotid plaque MRI.
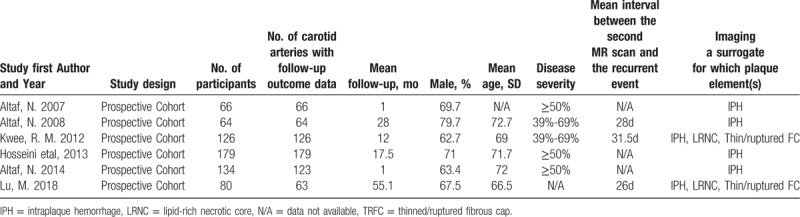
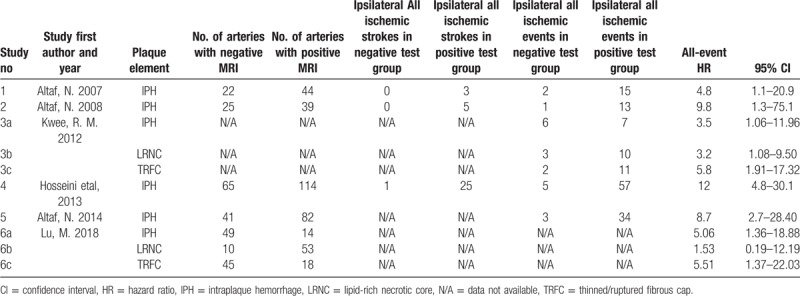
Five studies were performed on 1.5-T MRI, 1 study on 3.0-T MRI. In the IPH studies, 4 studies used multiple sequences to assess carotid plaque, and 2 studies used a separate standard carotid coil to detect IPH. In the original 6 studies, only 3 studies divided the results into ipsilateral stroke and ipsilateral TIA, whereas the remaining 3 studies did not distinguish the results. More detailed classification information such as MRI test methods and outcome indicators are provided in Tables 2 and 3.
Table 3.
Overview of MRI plaque testing characteristics and risk of recurrent ipsilateral cerebrovascular events.
3.3. Quality of included studies
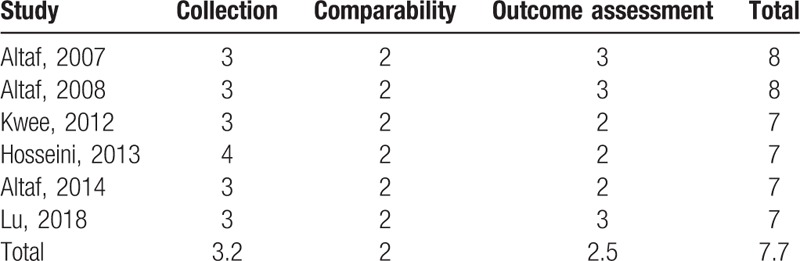
The quality evaluation of all the studies meeting the inclusion criteria of Meta were presented in Table 4. Both evaluators agreed on the quality of the study. All the studies were of high quality. The most common selection bias were derived from the determination of exposure factors. In terms of comparability bias, all the studies included adequate matching or adjustments (eg, age, and sex). The most common exposure bias were due to lack of integrity of follow-up records.
Table 4.
Quality of selected literature.
3.4. Meta-analysis results
All the included studies met the criteria for systematic meta-analysis, including 6 studies evaluating IPH, 2 studies evaluating LRNC, and 2 studies evaluating TRFC. In the IPH-characterized group, a total of 621 patients were meta-analyzed. In the LRNC-characterized group, 189 patients were meta-analyzed. Finally, in the TRFC-characterized group, a total of 189 patients were meta-analyzed. No significant heterogeneity was noted in the 2 primary analyses. We found a significant positive relationship between IPH, LRNC, and TRFC and the risk of recurrent ischemic events (stroke plus TIA), with a fixed-effects HR of 7.14 (95% CI, 4.32–11.82), 2.73 (95% CI, 1.04–7.16), and 5.68 (95% CI, 2.40–13.67), respectively, for each specific plaque element (Figs. 1–3). And no publication bias was found in funnel plot of IPH (Fig. 4).
Figure 1.
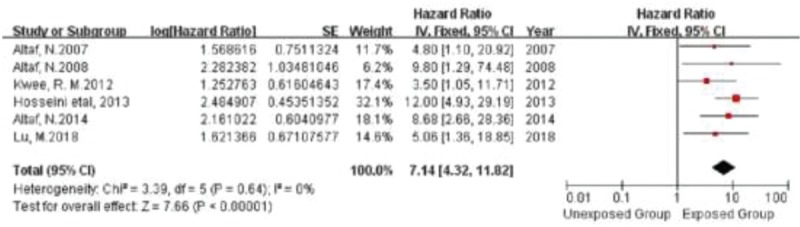
Forrest plot showing single studies included in the analysis for the risk associated with the presence of IPH.
Figure 3.
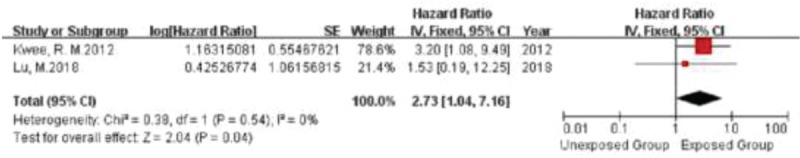
Forrest plot showing single studies included in the analysis for the risk associated with the presence of TRFC.
Figure 4.

Funnel plot of IPH.
Figure 2.

Forrest plot showing single studies included in the analysis for the risk associated with the presence of LRNC.
3.5. Subset analyses
Because there were only 2 studies on TRFC and LRNC respectively, subgroup analysis could not be conducted. Furthermore, no significant heterogeneity was found in the subgroup analysis of IPH. No significant difference in HR was found in the grouping of IPH studies with or without the use of dedicated carotid coil multi-sequence MRI (Fig. 5). And no publication bias was found in funnel plot of the subgroup of IPH (Fig. 6).
Figure 5.
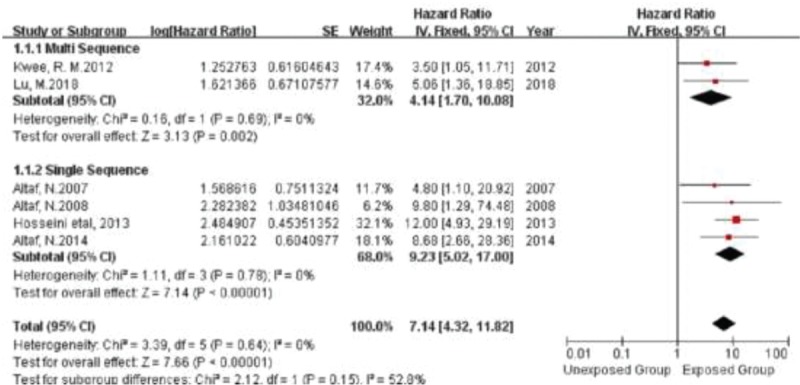
Forrest plot demonstrating multi sequence and single sequence studies included in the analysis for the risk associated with the presence of IPH.
Figure 6.
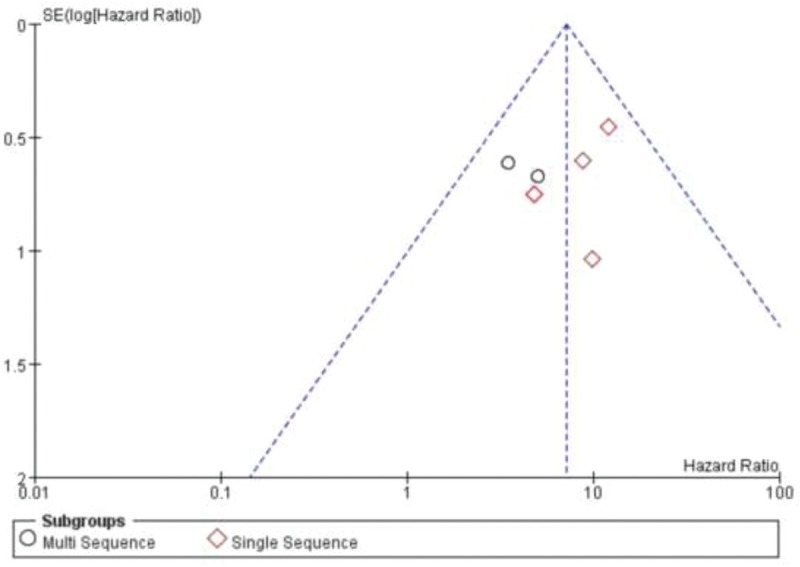
Funnel plot showing publication bias.
4. Discussion
The detection of carotid artery plaque has become the main sign of predicting the occurrence of stroke events and guiding the direction of clinical prevention and treatment.[13] In recent years, some studies have shown that the presence of carotid artery plaque characteristics can predict the occurrence of recurrent cerebral ischemic events.[14,15,18,19] However, at present, there is a lack of systematic evidence that the characteristic components of carotid artery plaques can predict the recurrence of cerebral ischemic events. Therefore, it is necessary to conduct a meta-analysis to present systematic evidence.
In our meta-analysis, we found that carotid artery plaques with IPH, TRFC, or LRNC were more likely to contribute to recurrence of ipsilateral ischemic events. In this comprehensive meta-analyses of the characteristic components of carotid artery plaques and ipsilateral recurrent ischemic events, we found that HRs of IPH, TRFC, and LRNC ranged from 2 to 8, among which 6 studies could be used for IPH meta-analysis,[9–11,14,15,18] and only 2 original researches data could be used for TRFC and LRNC analysis.[15,18] Till now, this is the first systematic meta-analysis that evaluate the effect of IPH, TRFC, and LRNC on recurrent ischemic events. In our present study, the HRs of IPH, TRFC and LRNC, and recurrent ischemic events were 7.14, 5.68, and 2.73, respectively. In this meta-analysis, data from 6 original studies were included.[9–11,14,15,18] A total of 621 patients with carotid artery plaque who had follow-up records were included, among whom male patients accounted for 73%.
HR for IPH was statistically significant when data analysis was limited to studies using multi-sequence or single-sequence carotid coil MRI. Meanwhile, the original studies on TRFC and LRNC were little, further subgroup analysis could not be achieved.[15,18]
In our study, the HRs of IPH, TRFC, and LRNC were statistically significant, while no significant heterogeneity was found. In addition, the study revealed that the HR was the highest in the presence of IPH (7.14), while HR was the lowest in the presence of LRNC (2.73). In addition, and HR was in the middle of the 2 plaque components in the presence of TRFC (5.68). Meanwhile, the rank of HRs of the 3 plaques in the analysis results was different from that of the progression of plaque components. According to the AHA classification scheme, carotid artery plaque progression, and formation occur before the atherosclerosis and lipid composition of deposits, thus forming artery atheromatous plaque. Plaque bleeding can occur in stable plaques and then become unstable plaque, the unstable plaque has a greater risk for FC broken and damaged surface patches, eventually lead to the carotid artery embolism.[21] In 6 studies, only 2 studies investigated the effect of 2 or more plaque elements on recurrent ischemic events,[15,18] 4 of which only studied IPH. None of the studies examined the significance of the 3 plaque elements in the composite plaque for the risk of recurrent ischemic events in a multi-parameter test. Moreover, no study separated TIA and Stroke from HRs of the 3 plaque elements, and only complex cerebral ischemic events related to HR of IPH, TRFC, and LRNC were finally obtained. Furthermore, the 6 studies did not further assess the possibility of a relationship between the temporal dynamics of plaque development and recurrent ischemic events.
Our study also analyzed whether different MRI sequences of carotid artery plaques could be the cause of the difference between the HRs of IPH and the actual results. In order to accurately obtain the presence signs of IPH, various studies did not necessarily use the same MRI technology for detection. Among the 6 studies focusing on IPH, 4 studies only used separate gradient echo MRI sequences to detect and analyze the existence of IPH,[9–11,14] and 2 studies used multi-sequence MRI to analyze IPH.[15,18] After our analysis, there was no statistically significant heterogeneity between the 2 subgroups, suggesting that the existing evidence was more supportive of MRI sequence differences and did not have a substantial impact on IPH HR. However, further work needs to evaluate the practical application of the testing tools if more accurate evidence is to be obtained. At the same time, due to the lack of studies with evaluating TRFC and LRNC, further subgroup analysis cannot be carried out in a more detailed manner, and the obstacle of MRI carotid plaque imaging as a routine risk stratification tool cannot be known.
Similar to the previous studies on MRI in carotid artery plaque studies, our study also exists some limitations. First of all, there were differences in the reported results of various plaque components among various studies. Most studies used the comprehensive measurement of stroke, TIA and other cerebral ischemic events, so we could not more accurately determine the individual HR of stroke and TIA. Second, TRFC, LRNC researches were not enough and could not go a step further to subgroup analysis, also we did not know whether there was a significant variation in team of TRFC and LRNC plaque imaging of MRI technology, whether we needed to discuss the question of what kind of technology was more suitable for risk stratification, 1.5 T and 3 T machine on the different characteristics of plaque. Third, our study indicated that there was no significant variation in the MRI technique for plaque imaging in the IPH group, which was consistent with the results of previous studies on carotid artery plaque characteristics and stroke. Fourth, none of the studies reported the possibility that changes in the morphology and composition of plaques over time might be associated with subsequent recurrent ischemic events. There was a large variation in the time interval between the initial event and MR plaque imaging across selected studies. Plaque composition and morphology may have changed over time. Fifth, it is not clear how many patients suffered recurrent ischemic events beyond the follow-up time due to inconsistency among studies. Sixth, since many studies have included a wide range of stenosis patients, more accurate comprehensive risk estimates that consider stenosis and plaque characteristics need to include studies with less heterogeneity in stenosis degree. Seventh, differences and heterogeneity may arise due to different secondary prevention strategies, such as different drugs and blood pressure control applied in different studies in patients with ischemic events in the time period between the first episode and recurrence. Finally, most studies excluded patients who underwent surgical revascularization, and we could not rule out the absence of carotid plaque characteristics and risk of recurrent ischemic events in these patients, which introduces selection bias into non-randomized cohort studies. Of course, our study needs to be further improved by more original studies.
In summary, our present study indicated that MRI detection of specific plaque components such as IPH, TRFC, and LRNC could help us to screen out patients who had experienced the first stroke/TIA event and were more likely to have recurrent ischemic events. For lessons, unnecessary surgery should be avoided to prevent recurrent ischemic events provides guidance for patients to receive more effective preventive measures.
Author contributions
Conceptualization: Kang Li, Qingjun Yang.
Data analysis: Fengbin Deng.
Data curation: Ling Yang, Changping Mu.
Data extraction: Ling Yang, Changping Mu.
Formal analysis: Fengbin Deng, Qingjun Yang.
Investigation: Fengbin Deng.
Methodology: Fengbin Deng, Huaqiang Li.
Software: Fengbin Deng, Huaqiang Li.
Supervision: Kang Li, Qingjun Yang.
Writing – original draft: Fengbin Deng, Changping Mu.
Writing – review & editing: Fengbin Deng, Changping Mu.
Kang Li orcid: 0000-0003-4637-6620.
Footnotes
Abbreviations: 95%CI = 95% confidence interval, HR = hazard ratio, IPH = intraplaque hemorrhage, LRNC = lipid-rich necrotic core, PRISMA = Preferred Reporting Items for Systematic review and Meta-Analysis, TIA = transient ischemic attack, TRFC = thinning/rupture of the fibrous cap.
How to cite this article: Deng F, Mu C, Yang L, Li H, Xiang X, Li K, Yang Q. Carotid plaque Magnetic Resonance Imaging and Recurrent stroke Risk: a systematic review and meta-analysis. Medicine. 2020;99:13(e19377).
Fengbin Deng and Changping Mu contributed equally to this work and are considered as co-first authors.
This study has been supported by Science and technology innovation program of Chongqing General Hospital (Y2017MSXM15, Y2019ZDXM01), Chongqing TCM science and technology program (ZY2017020430, 2019ZDXM008) and Chongqing yuzhong district science and technology program (20180107, 20190152).
The authors report no conflicts of interest.
References
- [1].Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England) 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bos D, Portegies ML, van der Lugt A, et al. Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam study. JAMA Neurol 2014;71:405–11. [DOI] [PubMed] [Google Scholar]
- [3].Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet (London, England) 2010;376:1074–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sadat U, Weerakkody RA, Bowden DJ, et al. Utility of high resolution MR imaging to assess carotid plaque morphology: a comparison of acute symptomatic, recently symptomatic and asymptomatic patients with carotid artery disease. Atherosclerosis 2009;207:434–9. [DOI] [PubMed] [Google Scholar]
- [5].Singh N, Moody AR, Gladstone DJ, et al. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology 2009;252:502–8. [DOI] [PubMed] [Google Scholar]
- [6].den Hartog AG, Bovens SM, Koning W, et al. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur J Vasc Endovasc Surg 2013;45:7–21. [DOI] [PubMed] [Google Scholar]
- [7].Mono ML, Karameshev A, Slotboom J, et al. Plaque characteristics of asymptomatic carotid stenosis and risk of stroke. Cerebrovasc Dis 2012;34:343–50. [DOI] [PubMed] [Google Scholar]
- [8].Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke 2009;40:e573–83. [DOI] [PubMed] [Google Scholar]
- [9].Altaf N, Daniels L, Morgan PS, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg 2008;47:337–42. [DOI] [PubMed] [Google Scholar]
- [10].Altaf N, Kandiyil N, Hosseini A, et al. Risk factors associated with cerebrovascular recurrence in symptomatic carotid disease: a comparative study of carotid plaque morphology, microemboli assessment and the European Carotid Surgery Trial risk model. J Am Heart Assoc 2014;3:e000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Altaf N, MacSweeney ST, Gladman J, et al. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 2007;38:1633–5. [DOI] [PubMed] [Google Scholar]
- [12].Cai JM, Hatsukami TS, Ferguson MS, et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002;106:1368–73. [DOI] [PubMed] [Google Scholar]
- [13].Gupta A, Baradaran H, Schweitzer AD, et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013;44:3071–7. [DOI] [PubMed] [Google Scholar]
- [14].Hosseini AA, Kandiyil N, Macsweeney ST, et al. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann Neurol 2013;73:774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kwee RM, van Oostenbrugge RJ, Mess WH, et al. MRI of carotid atherosclerosis to identify TIA and stroke patients who are at risk of a recurrence. J Magn Reson Imaging 2013;37:1189–94. [DOI] [PubMed] [Google Scholar]
- [16].Saam T, Hetterich H, Hoffmann V, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 2013;62:1081–91. [DOI] [PubMed] [Google Scholar]
- [17].Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke 2006;37:818–23. [DOI] [PubMed] [Google Scholar]
- [18].Lu M, Peng P, Cui Y, et al. Association of progression of carotid artery wall volume and recurrent transient ischemic attack or stroke: a magnetic resonance imaging study. Stroke 2018;49:614–20. [DOI] [PubMed] [Google Scholar]
- [19].Marnane M, Prendeville S, McDonnell C, et al. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. Stroke 2014;45:801–6. [DOI] [PubMed] [Google Scholar]
- [20].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol 1995;15:1512–31. [DOI] [PubMed] [Google Scholar]


