Abstract
Cerebellar ataxia can be caused by a variety of disorders, including degenerative processes, auto-immune and paraneoplastic illness as well as by gene mutations inherited in autosomal dominant, autosomal recessive or X-linked fashions. As we broaden our knowledge of the causes of cerebellar ataxia, we have also vastly increased our ability to treat cerebellar diseases, both symptomatically and targeting specific disease types. In this review, we highlight the treatments for cerebellar ataxia in a systematic way, to provide guidance for clinicians to treat patients with cerebellar ataxia. In addition, we review therapies currently under development for ataxia, which is one of the most exciting fields in neurology. Because strong genetic components underlie many types of ataxia, identifying the causes and developing individualized treatment for each ataxia patient is the key for patient care and research. Therefore, ataxia can also be considered a prototypical model for personalized medicine development. The advancement of neuroscience and our ever-increasing understanding of the cerebellum has led to many emerging therapies for ataxia, bringing with it the hope that soon we will have even more ways to improve the quality of life and possibly modify the disease trajectory of patients living with cerebellar ataxia.
Keywords: Cerebellum, Ataxia, Multiple system atrophy, Spinocerebellar ataxia, Friedreich Ataxia, Ataxia Treatment
1. Introduction
The cerebellum is responsible for a multitude of motor functions through the coordination of movements by prediction and is modulated by sensory feedback. Cerebellar ataxia refers to the dysfunction of the cerebellum that leads to problems with gait and balance, eye movements, speech, and hand dexterity1. The cerebellum is comprised of two hemispheres separated by the vermis. The 10 lobules of the cerebellum are grouped in three lobes; lobules I to V make up the anterior lobe of the cerebellum, lobules VI-IX make up the posterior lobe and lobule X is the flocculonodular lobe. From functional imaging studies of the cerebellum, the anterior lobe is thought to be important for motor control while the posterior lobe is more involved in cognitive processing2. Thus, patients with cerebellar ataxia may have a variety of motor and non-motor symptoms impacting their daily activities.
The prevalence of ataxia varies depending on ethnic background and geographic region, as different populations have founder effects for certain types of hereditary ataxias. Most studies have focused on the prevalence of hereditary ataxias. However, some estimates suggest that the prevalence of ataxia ranges from 2.7 to 38.35 per 100,0003 A study looking at the global distribution of hereditary ataxias found that the most common autosomal dominant (AD) cerebellar ataxia is spinocerebellar ataxia typa 3 (SCA3), and the most common autosomal recessive (AR) ataxia is Friedreich ataxia4.
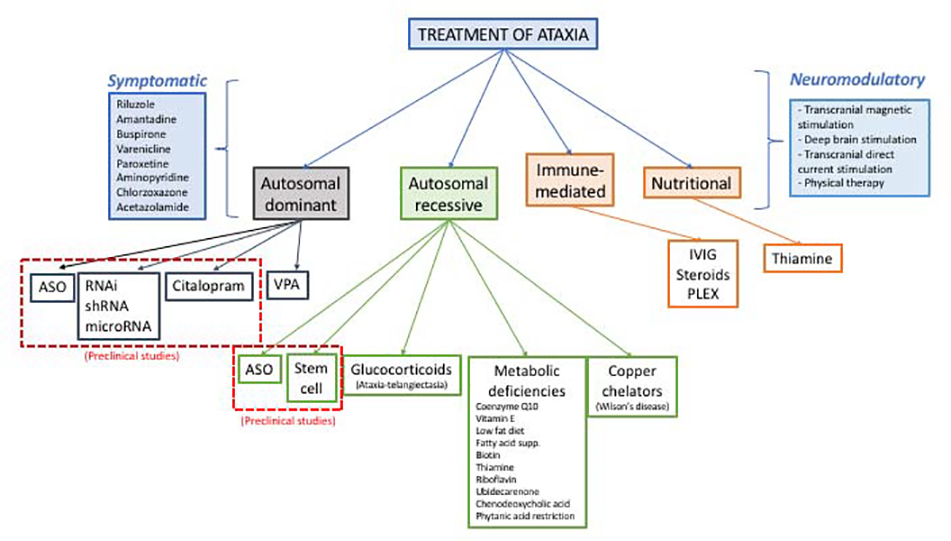
Cerebellar dysfunction can be caused by nutritional deficiencies, immune-mediated cerebellar degeneration, gene defects inherited in AD, AR or X-linked fashion, as well as neurodegenerative conditions. In the work-up of ataxia, it is important to understand the age of onset and time course of ataxia, family history, and associated medical conditions and signs and symptoms. There are several excellent papers describing the work-up of ataxia5–7, and the focus of this paper is not to re-summarize an ataxia work-up but instead to highlight current treatments for ataxia as well as those looming on the horizon. Figure 1 summarizes current and potential treatments for ataxia.
Figure 1.
Summary of current and potential treatments for ataxia.
In order to understand the development of therapy for ataxia, it is important to know how the severity of ataxia is measured. There are two rating scales commonly used to measure ataxia severity. The Scale for the Assessment and Rating of Ataxia (SARA) score is a 40-point scale taking into account gait, stance, and upper and lower extremity motor deficits8. It is relatively easy to use in a clinical setting as it is not particularly time consuming. The annual rate of SARA score increase has been found to be 2.11 in SCA1, 1.49 in patients with SCA2, 1.56 in SCA3 patients and 0.80 in SCA6 patients in a natural history study in Europe9. A US natural history study of SCA also showed comparable disease progression using SARA10. Another commonly used scale is the International Cooperative Ataxia Rating Scale (ICARS), which is a 100-point measure of cerebellar dysfunction taking into account eye movement abnormalities, upper and lower extremity coordination deficits, speech, stance and gait11. A study of 18 patients with static and progressive cerebellar lesions found that the ICARS score can differentiate between the two on certain measures and can detect yearly changes in ataxia in the patients with degenerative cerebellar diseases12. Although ICARS was developed earlier than the SARA score, the latter is now the more commonly used clinical measure for ataxia.
2a. Symptomatic Treatment of Ataxia
Many medications have been examined in an effort to find symptomatic treatment for ataxia, by modulating cerebellar function through changes in ion channel function and/or cerebellar physiology. However, most symptomatic treatments have not been tested for an extended period of time, and there is a lack of replicate studies. It is also unknown whether these medications can have additional disease-modifying effects. Nonetheless, the effects of these treatments may provide some symptomatic relief and improve quality of life. Table 1 summarizes the symptomatic treatments for cerebellar ataxias13–19, a few of which we will highlight below. Supplementary table 1 lists medications that have been tested and were proven non-effective.
Table 1:
Symptomatic treatment for ataxia
| Treatment | Ataxia type | Evidence | References |
|---|---|---|---|
| Riluzole | SCA, FA | Randomized, double-blind, placebo-controlled trial | (Ristori et al., 2010; Romano et al., 2015) |
| Varenicline | SCA3 | A randomized, double-blind, placebo-controlled trail | (Zesiewicz et al., 2012) |
| Paroxetine | MSA | A randomized, double-blind, placebo-controlled study | (Friess et al., 2006) |
| Aminopyridine | EA2 | A randomized, double-blind, placebo-controlled, crossover study | (Strupp et al., 2011) |
| SCA6, ADCA | Observational studies | (Tsunemi et al., 2010) | |
| Amantadine (IV) | MSA | A short-term, open label study | (Youn et al., 2012) |
| Buspirone | MSA-C | Open label studies | (Heo et al., 2008) |
| Acetazolamide | SCA6, EA | Open label studies and case reports | (Baloh and Winder, 1991; Griggs et al., 1978; Harno et al., 2004) (Yabeet al., 2001) |
| PMM2-CDG | A single-blind, randomized withdrawal trial | (Martinez-Monseny etal.,2019) | |
| Citalopram | FA | Case series (2 patients) | (Rohretal., 1999) |
| Chlorzoxazone | SCA1, SCA2 | Rodent studies | (Egorova et al., 2016) (Bushart et al., 2018) |
EA2: episodic ataxia type 2, FA: Friedreich ataxia, MSA: multiple system atrophy, SCA: spinocerebellar ataxia, PMM2-CDG: phosphomannomutase congenital disorder of glycosylation
Riluzole has been shown to improve function in patients with ataxia as measured by SARA and ICARS scores. It mostly affects axial domains by improving speech and gait. Side effects of riluzole include mild liver enzyme increases and transient vertigo but it is generally well-tolerated. Although the initial study only observed the effects of riluzole over 8 weeks, a longer study over 12 months demonstrated continued benefits20,21. Riluzole is thought to modulate SK channels, which are enriched in Purkinje cells, thereby partially normalizing neuronal firing patterns.
Varenicline is a partial agonist of the alpha-4 beta-2 nicotinic acetylcholine receptor, and was studied in 20 SCA3 patients. A randomized, double-blind, placebo-controlled trial of varenicline demonstrated improved axial symptoms and rapid alternating movements measured by SARA scores. Although relatively well tolerated, varenicline was associated with depression and irritability22.
Acetazolamide has been shown to reduce the severity of ataxia in SCA6 patients, who have repeat expansions in the CACNA1A gene23. The CACNA1A gene encodes a voltage-dependent calcium channel, and acetazolamide is thought to be helpful in channelopathies because it lowers the pH and may thus change channel properties. An open label trial of 6 SCA6 patients treated with acetazolamide had improvement in ataxia rating scale scores and decreased body sway but the effects were somewhat lessened after 1 year. Similarly, patients with episodic ataxia 2, which is also caused by CACNA1A gene mutations, had improvement in cerebellar symptoms with acetazolamide24–26. Acetazolamide has also been shown to be effective in treating patients with PMM2 congenital disorder of glycosylation (PMM2-CDG), which causes a cerebellar syndrome that may be mediated by abnormal glycosylation of calcium channels27. PMM2-CDG is inherited in an AR manner. Side effects of acetazolamide include low bicarbonate levels and paresthesia.
2b. Neuromodulation of Ataxia
Neuromodulation of the cerebellum has shown promising results for treating cerebellar ataxia28. Various studies have used transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), as well as deep brain stimulation (DBS) to test the effects on cerebellar ataxia. While DBS involves surgical implantation of electrodes, both TMS and tDCS are noninvasive and all have relatively few side effects. TMS can directly induce action potentials whereas tDCS can modulate local membrane potentials and neuronal plasticity29. Studies of cerebellar neuromodulation are highlighted in Table 230–40, but we will focus on a few tDCS studies in more depth here because tDCS is most studied in cerebellar ataxia with a potential for larger-scaled studies in the future.
Table 2:
Neuromodulation and physical exercise for ataxia
| Treatment | Ataxia type | Evidence | References |
|---|---|---|---|
| Physical merapy | SCA7, SCA2, FA | A randomized, open label study | (llg et al., 2009; Tercero-Pérez et al., 2019; Velázquez-Pérez et al., 2019) |
| Transcranial magnetic stimulation | SCA1, SCA6, SCA7 | A randomized, sham-controlled study and case reports | (Kawamura et al., 2018; Shiga et al., 2002; Shimizu et al., 1999) |
| Posterior circulation stroke | A randomized, double-blind trial | (Kim et al., 2014) | |
| Transcranial direct current stimulation | SCA, MSA, FA | A randomized, double-blind, sham-controlled study and case report | (Benussi et al., 2015, 2018; Grimaldi and Manto, 2013) |
| Deep brain stimulation | SCA | Case report | (Hashimoto et al., 2018) |
| FXTAS | Case report | (dos Santos Ghilardi et al., 2015) | |
| Cerebellar stroke | Case report | (Teixeira et al., 2015; Weiss et al., 2015) |
FA: Friedreich ataxia, FXTAS: Fragile X-associated tremor/ataxia syndrome, MSA: multiple system atrophy, SCA: spinocerebellar ataxia
A case report of two patients with SCA2 showed improvement in SARA score, tremor and upper limb dysmetria immediately after cerebellar tDCS41. Nineteen patients with various forms of neurodegenerative ataxias received anodal cerebellar tDCS in a double blind, sham-controlled crossover study35. There was a statistically significant improvement in 1.40 points of SARA score and 4.37 points in ICARS score between the sham and stimulation trials. A drawback of these studies was that the effect was only tested immediately after stimulation, making it unclear the duration of symptomatic improvement in these patients after cessation of tDCS.
A follow-up double blind, randomized, sham-controlled crossover study looked at cerebellar anodal and spinal cord cathodal tDCS stimulation in 21 patients with degenerative ataxias42. This study had a longer stimulation period (2 weeks of stimulation) and had SARA and ICARS performed before the stimulation or sham stimulation, immediately after the 2-week stimulation, and then 1 and 3 months after the stimulation. There was an improvement in SARA and ICARS scores in the stimulation group at all time points post-stimulation when compared to pre-stimulation and also when compared to sham. This study provides evidence of long-duration responses of ataxia with tDCS to the cerebellar region. The ease of use of tDCS and its relatively longer-lasting effects may be a useful adjunct to symptomatic medical therapy, especially in those patients who do not experience improvement in ataxia from medical therapy.
2c. Exercise as treatment for ataxia
Exercise or physical therapy has been a cornerstone in helping patients with cerebellar ataxia. Studies of intensive rehabilitation have shown long-lasting effects physical therapy. A study of 42 patients with degenerative ataxia were separated into two groups to receive either 4 weeks of intensive rehabilitation or a delayed start of the same rehabilitation schedule43. The immediate rehabilitation group had a greater improvement in SARA scores compared to the delayed start group at 4 weeks post-rehabilitation. Although the improvement was attenuated at 12 and 24 weeks post-rehabilitation, a significant number of patients still had improvement compared to baseline, which is notable given the progressive worsening of symptoms in degenerative ataxia.
A study of 38 SCA2 patients, randomized into a 24-week neurorehabilitation therapy program or no therapy, showed that there was a significant improvement in SARA scores in those receiving rehabilitation44. Notably the improvement in SARA scores between the therapy and no-therapy groups were in the sub-scores of gait, stance and sitting, and this improvement occurred in patients across different disease duration. Another study randomized 30 preclinical SCA2 patients into either a neurorehabilitation or control group45. While the study did not find differences in SARA scores between the two groups after the intervention, there was an improvement in tandem gait, finger-to-nose and heel-shin tasks in the rehabilitation group only.
Of note, a diverse range of physical activity has been shown to be helpful in improving cerebellar dysfunction. A study showed that intensive cycling in 20 patients with various SCAs led to an improvement in ICARS scores after 4 weeks of cycling46. Patients with cerebellar ataxia might be able to engage in diverse exercise and physical therapy, such as rock climbing47. Working through rehabilitation on physical functions as basic as swallowing may even be helpful in patients with cerebellar ataxia48. These studies highlight the long-lasting effects of exercise and physical therapy in improving ataxia, and may point to a neuromodulatory effect of exercise or to enhance other brain regions.
3. Disease modifying treatments for ataxia
The cerebellum is thought to be somewhat flexible in its ability to recover from injury and some investigators have even coined the phrase “time is cerebellum” to describe the abundance of cellular and synaptic plasticity within the cerebellum in ataxia patients, which can be enhanced by treatment49, when administered in the early stage of the diseases. We will highlight some current treatments that may be disease modifying as well as hopeful looming prospects in the next few sections. Perhaps the most important message is that identifying the cause of a patient’s cerebellar ataxia can be incredibly important as there are a multitude of treatments that can slow down and even halt the progression of ataxia.
3a. Treatment for nutritional and immune-mediated ataxias
Table 3 lists common causes of nutritional and immune-mediated ataxias, which occur on a time course of weeks to months50–52. It is critically important to examine serum B12 and B1 levels and potential auto-immune antibodies in a patient with cerebellar ataxia as these causes could potentially be reversed with the appropriate treatment especially early on in the disease course. The classic triad of Wernicke encephalopathy is altered mental status, ophthalmoplegia and cerebellar ataxia53, and often occurs in the setting of chronic alcoholism. Cancer and gastric bypass surgeries can be causes for Wernicke encephalopathy54. Prompt thiamine administration is the treatment for Wernicke encephalopathy.
Table 3:
Treatment for immune-mediated and nutritional ataxias.
| Treatment | Ataxia type | Evidence | References |
|---|---|---|---|
| IVIG | Opsoclonus myoclonus ataxia syndrome | A randomized, open label study | (de Alarcon et al., 2018) |
| Gluten-free diet, IVIG, steroids | Anti-DGP, anti-gliadin cerebellar ataxias | Open label | (Nanri et al., 2016) |
| IVIG, steroids | Anti-GAD, anti-TPO | Open label | (Nanri et al., 2016) |
| Thiamine | Wernicke's encephalopathy | Case reports | (Chataway and Hardman, 1995; Sinha et al., 2019) |
| Vitamin B12 | Pernicious anemia, dietary deficiency | Case reports | (Chakrabarty et al., 2014; Stabler, 2013) |
Anti-DGP: anti-deamidated gliadin peptide, anti-GAD: anti-glutamic acid decarboxylase antibody, anti-TPO: anti-thyroperoxidase antibody, IVIG: intravenous immunoglobulin
The most common cause of vitamin B12 deficiency is pernicious anemia, which is an autoimmune atrophic gastritis55. Although the gait dysfunction in vitamin B12 deficiency is due to both demyelination of the dorsal columns and central nervous system involvement, there have been cases of vitamin B12 deficiency that cause cerebellar degeneration only56. Prompt detection of vitamin B12 deficiency in a patient with cerebellar ataxia is critical to prevent clinical worsening and may even reverse the symptoms.
Finally, there are several auto-antibodies and paraneoplastic syndromes associated with cerebellar ataxia, and the identification of these causes are important in order to administer treatment promptly. The most common paraneoplastic autoantibodies are the anti-Yo (associated with breast, uterine and ovarian cancers), anti-Hu (associated with small cell lung cancer), anti-Tr (associated with Hodgkin’s lymphoma), and anti-CV2 (associated with SCLC and thymoma) antibodies. Guidelines on the treatment for paraneoplastic ataxias suggest that if treating the underlying cancer does not help, a round of immunotherapy may be helpful. Patients who have autoantibodies detected in the serum may receive a spinal tap to further investigate whether these antibodies are present in the cerebrospinal fluid. These patients with autoantibodies can be treated with either immunoglobulin therapy (IVIG), steroids or other immuno-modulatory agents. More randomized controlled trials need to be conducted to examine autoantibody-mediated cerebellar ataxia to determine the optimal immunotherapy tailored to ataxia associated with each individual autoantibody.
3b. Treatment for AR ataxias
AR ataxias are comprised of a diverse group of progressive gataxias often resulting from dysfunctional metabolic pathways. Most AR ataxias can be categorized in one of three subgroups: defective mitochondrial metabolism, dysfunctional lipid metabolism and impaired DNA repair58. Some of these AR mutations lead to accumulation of toxic downstream metabolites and reduction of these metabolites can ameliorate the symptoms of the illness and/or modify the disease course.
Wilson disease, which causes a multitude of neurologic, psychiatric and ophthalmologic symptoms including ataxia due to copper accumulation in the brain and liver resulting from a mutation in the ATP7B gene that encodes a protein for copper transport. Lifelong treatment with oral copper chelators such as penicillamine and trientene may reverse symptoms59.
The clinical hallmarks of cerebrotendinous xanthomatosis (CTX) include tendon xanthomas, cerebellar ataxia, dementia, cataracts, premature atherosclerosis, and pulmonary dysfunction, with elevated cholestenol and bile alcohols found in serum and urine60. Supplementation with chenodeoxycholic acid (CDCA) inhibits abnormal bile acid synthesis by providing feedback inhibition which is deficient in these patients. A hallmark 1984 study showed that in 17 CTX patients given CDCA, several patients had improvement of dementia and cerebellar ataxia61. A more recent study in two patients with CTX started on CDCA at 3 and 5 months showed that the neurologic deficits could be improved but not completely reversed, suggesting that there even earlier detection and treatment of CTX may lead to normal development62.
Niemann Pick type C (NPC) is caused by a mutation in the NPC1 or NPC2 gene, and the adolescent onset forms of NPC usually result in cerebellar dysfunction including ataxia, dysarthria, dysmetria and dysphagia, as well as gelastic cataplexy63. On exam, NPC patients have characteristic impaired vertical saccades64. Miglustat inhibits the synthesis of glycosphingolipids and has been approved for the treatment of NPC. Miglustat improves swallowing function and stabilizes other neurologic manifestations in the years following its administration in NPC patients65. Other potential treatments for NPC include 2-hydroxypropyl-beta-cyclodextrins which may delay Purkinje cell loss and slows disease progression in patients with NPC66.
Other AR ataxias that are due to nutritional deficiencies may be stabilized or improved with correct nutritional supplementation. In a patient with ataxia, dysarthria, vision loss due to retinitis pigmentosa, head tremor and areflexia, ataxia with vitamin E deficiency (AVED) must be considered. AVED patients have mutations in the alpha tocopherol transfer protein (alpha-TTP) lead to reduced intrahepatocyte vitamin E incorporation into very low density lipoproteins. Serum vitamin E levels are very low in AVED patients, and early supplementation of vitamin E in these patients can improve cerebellar ataxia67. AR cerebellar ataxia type 2 (ARCA2) is caused by a mutation in the ADCK3 gene which results in coenzyme Q10 deficiency. Studies show that patients seem to respond to idebenone supplementation68.
A success in AR cerebellar ataxia treatment is seen in patients with biotinidase deficiency identified on newborn screen, who subsequently received biotin supplementation69. A study following 44 patients started on biotin supplementation as infants lived normal adult lives, significantly different from the natural history of biotinidase deficiency. Interestingly, biotin supplementation seems only to slow or stop disease progression but cannot reverse symptoms. A case report described patients with novel mutations in the SLC19A3 gene that have recurrent episodes of encephalopathy and at baseline have generalized dystonia, epilepsy and bilateral caudate and putaminal hyperintensities had clinical improvement during encephalopathic episodes with administration of high doses of biotin and thiamine70.
The most common AR cerebellar ataxia is Friedreich ataxia, accounting for ~25% of all AR cerebellar ataxias. Friedreich ataxia is caused by a homozygous GAA trinucleotide repeat in intron 1 of the FXN gene on chromosome 9q1371, which reduces expression of the protein products, frataxin. Several pre-clinical studies in rodents and cell lines have shown that administration of wild-type frataxin either through bone-marrow transplants72, administration of antisense oligonucleotides (ASOs)73, or injecting FXN expressing adeno-associated virus74 can improve some of the symptoms of Friedreich ataxia in preclinical models.
Table 4 provides a more comprehensive summary of the different AR cerebellar ataxias and their treatments75–84. The majority of the evidence for the treatments of AR cerebellar ataxias rely on case reports and open label studies, likely because of the rarity of the illnesses. However, it is still important to consider the possibility of AR cerebellar ataxias in patients with early onset ataxia with or without sensory neuropathy, since many AR ataxias can stabilize or improve if treatments are started early. The potential for gene therapy or strategies to enhance FXN expression in the treatment of Friedreich ataxia portends great hope for the treatment of AR cerebellar ataxias.
Table 4:
Disease modifying therapies for autosomal recessive ataxias
| Ataxia type | Treatment | Evidence | Mechanism of action | References |
|---|---|---|---|---|
| Wilson disease | Copper chelators | Observational studies | Blockade of intestinal copper absorption | (Aggarwal and Bhatt, 2018) |
| Niemann-Pick Disease | Miglustat | A prospective randomized control studies | Inhibition of glycosphingolipid synthesis | (Pineda et al., 2018) |
| Niemann-Pick Disease | Intrathecal 2-hydroxypropyl-β-cyclodextrins | An open label, observational study | Unclear | (Ory et al., 2017) |
| Ataxia Telangiectasia | Glucocorticoids | An prospective, cohort study | Unclear, possible restoration of ATM gene expression | (Menotta et al., 2017; Zannolli et al., 2012) |
| Cerebrotendinous xanthomatosis | Chenodeoxycholic acid supplementation | An open label, observational study | Replace missing bile acids; lipid metabolism deficit | (Berginer et al., 1984; Nie et al., 2014; Pierre et al., 2008) |
| Ataxia with vitamin E deficiency | Vitamin E | An open label, observational study | Replacement therapy | (Gabsi et al., 2001) |
| Riboflavin transporter deficiency neuronopathy (SLC52A2 gene mutation) | Riboflavin | An open label, observational study | Supplementation to boost absorption | (Foley et al., 2014; Guissart et al., 2016) |
| Autosomal recessive cerebellar ataxia 2 | Ubidecarenone | An open label, observational study | Replacement therapy | (Mignot et al., 2013) |
| Abetalipoproteinemia | Low-fat diet, essential fatty acid supplementation | A case series | Replacement therapy | (Chardon et al., 2009; Lee and Hegele, 2014) |
| Biotinidase deficiency | Biotin | A case series | Replacement therapy | (Wolf, 2017) |
| SLC19A3 gene mutation | Biotin, thiamine | A case series | Replacement therapy | (Debs et al., 2010) |
| CoQ10, CoQ4 deficiency | Coenzyme Q10 | A case series | Replacement therapy | (Caglayan et al., 2019) |
| Refsum disease | Phytanic acid restriction, lipid apheresis | A case report and 2 case series | Toxic substrate reduction | (Baldwin et al., 2010; Gibberd et al., 1979; Zolotov et al., 2012) |
| Friedreich ataxia | Frataxin-expressing adeno-associated virus | A preclinical, rodent study | Frataxin replacement | (Piguet et al., 2018) |
| Friedreich ataxia | Allogenic stem cell transplantation | A preclinical, rodent study | Increasing frataxin levels | (Kemp et al., 2018) |
| Friedreich ataxia | ASO targeting triplet expansion in frataxin | A preclinical, rodent study | Increasing frataxin expression | (Li et al., 2018) |
CoQ4: Coenzyme Q4, CoQ10: Coenzyme Q10
3c. Treatment for AD cerebellar ataxias
In patients presenting with a slowly progressive cerebellar ataxia in middle-age who may have a family history of ataxia, the diagnosis of AD ataxias should be aggressively pursued. The majority of AD cerebellar ataxias are categorized as SCAs with exceptions such as dentatorubro-pallidoluysian atrophy (DRPLA) and episodic ataxias. The most common SCAs involve poly-glutamine expansion repeats, which lead to protein misfolding and aggregation85, and SCA1, 2, 3, 6, 7, and 17 belong to this group. There are no definitive disease-modifying therapies for AD cerebellar ataxias. Nonetheless, we list several relevant clinical and preclinical studies as potential therapies for AD cerebellar ataxias.
A randomized, open-label study of valproic acid in 12 SCA3 patients showed improvement in SARA scores, with dizziness and loss of appetite as side effects86, with two patients dropping out as a result of these side effects. The possible mechanism of action is thought to be inhibition of histone deacetylases to regulate the expression of pathological proteins. Proteins carrying poly-glutamine repeats are known to activate the mitochondrial apoptotic pathway leading to neuronal death; therefore, mitochondrial dysfunction might be one of the shared pathways for SCAs. A retrospective study demonstrated that SCA patients who took coenzyme Q10, a co-factor in the mitochondrial respiratory chain, had better SARA scores than those who did not. In addition, a dose-dependent effect was found in SCA3 patients87. This study suggests coenzyme Q10 may have disease-modifying effects for SCAs, and warrants further randomized, placebo-controlled trials.
Citalopram was initially identified in a screen of Food and Drug Administration-approved drugs to rescue neuronal dysfunction in an SCA3 C.elegans model. Citalopram, a serotonin reuptake inhibitor, was further demonstrated to reduce ataxin-3 neuronal inclusions, astrogliosis, as well as improving motor symptoms in SCA3 mouse models88. These preclinical animal studies provide strong rationale for future randomized placebo-controlled trials for citalopram in SCA3. Excessive glutamate-mediated neuronal transmission leading to neuronal toxicity is a major mechanism for neurodegenerative disorders; therefore, modulating glutamatergic neuronal transmission could be disease modifying in SCAs. Towards this goal, there is an ongoing clinical trial treating SCA patients with a glutamate modulator, troriluzole, to examine its effects on slowing disease progression.
As SCAs are monogenetic disorders, therapies specifically through ASO targeting mutated genes hold promise as disease modifying therapy. There are several preclinical studies showing that knocking down of mutated protein expression by gene therapy can be disease modifying therapy in animal models of SCAs. ASOs targeting ATXN2 in a SCA2 mouse model improved motor function and Purkinje cell firing89. A similar study in showed efficacy of ASOs in reducing ATXN1 protein in a SCA1 mouse model with improvement in motor coordination90. ASOs targeting ATXN3 also showed promising results in SCA3 mouse models91, while knockout of the entire ATXN3 gene is well-tolerated in a SCA3 mouse model92, which provide some preclinical safety evidence. Other than ASOs, intraventricular injections of shRNA silencing mutant ATXN3 have also been shown to improve motor symptoms in SCA3 mouse models93. In a SCA6 mouse model, delivery of microRNA against the toxic gene product can also improve the motor performance and halt Purkinje cell degeneration94.
Table 5 summarizes the potential disease modifying treatments highlighted above, as well as other promising preclinical studies not mentioned in the text95–99.
Table 5:
Disease modifying therapy in autosomal dominant ataxias
| Treatment | Ataxia type | Evidence | Mechanism of action | References |
|---|---|---|---|---|
| Valproic acid | SCA3 | A randomized, open-label, doseescalation | Histone deacetylase inhibition | (Lei et al., 2016) |
| Coenzyme Q10 | SCA 1, SCA3 | An observational study | Enhancing mitochondrial respiratory chain | (Lo et al., 2015) |
| Troriluzole (BHV4157) | SCA1, SCA2, SCA3, SCA6, SCA7, SCA8, SCA10 | An ongoing phase III, randomized, double-blind, placebo-controlled study | Modulation of glutamate neurotransmission | ClinicalTrials.gov Identifier: NCT03701399 |
| Citalopram | SCA3 | A preclinical, rodent study | Reduction of ATXN3 neuronal inclusions and astrogliosis | (Teixeira-Castro et al., 2015) |
| ASO targeting ATXN1 | SCA1 | A preclinical, rodent study | Downregulation of ATXN1 | (Friedrich et al., 2018) |
| ASO targeting ATXN2 | SCA2 | A preclinical, rodent study | Downregulation of ATXN2 | (Scoles et al., 2017) |
| ASO vitreal injections | SCA7 | A preclinical, rodent study | Downregulation of ATXN7 | (Niu et al., 2018) |
| ASO targeting ATXN3 | SCA3 | A preclinical, rodent study | Downregulation of ATXN3 | (Toonen et al., 2017) (Moore et al., 2017) |
| shRNA silencing ATXN3 | SCA3 | A preclinical, rodent study | Downregulation of ATXN3 | (Nóbrega et al., 2013, 2019) |
| RNAi targeting ATXN7 | SCA7 | A preclinical, rodent study | Reduction of WT and mutant ATXN7 | (Ramachandran et al., 2014) |
| MicroRNA blocking IRES driven translation CACNA1A second cistern | SCA6 | A preclinical, rodent study | Selective downregulation of toxic gene product | (Miyazaki et al., 2016) |
| Gluten-free diet | SCA35 | A case report | Toxic substrate reduction | (Lin et al., 2019) |
ASO: antisense oligonucleotide, SCA: spinocerebellar ataxia
3d. Treatments for idiopathic neurodegenerative ataxias
Multiple system atrophy (MSA) is a common diagnosis in patients with late onset, progressive cerebellar ataxia, and is often accompanied by parkinsonism and autonomic dysfunction. A recent genetic study found an association between a rare variant of COQ2 and MSA in East Asian populations100,101. COQ2 encodes for a protein that is essential in the synthesis of coenzyme Q10. Interestingly, reduction of coenzyme Q10 levels is observed both in the serum93, and in the postmortem cerebellum of MSA patients102. A clinical trial of ubiquinol, a form of coenzyme Q10, in the treatment of MSA is currently underway.
Other potential treatments for MSA include modulation of the serotonergic system with the selective serotonergic reuptake inhibitors, which have been shown to reduce alpha-synuclein uptake in neuronal and oligodendroglial cells and thus could be disease-modifying103. A small double-blind, placebo controlled study of 19 MSA patients showed that paroxetine, a serotonin reuptake inhibitor, treated patients showed better limb agility compared to placebo-treated patients104. Another promising potential treatment for MSA are myeloperoxidase inhibitors, which have been shown to improve motor function in an MSA mouse model and to reduce alpha-synuclein aggregates105. A phase III trial of an irreversible myeloperoxidase inhibitor, BHV-3241, is currently underway.
4. Conclusions
The purpose of this paper is to describe the wide range of treatments available for cerebellar ataxia and to highlight promising therapies on the horizon. With the recent advances in gene therapy and ASO approaches, targeted therapies for ataxia hold the promise of improving quality of life for patients with ataxia and possibly even slow or reverse the disease course. Moreover, continued improvement of clinical trial design for ataxia will also advance our ability to demonstrate therapeutic efficacy.
Several challenges remain to be addressed in the currently burgeoning field of ataxia research. First, while most studies rely on SARA or ICARS scores, additional patient-oriented outcome measures need to be developed to demonstrate improvement in quality of life for ataxia patients. Second, as we begin to understand the role of the cerebellum in cognitive and emotional processing, non-motor rating scales, such as the cerebellar cognitive affective scale106, will need to be implemented to comprehensively assess the aspects of patients’ lives affected by cerebellar dysfunction. Third, all clinical rating scales are limited by their ability to capture only a moment in time in the clinic and as such the development of wearable instruments are needed to more fully describe “real life” motor performance at home. Fourth, imaging and fluid biomarkers should be used as part of clinical trials for ataxia to test for target engagement and as additional evidence for disease modifying effects.
There are currently a multitude of treatments for ataxia that can be offered, both symptomatic therapies that may help regardless of the cause of ataxia as well as potential disease-modifying therapies targeting specific types of ataxia. It is not only the time to dispel the myth that there is no treatment for ataxia, but in fact there is a reason to be hopeful about the current state and future of ataxia treatments.
Supplementary Material
Key Points.
A review paper summarizing current treatments for cerebellar ataxia.
Review categorizing treatments for cerebellar ataxia by symptomatic treatment and disease modifying treatments.
We further separate disease modifying treatments into those for autosomal dominant, autosomal recessive and immune-mediated cerebellar ataxias.
We discuss preclinical and clinical trials currently underway for the treatment of cerebellar ataxia.
Synopsis.
Cerebellar ataxia can be caused by a variety of disorders, including degenerative processes, auto-immune and paraneoplastic illness as well as by gene mutations inherited in autosomal dominant, autosomal recessive or X-linked fashions. In this review, we highlight the treatments for cerebellar ataxia in a systematic way, to provide guidance for clinicians to treat patients with cerebellar ataxia. In addition, we review therapies currently under development for ataxia, which is one of the most exciting fields in neurology.
Acknowledgments
Dr. Kuo is supported by the NINDS K08 NS083738 (Kuo), R01 NS104423 (Kuo), National Ataxia Foundation, Louis V. Gerstner Jr. Scholarship, Parkinson Foundation, Brain Research Foundation, and International Essential Tremor Foundation.
Footnotes
Disclosure Statement:
The authors have no relationship with a commercial company that has a direct financial interest in the subject matter or materials discussed in this article or with a company making a competing product.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Manto M, Bower JM, Conforto AB, et al. Consensus paper: Roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11(2):457–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoodley CJ, Schmahmann JD. Functional topography of the human cerebellum. Handb Clin Neurol. 2018;154:59–70. [DOI] [PubMed] [Google Scholar]
- 3.Subramony SH. Degenerative Ataxias: Challenges in clinical research. Ann Clin Transl Neurol. 2017;4(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: A systematic review of prevalence studies. Neuroepidemiology. 2014;42(3):174–183. [DOI] [PubMed] [Google Scholar]
- 5.Klockgether T Sporadic ataxia with adult onset: Classification and diagnostic criteria. Lancet Neurol. 2010;9(1):94–104. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfo M, Manto M. Cerebellar and afferent ataxias. Continuum (Minneap Minn).2013;19(5 Movement Disorders):1312–1343. [DOI] [PubMed] [Google Scholar]
- 7.Fogel BL, Perlman S. An approach to the patient with late-onset cerebellar ataxia. Nat Clin Pract Neurol. 2006;2(11):629–635; quiz 1 p following 635. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz-Hübsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: Development of a new clinical scale. Neurology. 2006;66(11):1717–1720. [DOI] [PubMed] [Google Scholar]
- 9.Jacobi H, du Montcel ST, Bauer P, et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: A longitudinal cohort study. Lancet Neurol. 2015;14(11):1101–1108. [DOI] [PubMed] [Google Scholar]
- 10.Ashizawa T, Figueroa KP, Perlman SL, et al. Clinical characteristics of patients with spinocerebellar ataxias 1, 2, 3 and 6 in the US; a prospective observational study. Orphanet J Rare Dis. 2013;8:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145(2):205–211. [DOI] [PubMed] [Google Scholar]
- 12.Morton SM, Tseng Y-W, Zackowski KM, Daline JR, Bastian AJ. Longitudinal tracking of gait and balance impairments in cerebellar disease. Mov Disord. 2010;25(12):1944–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strupp M, Kalla R, Claassen J, et al. A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias. Neurology. 2011;77(3):269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsunemi T, Ishikawa K, Tsukui K, Sumi T, Kitamura K, Mizusawa H. The effect of 3,4-diaminopyridine on the patients with hereditary pure cerebellar ataxia. J Neurol Sci. 2010;292(1–2):81–84. [DOI] [PubMed] [Google Scholar]
- 15.Youn J, Shin H, Kim JS, Cho JW. Preliminary study of intravenous amantadine treatment for ataxia management in patients with probable multiple system atrophy with predominant cerebellar ataxia. J Mov Disord. 2012;5(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heo J-H, Lee S-T, Chu K, Kim M. The efficacy of combined estrogen and buspirone treatment in olivopontocerebellar atrophy. J Neurol Sci. 2008;271(1–2):87–90. [DOI] [PubMed] [Google Scholar]
- 17.Rohr A, Eichler K, Hafezi-Moghadam N. Citalopram, a selective serotonin reuptake inhibitor, improves symptoms of Friedreich’s ataxia. Pharmacopsychiatry. 1999;32(3):113–114. [DOI] [PubMed] [Google Scholar]
- 18.Egorova PA, Zakharova OA, Vlasova OL, Bezprozvanny IB. In vivo analysis of cerebellar Purkinje cell activity in SCA2 transgenic mouse model. J Neurophysiol. 2016;115(6):2840–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bushart DD, Chopra R, Singh V, Murphy GG, Wulff H, Shakkottai VG. Targeting potassium channels to treat cerebellar ataxia. Ann Clin Transl Neurol. 2018;5(3):297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ristori G, Romano S, Visconti A, et al. Riluzole in cerebellar ataxia: A randomized, double-blind, placebo-controlled pilot trial. Neurology. 2010;74(10):839–845. [DOI] [PubMed] [Google Scholar]
- 21.Romano S, Coarelli G, Marcotulli C, et al. Riluzole in patients with hereditary cerebellar ataxia: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14(10):985–991. [DOI] [PubMed] [Google Scholar]
- 22.Zesiewicz TA, Greenstein PE, Sullivan KL, et al. A randomized trial of varenicline (Chantix) for the treatment of spinocerebellar ataxia type 3. Neurology. 2012;78(8):545–550. [DOI] [PubMed] [Google Scholar]
- 23.Yabe I, Sasaki H, Yamashita I, Takei A, Tashiro K. Clinical trial of acetazolamide in SCA6, with assessment using the Ataxia Rating Scale and body stabilometry. Acta Neurol Scand. 2001;104(1):44–47. [DOI] [PubMed] [Google Scholar]
- 24.Baloh RW, Winder A. Acetazolamide-responsive vestibulocerebellar syndrome: Clinical and oculographic features. Neurology. 1991;41(3):429–433. [DOI] [PubMed] [Google Scholar]
- 25.Griggs RC, Moxley RT, Lafrance RA, McQuillen J. Hereditary paroxysmal ataxia: Response to acetazolamide. Neurology. 1978;28(12):1259–1264. [DOI] [PubMed] [Google Scholar]
- 26.Harno H, Hirvonen T, Kaunisto MA, et al. Acetazolamide improves neurotological abnormalities in a family with episodic ataxia type 2 (EA-2). J Neurol. 2004;251(2):232–234. [DOI] [PubMed] [Google Scholar]
- 27.Martinez-Monseny AF, Bolasell M, Callejon-Poo L, et al. AZATAX: Acetazolamide safety and efficacy in cerebellar syndrome in PMM2 congenital disorder of glycosylation (PMM2-CDG). Ann Neurol. 2019;85(5):740–751. [DOI] [PubMed] [Google Scholar]
- 28.França C, de Andrade DC, Teixeira MJ, et al. Effects of cerebellar neuromodulation in movement disorders: A systematic review. Brain Stimul. 2018;11(2):249–260. [DOI] [PubMed] [Google Scholar]
- 29.Grimaldi G, Argyropoulos GP, Boehringer A, et al. Non-invasive cerebellar stimulationâ “A consensus paper. Cerebellum. 2014;13(1):121–138. [DOI] [PubMed] [Google Scholar]
- 30.Ilg W, Synofzik M, Brötz D, Burkard S, Giese MA, Schöls L. Intensive coordinative training improves motor performance in degenerative cerebellar disease. Neurology. 2009;73(22):1823–1830. [DOI] [PubMed] [Google Scholar]
- 31.Kawamura K, Etoh S, Shimodozono M. Transcranial magnetic stimulation for diplopia in a patient with spinocerebellar ataxia type 6: A case report. Cerebellum Ataxias. 2018;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiga Y, Tsuda T, Itoyama Y, et al. Transcranial magnetic stimulation alleviates truncal ataxia in spinocerebellar degeneration. J Neurol Neurosurg Psychiatry. 2002;72(1):124–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu H, Tsuda T, Shiga Y, et al. Therapeutic efficacy of transcranial magnetic stimulation for hereditary spinocerebellar degeneration. Tohoku J Exp Med. 1999;189(3):203–211. [DOI] [PubMed] [Google Scholar]
- 34.Kim W-S, Jung SH, Oh MK, Min YS, Lim JY, Paik N-J. Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: A pilot study. J Rehabil Med. 2014;46(5):418–423. [DOI] [PubMed] [Google Scholar]
- 35.Benussi A, Koch G, Cotelli M, Padovani A, Borroni B. Cerebellar transcranial direct current stimulation in patients with ataxia: A double-blind, randomized, sham-controlled study. Mov Disord. 2015;30(12):1701–1705. [DOI] [PubMed] [Google Scholar]
- 36.Grimaldi G, Manto M. Anodal transcranial direct current stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann Biomed Eng. 2013;41(11):2437–2447. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto T, Muralidharan A, Yoshida K, et al. Neuronal activity and outcomes from thalamic surgery for spinocerebellar ataxia. Ann Clin Transl Neurol. 2018;5(1):52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.dos Santos Ghilardi MG, Cury RG, dos Ângelos JS, et al. Long-term improvement of tremor and ataxia after bilateral DBS of VoP/zona incerta in FXTAS. Neurology. 2015;84(18):1904–1906. [DOI] [PubMed] [Google Scholar]
- 39.Teixeira MJ, Cury RG, Galhardoni R, et al. Deep brain stimulation of the dentate nucleus improves cerebellar ataxia after cerebellar stroke. Neurology. 2015;85(23):2075–2076. [DOI] [PubMed] [Google Scholar]
- 40.Weiss D, Mielke C, Wächter T, et al. Long-term outcome of deep brain stimulation in fragile X-associated tremor/ataxia syndrome. Parkinsonism Relat Disord. 2015;21(3):310–313. [DOI] [PubMed] [Google Scholar]
- 41.Grimaldi G, Oulad Ben Taib N, Manto M, Bodranghien F. Marked reduction of cerebellar deficits in upper limbs following transcranial cerebello-cerebral DC stimulation: Tremor reduction and re-programming of the timing of antagonist commands. Front Syst Neurosci. 2014;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benussi A, Dell’Era V, Cantoni V, et al. Cerebello-spinal tDCS in ataxia: A randomized, double-blind, sham-controlled, crossover trial. Neurology. 2018;91(12):e1090–e1101. [DOI] [PubMed] [Google Scholar]
- 43.Miyai I, Ito M, Hattori N, et al. Cerebellar ataxia rehabilitation trial in degenerative cerebellar diseases. Neurorehabil Neural Repair. 2012;26(5):515–522. [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez-Díaz JC, Velázquez-Pérez L, Rodríguez Labrada R, et al. Neurorehabilitation therapy in spinocerebellar ataxia type 2: A 24-week, rater-blinded, randomized, controlled trial. Mov Disord. 2018;33(9):1481–1487. [DOI] [PubMed] [Google Scholar]
- 45.Velázquez-Pérez L, Rodríguez-Diaz JC, Rodríguez-Labrada R, et al. Neurorehabilitation Improves the Motor Features in Prodromal SCA2: A Randomized, Controlled Trial. Mov Disord April 2019. [DOI] [PubMed] [Google Scholar]
- 46.Chang Y-J, Chou C-C, Huang W-T, Lu C-S, Wong AM, Hsu M-J. Cycling regimen induces spinal circuitry plasticity and improves leg muscle coordination in individuals with spinocerebellar ataxia. Arch Phys Med Rehabil. 2015;96(6):1006–1013. [DOI] [PubMed] [Google Scholar]
- 47.Lin C-Y, Kuo S-H. The role of the cerebellum in rock climbing. J Neurol Sci. 2017;383:158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry SE, Sevitz JS, Curtis JA, Kuo S-H, Troche MS. Skill Training Resulted in Improved Swallowing in a Person with Multiple System Atrophy: An Endoscopy Study. Mov Disord Clin Pract. 2018;5(4):451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitoma H, Manto M, Hampe CS. Time Is Cerebellum. Cerebellum. 2018;17(4):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Alarcon PA, Matthay KK, London WB, et al. Intravenous immunoglobulin with prednisone and risk-adapted chemotherapy for children with opsoclonus myoclonus ataxia syndrome associated with neuroblastoma (ANBL00P3): A randomised, open-label, phase 3 trial. Lancet Child Adolesc Health. 2018;2(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nanri K, Okuma M, Sato S, et al. Prevalence of Autoantibodies and the Efficacy of Immunotherapy for Autoimmune Cerebellar Ataxia. Intern Med. 2016;55(5):449–454. [DOI] [PubMed] [Google Scholar]
- 52.Chataway J, Hardman E. Thiamine in Wernicke’s syndromeâ “How much and how long? Postgrad Med J. 1995;71(834):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinha S, Kataria A, Kolla BP, Thusius N, Loukianova LL. Wernicke Encephalopathy-Clinical Pearls. Mayo Clin Proc. 2019;94(6):1065–1072. [DOI] [PubMed] [Google Scholar]
- 54.Kuo S-H, Debnam JM, Fuller GN, de Groot J. Wernicke’s encephalopathy: An underrecognized and reversible cause of confusional state in cancer patients. Oncology. 2009;76(1):10–18. [DOI] [PubMed] [Google Scholar]
- 55.Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368(2):149–160. [DOI] [PubMed] [Google Scholar]
- 56.Chakrabarty B, Dubey R, Gulati S, Yoganathan S, Kumar A, Kumar A. Isolated cerebellar involvement in vitamin B12 deficiency: A case report. J Child Neurol. 2014;29(11):NP161–163. [DOI] [PubMed] [Google Scholar]
- 57.Mitoma H, Hadjivassiliou M, Honnorat J. Guidelines for treatment of immune-mediated cerebellar ataxias. Cerebellum Ataxias. 2015;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Synofzik M, Puccio H, Mochel F, Schols L. Autosomal Recessive Cerebellar Ataxias: Paving the Way toward Targeted Molecular Therapies. Neuron. 2019;101(4):560–583. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal A, Bhatt M. Advances in Treatment of Wilson Disease. Tremor Other Hyperkinet Mov (N Y) 2018;8:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nie S, Chen G, Cao X, Zhang Y. Cerebrotendinous xanthomatosis: A comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2014;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berginer VM, Salen G, Shefer S. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med. 1984;311(26):1649–1652. [DOI] [PubMed] [Google Scholar]
- 62.Pierre G, Setchell K, Blyth J, Preece MA, Chakrapani A, McKiernan P. Prospective treatment of cerebrotendinous xanthomatosis with cholic acid therapy. J Inherit Metab Dis. 2008;31 Suppl 2:S241–245. [DOI] [PubMed] [Google Scholar]
- 63.Pedroso JL, Fusão EF, Ladeia-Frota C, et al. Teaching video neuroimages: Gelastic cataplexy as the first neurologic manifestation of Niemann-Pick disease type C. Neurology. 2012;79(22):e189. [DOI] [PubMed] [Google Scholar]
- 64.Gupta DK, Blanco-Palmero VA, Chung WK, Kuo S-H. Abnormal Vertical Eye Movements as a Clue for Diagnosis of Niemann-Pick Type C. Tremor Other Hyperkinet Mov (N Y). 2018;8:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pineda M, Walterfang M, Patterson MC. Miglustat in Niemann-Pick disease type C patients: A review. Orphanet J Rare Dis. 2018;13(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ory DS, Ottinger EA, Farhat NY, et al. Intrathecal 2-hydroxypropyl-β-cyclodextrin decreases neurological disease progression in Niemann-Pick disease, type C1: A non-randomised, open-label, phase 1–2 trial. Lancet. 2017;390(10104):1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gabsi S, Gouider-Khouja N, Belal S, et al. Effect of vitamin E supplementation in patients with ataxia with vitamin E deficiency. Eur J Neurol. 2001;8(5):477–481. [DOI] [PubMed] [Google Scholar]
- 68.Mignot C, Apartis E, Durr A, et al. Phenotypic variability in ARCA2 and identification of a core ataxic phenotype with slow progression. Orphanet J Rare Dis. 2013;8:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf B Successful outcomes of older adolescents and adults with profound biotinidase deficiency identified by newborn screening. Genet Med. 2017;19(4):396–402. [DOI] [PubMed] [Google Scholar]
- 70.Debs R, Depienne C, Rastetter A, et al. Biotin-responsive basal ganglia disease in ethnic Europeans with novel SLC19A3 mutations. Arch Neurol. 2010;67(1):126–130. [DOI] [PubMed] [Google Scholar]
- 71.Campuzano V, Montermini L, Molto MD, et al. Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271(5254):1423–1427. [DOI] [PubMed] [Google Scholar]
- 72.Kemp KC, Hares K, Redondo J, et al. Bone marrow transplantation stimulates neural repair in Friedreich’s ataxia mice. Ann Neurol. 2018;83(4):779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L, Shen X, Liu Z, et al. Activation of Frataxin Protein Expression by Antisense Oligonucleotides Targeting the Mutant Expanded Repeat. Nucleic Acid Ther. 2018;28(1):23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Piguet F, de Montigny C, Vaucamps N, Reutenauer L, Eisenmann A, Puccio H. Rapid and Complete Reversal of Sensory Ataxia by Gene Therapy in a Novel Model of Friedreich Ataxia. Mol Ther. 2018;26(8):1940–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menotta M, Biagiotti S, Spapperi C, et al. ATM splicing variants as biomarkers for low dose dexamethasone treatment of A-T. Orphanet J Rare Dis. 2017;12(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zannolli R, Buoni S, Betti G, et al. A randomized trial of oral betamethasone to reduce ataxia symptoms in ataxia telangiectasia. Mov Disord. 2012;27(10):1312–1316. [DOI] [PubMed] [Google Scholar]
- 77.Foley AR, Menezes MP, Pandraud A, et al. Treatable childhood neuronopathy caused by mutations in riboflavin transporter RFVT2. Brain. 2014;137(Pt 1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guissart C, Drouot N, Oncel I, et al. Genes for spinocerebellar ataxia with blindness and deafness (SCABD/SCAR3, MIM# 271250 and SCABD2). Eur J Hum Genet. 2016;24(8):1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chardon L, Sassolas A, Dingeon B, et al. Identification of two novel mutations and long-term follow-up in abetalipoproteinemia: A report of four cases. Eur J Pediatr. 2009;168(8):983–989. [DOI] [PubMed] [Google Scholar]
- 80.Lee J, Hegele RA. Abetalipoproteinemia and homozygous hypobetalipoproteinemia: A framework for diagnosis and management. J Inherit Metab Dis. 2014;37(3):333–339. [DOI] [PubMed] [Google Scholar]
- 81.Caglayan AO, Gumus H, Sandford E, et al. COQ4 Mutation Leads to Childhood-Onset Ataxia Improved by CoQ10 Administration. Cerebellum. 2019;18(3):665–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baldwin EJ, Gibberd FB, Harley C, Sidey MC, Feher MD, Wierzbicki AS. The effectiveness of long-term dietary therapy in the treatment of adult Refsum disease. J Neurol Neurosurg Psychiatry. 2010;81(9):954–957. [DOI] [PubMed] [Google Scholar]
- 83.Gibberd FB, Billimoria JD, Page NG, Retsas S. Heredopathia atactica polyneuritiformis (refsum’s disease) treated by diet and plasma-exchange. Lancet. 1979;1(8116):575–578. [DOI] [PubMed] [Google Scholar]
- 84.Zolotov D, Wagner S, Kalb K, Bunia J, Heibges A, Klingel R. Long-term strategies for the treatment of Refsum’s disease using therapeutic apheresis. J Clin Apher. 2012;27(2):99–105. [DOI] [PubMed] [Google Scholar]
- 85.Sullivan R, Yau WY, O’Connor E, Houlden H. Spinocerebellar ataxia: An update. J Neurol. 2019;266(2):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lei L-F, Yang G-P, Wang J-L, et al. Safety and efficacy of valproic acid treatment in SCA3/MJD patients. Parkinsonism Relat Disord. 2016;26:55–61. [DOI] [PubMed] [Google Scholar]
- 87.Lo RY, Figueroa KP, Pulst SM, et al. Coenzyme Q10 and spinocerebellar ataxias. Mov Disord. 2015;30(2):214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teixeira-Castro A, Jalles A, Esteves S, et al. Serotonergic signalling suppresses ataxin 3 aggregation and neurotoxicity in animal models of Machado-Joseph disease. Brain. 2015;138(Pt 11):3221–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Scoles DR, Meera P, Schneider MD, et al. Antisense oligonucleotide therapy for spinocerebellar ataxia type 2. Nature. 2017;544(7650):362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Friedrich J, Kordasiewicz HB, O’Callaghan B, et al. Antisense oligonucleotide-mediated ataxin-1 reduction prolongs survival in SCA1 mice and reveals disease-associated transcriptome profiles. JCI Insight. 2018;3(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toonen LJA, Rigo F, van Attikum H, van Roon-Mom WMC. Antisense Oligonucleotide-Mediated Removal of the Polyglutamine Repeat in Spinocerebellar Ataxia Type 3 Mice. Mol Ther Nucleic Acids. 2017;8:232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moore LR, Rajpal G, Dillingham IT, et al. Evaluation of Antisense Oligonucleotides Targeting ATXN3 in SCA3 Mouse Models. Mol Ther Nucleic Acids. 2017;7:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitsui J, Matsukawa T, Yasuda T, Ishiura H, Tsuji S. Plasma Coenzyme Q10 Levels in Patients With Multiple System Atrophy. JAMA Neurol. 2016;73(8):977–980. [DOI] [PubMed] [Google Scholar]
- 94.Miyazaki Y, Du X, Muramatsu S-I, Gomez CM. An miRNA-mediated therapy for SCA6 blocks IRES-driven translation of the CACNA1A second cistron. Sci Transl Med. 2016;8(347):347ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niu C, Prakash TP, Kim A, et al. Antisense oligonucleotides targeting mutant Ataxin-7 restore visual function in a mouse model of spinocerebellar ataxia type 7. Sci Transl Med. 2018;10(465). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nóbrega C, Codêsso JM, Mendonça L, Pereira de Almeida L. RNA Interference Therapy for Machado-Joseph Disease: Long-Term Safety Profile of Lentiviral Vectors Encoding Short Hairpin RNAs Targeting Mutant Ataxin-3. Hum Gene Ther. May 2019. [DOI] [PubMed] [Google Scholar]
- 97.Nóbrega C, Nascimento-Ferreira I, Onofre I, et al. Silencing mutant ataxin-3 rescues motor deficits and neuropathology in Machado-Joseph disease transgenic mice. PLoS ONE. 2013;8(1):e52396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramachandran PS, Boudreau RL, Schaefer KA, La Spada AR, Davidson BL. Nonallele specific silencing of ataxin-7 improves disease phenotypes in a mouse model of SCA7. Mol Ther. 2014;22(9):1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin C-C, Gan S-R, Gupta D, Alaedini A, Green PH, Kuo S-H. Hispanic Spinocerebellar Ataxia Type 35 (SCA35) with a Novel Frameshift Mutation. Cerebellum. 2019;18(2):291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Multiple-System Atrophy Research Collaboration. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369(3):233–244. [DOI] [PubMed] [Google Scholar]
- 101.Kuo S-H, Quinzii CM. Coenzyme Q10 as a Peripheral Biomarker for Multiple System Atrophy. JAMA Neurol. 2016;73(8):917–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barca E, Kleiner G, Tang G, et al. Decreased Coenzyme Q10 Levels in Multiple System Atrophy Cerebellum. J Neuropathol Exp Neurol. 2016;75(7):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Konno M, Hasegawa T, Baba T, et al. Suppression of dynamin GTPase decreases α-synuclein uptake by neuronal and oligodendroglial cells: A potent therapeutic target for synucleinopathy. Mol Neurodegener. 2012;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Friess E, Kuempfel T, Modell S, et al. Paroxetine treatment improves motor symptoms in patients with multiple system atrophy. Parkinsonism Relat Disord. 2006;12(7):432–437. [DOI] [PubMed] [Google Scholar]
- 105.Stefanova N, Georgievska B, Eriksson H, Poewe W, Wenning GK. Myeloperoxidase inhibition ameliorates multiple system atrophy-like degeneration in a transgenic mouse model. Neurotox Res. 2012;21(4):393–404. [DOI] [PubMed] [Google Scholar]
- 106.Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. 2018;141(1):248–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.