Abstract
Increased carotid intima-media thickness (cIMT) is reported in both adults and children with human immunodeficiency virus (HIV) in high income settings and is associated with an increased risk of cardiovascular disease, but data from sub-Saharan Africa is lacking.
We assessed cIMT using ultrasound in perinatally HIV-infected children aged 6 to 16 years taking antiretroviral therapy (ART) for ≥6 months compared with HIV-uninfected controls in Harare, Zimbabwe. Groups were compared using unpaired t test and potential predictors of cIMT were assessed using multiple linear regression.
A total of 117 participants with HIV, of whom 55 (45%) were female and 75 healthy uninfected controls were included. Participants with HIV were younger than uninfected controls, 10.7 (2.4) years versus 11.9 (2.6) years (P = .001). Mean cIMT was 0.40 (0.05) mm in those with HIV versus 0.40 (0.04) mm in healthy controls (P = .377). There was no association between cluster of differentiation 4 count, HIV viral load, and duration on ART and cIMT.
Children with HIV taking ART have similar cIMT to uninfected children. Increasing numbers of children with HIV are reaching adulthood and longitudinal studies to assess the effect of long-term HIV and ART on vascular changes are required.
Keywords: adolescents, antiretroviral therapy, carotid-intima media thickness, HIV
1. Introduction
Carotid intima media thickness (cIMT) is a surrogate marker for atherosclerosis.[1] Subclinical arterial wall alterations may be progressive and precede the development of atherosclerosis. human immunodeficiency virus (HIV) is associated with increased cIMT in both adults and children in reports from high income settings.[2,3] Few studies have been conducted in children in Sub-Saharan African (SSA), where 90% of the world's children with HIV live, and findings from high-income settings may differ to SSA children due to variations in access to antiretroviral therapy (ART) as well as racial differences that have been described before.[4] Notably, a significant proportion of children and adolescents with perinatal HIV in SSA have had delayed diagnosis of HIV and/or ART initiation compared with those from high income settings, and this may impact on cardiovascular disease risk. Therefore, the aim of this study was to assess cIMT in children with HIV and receiving ART in an SSA setting.
2. Methods
The study was part of a larger cohort study to investigate cardiorespiratory disease in older children with HIV taking ART. Cardiac and lung findings have been published and the full protocol has been described elsewhere.[5,6] Participants were recruited from the pediatric HIV clinic at Harare Hospital, the main public sector hospital in the city. Inclusion criteria included age between 6 and 16 years and taking ART for at least 6 months. HIV uninfected children with no known history of cardiac disease were enrolled from 7 primary care clinics in Harare serving the same catchment population as that of Harare Hospital and from the public attending general outpatients at Harare Hospital. Socio-demographic data, HIV-related history, clinical signs and symptoms were collected through a nurse-administered questionnaire. Assessments included heart rate, respiratory rate, blood pressure (BP), and pulse oximetry. Blood samples were collected for measurement of HIV-1 viral load and cluster of differentiation 4 (CD4) count testing. Carotid ultrasound was performed using a Mindray DC N6 ultrasound machine (Mindray, Shenzhen, China). Participants were scanned in supine position with a high frequency linear array probe of 12 MHz. With the patient's head tilted upwards slightly and at 45°, the left and right common carotid arteries were examined respectively. Using two-dimensional images in longitudinal view the carotid bulb was identified and cine loops (10 seconds long) were recorded in Dicom format for later off-line analysis using an automated edge detection system. Intima media thickness measurements were made at approximately 1 to 2 cm from the carotid bulb in triplicate for each side and the average used for analysis.
The study had 80% power to detect a mean difference in cIMT of 0.03 mm and an alpha of 0.05. Unpaired t test was used to compare normally distributed variables and Wilcoxon rank-sum test for variables not normally distributed between the 2 groups. Carotid IMT measures were compared between HIV-infected versus uninfected participants and between HIV-infected participants receiving a protease inhibitor (PI) based regimen compared with a non-PI regimen, using unpaired t tests. The relationship between cIMT and clinical and HIV related factors including CD4 count, viral load, duration of ART, age, body mass index (BMI)-for-age-z-score and systolic BP and diastolic BP were analyzed using multiple linear regression using backward stepwise regression.
Ethical approval for this study was obtained from the Medical Research Council of Zimbabwe, the London School of Hygiene and Tropical Medicine Ethics Committee, the Biomedical Research and Training Institute Institutional Review Board, and the Harare Central Hospital Ethics Committee.
3. Results
A total of 117 participants with HIV, and 75 HIV uninfected controls underwent cIMT scans. There was no difference by sex between the 2 groups but participants with HIV were younger than controls (mean [SD] age 10.7 [2.4] years vs 11.9 [2.6] years [P = .001]); shorter (mean height-for-age-z-score –1.22 [1.0] vs –0.29 [1.0] [P < .001]); and had lower median body mass index (BMI) for-age z-score (–0.47 [–1.09–0.13] vs –0.17 [–0.09–0.63] [P < .019]) (Table 1). All participants with HIV were perinatally infected with a median age at HIV diagnosis of 5 (interquartile range, 3–7) years. The median duration on ART was 4.8 (interquartile range 2.5–6.4) years and 24 (21%) of participants were receiving PI as part of their ART regimen. All participants on the PI-regimen (n = 24) received atazanavir drug boosted with ritonavir. More than 3-quarters of participants with HIV were virally suppressed, 93 (80%).
Table 1.
Clinical characteristics of the participants.
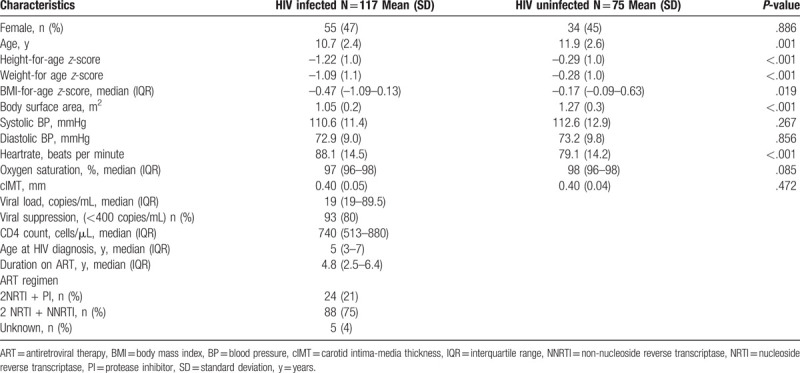
Carotid IMT did not differ between the 2 groups: mean (SD) 0.40 (0.05) mm in those with HIV and 0.40 (0.04) mm in HIV controls (P = .377). There was also no difference in mean cIMT between participants who were and those who were not receiving a PI regimen, 0.40 mm versus 0.40 mm (P = .809) and between virally and non-virally suppressed (P = .514). Age, systolic BP, BMI-for-age-z-score, CD4 count, HIV viral load, and duration on ART were not associated with cIMT. However, diastolic BP was associated with cIMT P = .043.
4. Discussion
We found that older children and adolescents with HIV and receiving ART have similar cIMT to uninfected children. This finding is consistent with other reports of children who have had long-term exposure to ART from Indonesia and France.[7,8] Our findings that ART-experienced children have comparable cIMT to uninfected children could suggest that ART may protect against atherosclerotic changes in childhood. This may be due to slowing of arterial wall structural changes that are associated with HIV infection. A fifth of the participants with HIV were on PI-containing regimen, receiving atazanavir drug, which has been reported to be less atherogenic than other PIs.[9] Data in children from SSA is limited. In one study conducted among Zambian and Ugandan children with HIV, ART-naïve children had significantly higher cIMT, which then improved after 96 weeks of ART initiation.[10] However, the second study conducted among Ethiopian children found that cIMT was similar in the participants regardless of treatment status or ART regimen.[11]
Several studies elsewhere have investigated the association between HIV and cIMT in children and contradicting findings have been reported. Sainz et al,[12] found that Spanish HIV infected children and adolescents, 97% of whom were on ART had increased cIMT thickness compared with uninfected controls. Other studies have found increased cIMT in adult patients receiving PI based ART regimen compared with non-PI treated or uninfected controls.[13] Inconsistent findings in studies investigating cIMT in HIV may be because of different approaches in assessing cIMT,[2,3,12] the operator-dependency of the technique or due to different underlying drivers of vessel thickening in early life. It is also likely that the contradictory findings in previous studies are due to the operator dependency of ultrasound imaging.
Age and ART were found to be associated with increased cIMT in a study conducted among children in United Kingdom.[2] Vigano et al,[14] reported that HIV infection, longer duration on ART, and male sex were associated with increased cIMT in their cohort aged 17 to 23 from Italy. In this study, diastolic BP was associated with cIMT and no HIV-related factors were associated with cIMT. It is possible that our study was underpowered to detect other associations, or it may be that differences in cIMT at this early age may predominantly represent hypertrophy of intimal and/or medial layers as an adaptive response to blood flow and intraluminal pressure rather than subclinical atherosclerosis.[15]
Causes of subclinical atherosclerosis in HIV are likely multifactorial. Both HIV and traditional cardiovascular risk factors reportedly contribute to atherosclerosis in adults[4] and others speculate that chronic inflammation may be the culprit. Children allow the opportunity to investigate risk factors and mechanisms of subclinical atherosclerosis without confounding by traditional cardiovascular risk factors. There are some limitations to this study. Ultrasound is limited as a technique by its operator dependency. Data on lipid profiles and other cardiovascular biomarkers are lacking and the study was cross-sectional and therefore, no causality can be attributed to the factors that were associated with cIMT.
5. Conclusion
Older children and adolescents with HIV and receiving ART have similar cIMT to children without HIV. Longitudinal investigations of vascular structure and function are required to understand whether children and adolescents in SSA receiving ART are at increased risk of subclinical atherosclerosis.
Acknowledgments
The authors would like to thank Harare Hospital, Paediatric HIV clinic, research staff, participants and their families, and HIV Research Trust. Many thanks to University College London, Institute of Cardiovascular Science (Vascular Physiology Unit), London, United Kingdom for assistance with analysis of the carotid scans.
Author contributions
EDM and RAF designed the study. EDM collected and analysed the data with input from STC. All authors contributed to editing and approved the final version of the manuscript.
Scott T. Chiesa: 0000-0003-4323-2189.
Hilda Mujuru: 0000-0003-1615-3856.
Jon Odland: 0000-0002-2756-0732.
Juan P. Kaski: 0000-0002-0014-9927.
Rashida Ferrand: 0000-0002-7660-9176.
Edith Majonga orcid: 0000-0002-9021-0698.
Footnotes
Abbreviations: ART = antiretroviral therapy, BMI = body mass index, BP = blood pressure, cIMT = carotid intima-media thickness, NNRTI = non-nucleoside reverse transcriptase, NRTI = nucleoside reverse transcriptase, PI = protease inhibitor, SSA = Sub-Saharan Africa.
How to cite this article: Majonga ED, Chiesa ST, McHugh G, Mujuru H, Nathoo K, Odland JO, Kaski JP, Ferrand RA. Carotid intima media thickness in older children and adolescents with HIV taking antiretroviral therapy. Medicine. 2020;99:17(e19554).
This work was supported by the Nina Ireland Program for Lung Health and the Wellcome Trust (095878/Z/11/Z).
The authors have no conflicts of interest to disclose.
References
- [1].Eckard AR, Raggi P, Ruff JH, et al. Arterial stiffness in HIV-infected youth and associations with HIV-related variables. Virulence 2017;8:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Charakida M, Donald AE, Green H, et al. Early structural and functional changes of the vasculature in HIV-infected children: impact of disease and antiretroviral therapy. Circulation 2005;112:103–9. [DOI] [PubMed] [Google Scholar]
- [3].Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004;109:1603–8. [DOI] [PubMed] [Google Scholar]
- [4].Whincup PH, Nightingale CM, Owen CG, et al. Ethnic differences in carotid intima-media thickness between UK children of black African-Caribbean and white European origin. Stroke 2012;43:1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Majonga ED, Rehman AM, Simms V, et al. High prevalence of echocardiographic abnormalities in older HIV-infected children taking antiretroviral therapy. AIDS 2018;32:2739–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rylance J, McHugh G, Metcalfe J, et al. Chronic lung disease in HIV-infected children established on antiretroviral therapy. AIDS 2016;30:2795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Idris NS, Grobbee DE, Burgner D, et al. Effects of paediatric HIV infection on childhood vasculature. Eur Heart J 2016;37:3610–6. [DOI] [PubMed] [Google Scholar]
- [8].Bonneta D, Aggouna Y, Szezepanski I, et al. Arterial stiffness and endothelial dysfunction in HIV-infected children. AIDS 2004;18:1037–41. [DOI] [PubMed] [Google Scholar]
- [9].Cahn PE, Gatell JM, Squires K, et al. Atazanavir--a once-daily HIV protease inhibitor that does not cause dyslipidemia in newly treated patients: results from two randomized clinical trials. J Int Assoc Physicians AIDS Care (Chic) 2004;3:92–8. [DOI] [PubMed] [Google Scholar]
- [10].Kenny J, Cook A, Rapala A, et al. Structural Cardiovascular Changes Are Reversible in HIV-Infected Children in Zambia and Uganda. Conference on Retroviruses and Opportunistic Infections. 2015;abstract #37. [Google Scholar]
- [11].Rosebush JC, Gleason RL, Caulk A, et al. Preclinical Atherosclerosis in Eastern Africa: Results From a Pediatric Ethiopian Cohort. 21st Conference on Retroviruses and Opportunistic Organisms in Boston. 2014;Abstract #917. [Google Scholar]
- [12].Sainz T, Álvarez-Fuente M, Navarro ML, et al. Subclinical atherosclerosis and markers of immune activation in HIV-infected children and adolescents: the CaroVIH Study. J Acquir Immune Defic Syndr 2014;65:42–9. [DOI] [PubMed] [Google Scholar]
- [13].Johnsen S, Dolan SE, Fitch KV, et al. Carotid intimal medial thickness in human immunodeficiency virus-infected women: effects of protease inhibitor use, cardiac risk factors, and the metabolic syndrome. J Clin Endocrinol Metab 2006;91:4916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vigano A, Bedogni G, Cerini C, et al. Both HIV-infection and long term antiretroviral therapy are associated with increased common carotid intima-media thickness in HIV infected adolescents and young adults. Curr HIV Res 2010;8:411–7. [DOI] [PubMed] [Google Scholar]
- [15].Bots ML, Hofman A, Grobbee DE. Increased common carotid intima-media thickness. Adaptive response or a reflection of atherosclerosis? Findings from the Rotterdam Study. Stroke 1997;28:2442–7. [DOI] [PubMed] [Google Scholar]


