Supplemental Digital Content is available in the text
Keywords: activation likelihood estimation, insomnia disorder, meta-analysis, multimodal neuroimaging
Abstract
Inconsistent results for comparison between insomnia disorder (ID) patients and healthy controls (HC) were obtained from previous neuroimaging studies. An activation likelihood estimation (ALE) meta-analysis was made for multimodal neuroimaging in ID. ALE analysis indicated that ID patients showed significant gray matter reductions in the right middle frontal gyrus (MFG), compared to HC. Regarding positron emission tomography studies, ALE analysis showed reduced relative cerebral glucose metabolism in the right amygdala, the right anterior cingulate cortex (ACC), and the right posterior cingulate gyrus (PCG) in ID patients, compared to HC. Regarding diffusion tensor imaging studies, the present study indicated that ID patients showed reduced fractional anisotropy values in the left putamen and the right caudate body, compared to HC. Additionally, ID patients showed reduced amplitude of low frequency fluctuations (ALFF) in the left fusiform gyrus (FG), the left middle temporal gyrus (MTG), the right MTG, the right anterior lobe (AL), and the left PCG, compared to HC. ID patients showed increased ALFF in the left MFG, compared to HC. ID patients showed reduced regional homogeneity (ReHo) in the left parahippocampal gyrus, the left sublobar, the left cuneus, the left precentral gyrus (PCG), the right AL, the right ACC, and the right PCG, compared to HC. ID patients showed increased ReHo in the left FG, the left precuneus, and the right cingulate gyrus, compared to HC. Moreover, the ALE analysis showed hypoactivation relative to HC in the left superior temporal gyrus (STG), the left MTG, the right inferior frontal gyrus, the right cuneus, and the right STG in ID patients. Via this ALE meta-analysis, we obtained these key regions suffering from deficits in ID.
1. Introduction
Insomnia disorder (ID) is marked by the difficulty in initiating or maintaining sleep or when the sleep is nonrefreshing or of poor quality.[1] ID is a common sleep problem in adults with a prevalence ranging from 4% to 22% in the worldwide population.[2] Additionally, ID has been considered as the second most common mental disorder in Europe.[3] ID was associated with a reduced quality of life, and may show an increased risk of road and motor vehicle accidents.[4] However, the neurobiology of ID remains unclear.
Magnetic resonance imaging (MRI) is a noninvasive tool with high spatial resolutions. Over the past few decades, various imaging modalities were implemented in ID studies. These different modalities included: structural MRI, diffusion tensor imaging (DTI), functional MRI (fMRI), and positron emission tomography (PET).[5] Most studies adopted a separate modality to explore ID. More efforts should be made to concentrate on integration of different imaging modalities to enhance accuracy of ID diagnosis. Additionally, inconsistent results for comparison between ID and healthy controls (HC) were obtained from studies with specific modality especially from fMRI studies. Therefore, we applied activation likelihood estimation (ALE) method, which is a powerful voxel-based technique for neuroimaging meta-analysis, to explore the key regions of brain pathology in ID.
The present study aimed to perform a comprehensive review of multimodal MRI studies on ID to explore the structural and functional brain changes of ID and key regions suffering from deficits in ID.
2. Methods
The present investigation was performed on the basis of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[6] The study is a meta-analysis, an analysis with secondary processing. Thus, ethical approval was not necessary in the study.
2.1. Search strategy
We searched on PubMed and Web of Science databases for articles in English published until August 2019. Search terms used were: (“insomnia disorder") AND (“neuroimaging" OR “magnetic resonance imaging" OR “MRI"). After removing duplicates, a total of 63 articles were included.
2.2. Inclusion and exclusion criteria
All structural MRI, PET, DTI, and fMRI investigations on ID were included in our study. Additionally, included studies should include both HC and ID as participants. Moreover, included investigations should provide Talairach or Montreal Neurologic Institute (MNI) coordinates for comparisons between HC and ID.
We excluded secondary processing of literature including reviews and meta-analysis articles. Additionally, we excluded case studies without group-level statistics. Moreover, the present study excluded region of interest analyses and seed-based functional connectivity analyses.
2.3. Data collection
Two independent individuals read the titles and abstracts of the finally included 18 articles. We recorded following data from these full-texts: author, publication years, imaging modality and analysis methods, participant demographics (sample size, age, and gender), tasks for task-related fMRI studies, contrasts of included studies, foci, correction for multiple comparisons, and covariates.
2.4. Meta-analysis procedures
We performed the ALE meta-analysis with Java-based version of GingerALE 2.3.6 (http://www.brainmap.org/ale). ALE studies were performed for structural MRI, PET, DTI, rs-fMRI, and working memory-related fMRI investigations in ID. We converted Talairach coordinates to corresponding MNI coordinates with icbm2tal.[7,8] We applied ALE to evaluate the convergence of difference between ID and HC in terms of foci across studies. The foci data were recorded in a text file and read into the software. We acquired statistical significance using a permutation test (5000 permutations) on randomly distributed foci. According to subjects numbers in each study, we calculated full-width-half-maximum.[9] ALE maps were set a threshold at P < .05 using the false discovery rate with an extent threshold >200 mm3. Finally, ALE maps were overlaid onto the MNI 152 template and viewed with Mango software (http://rii.uthscsa.edu/mango).
3. Results
3.1. Search results
The search results and inclusion procedures were showed in Figure 1. Additionally, we summarized study characteristics and results in supplementary Table S1. In finally include n = 18 articles, n = 3 studies[10–12] made analysis of ID-related gray matter (GM) abnormalities with whole-brain voxel-based morphometry (VBM), n = 2 PET experiments[13,14] were included in the present study, n = 2 studies[15,16] investigated disturbed white matter integrity in ID. n = 7 resting-state fMRI studies[10,17–22] (n = 4 studies applied the amplitude of low frequency fluctuations [ALFF] method, n = 2 studies used regional homogeneity [ReHo], n = 1 study applied whole brain functional connectivity) were included in the present study. n = 4 task-related fMRI investigations[23–26] explored brain activation abnormalities in ID patients (n = 2 studies applied working memory tasks, n = 1 study used sleep-related stimuli, n = 1 study applied letter fluency task and category fluency task).
Figure 1.
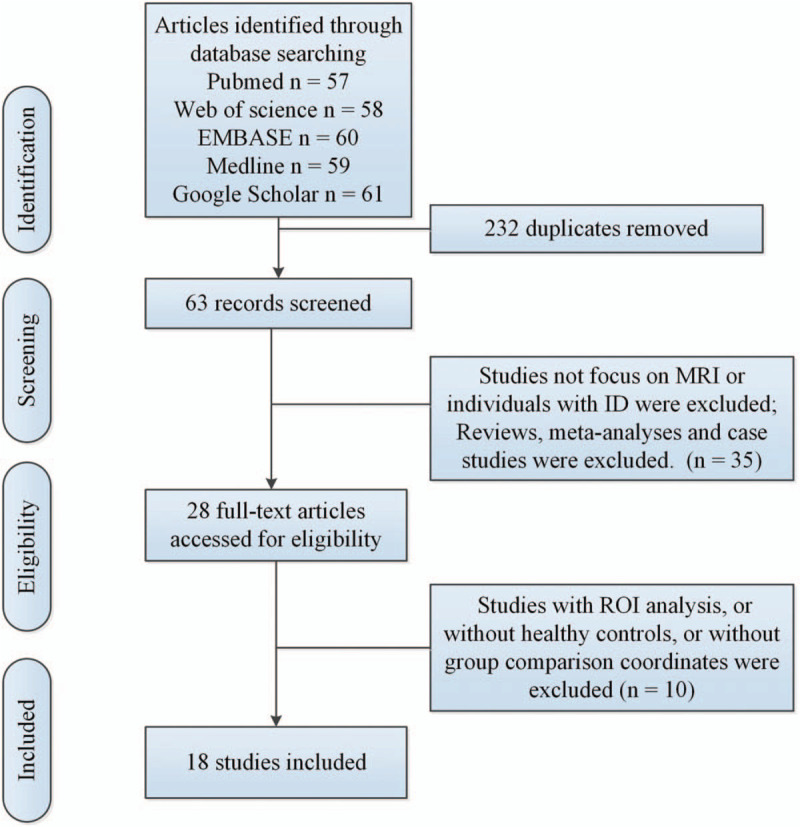
Flow of information through the different phases of a meta-analysis. ID = insomnia disorder, MRI = magnetic resonance imaging, ROI = region of interest.
3.2. Meta-analysis results
ALE analysis indicated that ID patients showed significant GM reductions in the right middle frontal gyrus (MFG), compared to HC (see Fig. 2A and supplementary Table S2). Regarding PET studies, ALE analysis showed reduced relative cerebral glucose metabolism in the right amygdala (AMYG), the right anterior cingulate cortex (ACC), and the right posterior cingulate gyrus (PCG) in ID patients, compared to HC (see Fig. 2B and supplementary Table S2). Regarding DTI studies, the present study indicated that ID patients showed reduced fractional anisotropy values in the left putamen and the right caudate body, compared to HC (see Fig. 2C and supplementary Table S2).
Figure 2.
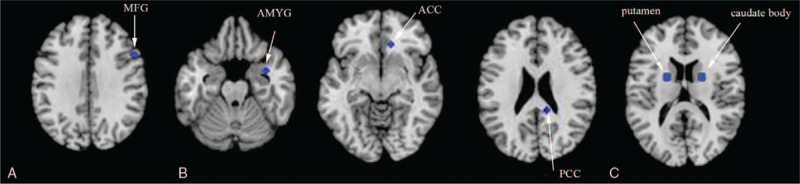
(A) Gray matter reduction in ID patients compared to HC (in blue). (B) Reduced relative cerebral glucose metabolism in ID patients compared to HC (in blue). (C) Lower FA in ID patients relative to HC (in blue). ACC = anterior cingulate cortex, AMYG = amygdala, FA = fractional anisotropy, GM = gray matter, HC = healthy controls, ID = insomnia disorder, MFG = middle frontal gyrus, PCG = posterior cingulate gyrus.
Additionally, ID patients showed reduced ALFF in the left fusiform gyrus (FG), the left middle temporal gyrus (MTG), the right MTG, the right anterior lobe (AL) and the left PCG, compared to HC (see Fig. 3A and supplementary Table S2). ID patients showed increased ALFF in the left MFG, compared to HC (see Fig. 3B and supplementary Table S2). ID patients showed reduced ReHo in the left parahippocampal gyrus (PHG), the left sub-lobar, the left cuneus, the left precentral gyrus (PCG), the right AL, the right ACC, and the right PCG, compared to HC (see Fig. 3C and supplementary Table S2). ID patients showed increased ReHo in the left FG, the left precuneus, and the right cingulate gyrus, compared to HC (see Fig. 3D and supplementary Table S2). Moreover, the ALE analysis showed hypoactivation relative to HC in the left superior temporal gyrus (STG), the left MTG, the right inferior frontal gyrus, the right cuneus, and the right STG in ID patients (see Fig. 3E and supplementary Table S2) in task-related fMRI investigations.
Figure 3.
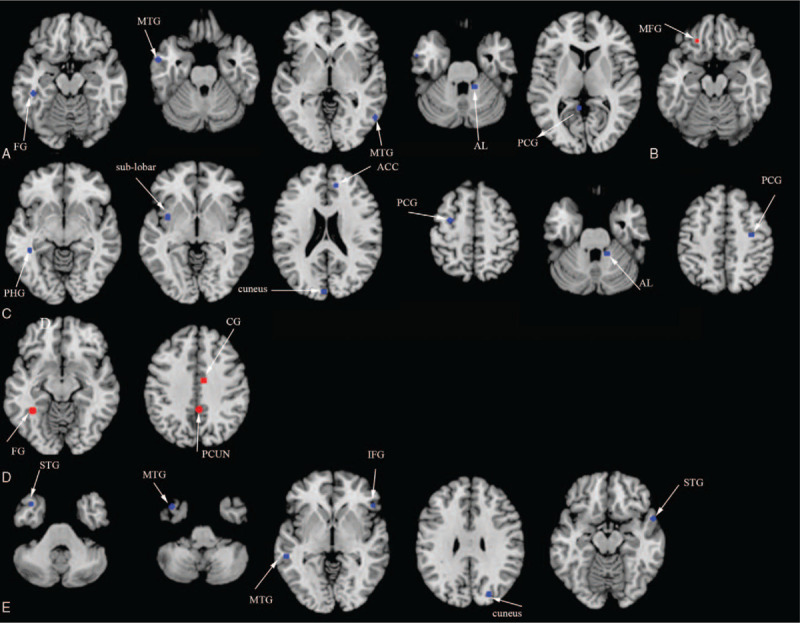
(A) Resting-state hypoactivation (in blue) with ALFF algorithm in ID patients relative to HC. (B) Resting-state hyperactivation (in red) with ALFF algorithm in ID patients relative to HC. (C) Resting-state hypoactivation (in blue) with ReHo algorithm in ID patients relative to HC. (D) Resting-state hyperactivation (in red) with ReHo algorithm in ID patients relative to HC. (E) Hypoactivation (in blue) in ID relative to HC during tasks. ACC = anterior cingulate cortex, AL = anterior lobe, ALFF = amplitude of low frequency fluctuations, CG = cingulate gyrus, FG = fusiform gyrus, HC = healthy controls, ID = insomnia disorder, IFG = inferior frontal gyrus, MTG = middle temporal gyrus, PCG = posterior cingulate gyrus, PCG = precentral gyrus, PCUN = precuneus, PHG = parahippocampal gyrus, ReHo = regional homogeneity, STG = superior temporal gyrus.
4. Discussion
The ALE results reflected different aspects (brain atrophy, microstructural abnormity, neuronal network dysfunctions) of ID-related pathological changes.
The present study indicated that ID patients showed significant GM reductions in the right MFG, compared to HC. The prefrontal cortex is considered to be associated with alertness, attention, and executive function.[27] Some other brain morphological studies also supported structural abnormalities in the prefrontal cortex in primary insomnia (PI) patients. A VBM study[12] reported that PI patients showed significantly reduced GM volumes in medial frontal and middle temporal gyri, compared to HC. A PET study[14] indicated PI patients showed reduced metabolism in the prefrontal cortex when awake. Additionally, fMRI studies also support our results. Altena et al[23] reported that chronic ID patients showed hypoactivation in the medial and inferior prefrontal cortical areas during a category and a letter fluency task. A rs-fMRI study with ALFF algorithm indicated that HC with sleep deprivation showed reduced ALFF in the right inferior parietal lobule, bilateral orbitofrontal cortex, and dorsolateral prefrontal cortex.[28] Our ALE meta-analysis for rs-fMRI studies with ALFF algorithm indicated that ID patients showed increased ALFF in the left MFG, compared to HC. These findings supported that ID patients showed damage in prefrontal cortex structure and function. A recent animal study[29] showed that dysfunction in prefrontal cortex might be associated with neural fatigue of locus coeruleus neurons, projecting to prefrontal cortex, after extended prolonged wakefulness.
The present study showed decreased ALFF and ReHo in temporal lobe in ID, compared to HC. The temporal lobe is memory-related brain lobe.[30] Epidemiological and experimental evidence supported that untreated ID may contribute to cognitive and behavioral symptoms.[31,32] Additionally, a previous study indicated that ID patients showed characteristic abnormalities in the left PHG, right PHG, and bilateral temporal cortex during spatial working memory.[25] Son et al[26] found higher levels of brain activation in the right lateral inferior frontal cortex and the right superior temporal pole in ID patients compared to HC during a working memory task. The dysfunction in the temporal lobe might be the link between ID and memory deficits. The present study showed structural deficits in the right MFG, the right AMYG, the right ACC, the PCG, the left putamen, and the right caudate body in ID patients, compared to HC. Meanwhile, a meta-analysis of 6 MRI and 4 PET studies by Sacher et al[33] claimed deficits in the AMYG, hippocampus, PHG, ACC, orbitofrontal cortex, and insula in major depressive disorder (MDD), compared to HC. The corresponding results obtained from ID and MDD patients could explain the structural association between the 2 diseases. Additionally, ID patients showed functional deficits in the left FG, the bilateral MTG, the right AL, the left PCG, the left PHG, the left sublobar, the left cuneus, the bilateral PCG, and the right ACC, compared to HC. Some of these regions were overlapped with the regions provided by Kuhn and Gallinat[34] that MDD patients showed functional deficits in the left PCG, left FG, and the left insula. The result provided the functional relationship between ID and MDD.
There are some limitations in the present study. First, ALE meta-analysis could not explore the heterogeneity between individual studies. But the present study tried to minimize the heterogeneity via the strict inclusion and exclusion criteria. Moreover, the ALE algorithm is based on a random-effects model which is more conservative than the fixed-effects model and incorporates both within-study and between-study variance. Second, meta-analysis for task-related fMRI reflects different facets of neural activation abnormity during different task, combined study might be not appropriate. Thirdly, ALE technique could not evaluate the significance level of contributing results.
5. Conclusions
Via this ALE meta-analysis, we obtained key regions suffering from different kinds of deficits in ID with multimodal MRI studies. Additionally, the regional abnormalities in MRI studies might serve as biomarkers for early diagnosis of ID.
Author contributions
Investigation: Yangyang Wu, Jun Qi.
Methodology: Yangyang Wu, Jun Qi.
Writing – original draft: Yangyang Wu.
Data curation: Yuan Zhuang.
Formal analysis: Yuan Zhuang.
Software: Yuan Zhuang.
Writing – review & editing: Jun Qi.
Supplementary Material
Footnotes
Abbreviations: ACC = anterior cingulate cortex, AL = anterior lobe, ALE = activation likelihood estimation, ALFF = amplitude of low frequency fluctuations, AMYG = amygdala, CG = cingulate gyrus, DTI = diffusion tensor imaging, FA = fractional anisotropy, FG = fusiform gyrus, fMRI = functional MRI, GM = gray matter, HC = healthy controls, ID = insomnia disorder, IFG = inferior frontal gyrus, MDD = major depressive disorder, MFG = middle frontal gyrus, MNI = Montreal Neurologic Institute, MRI = magnetic resonance imaging, MTG = middle temporal gyrus, PCG = posterior cingulate gyrus, PCG = precentral gyrus, PCUN = precuneus, PET = positron emission tomography, PHG = parahippocampal gyrus, PI = primary insomnia, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses, ReHo = regional homogeneity, ROI = region of interest, STG = superior temporal gyrus.
How to cite this article: Wu Y, Zhuang Y, Qi J. Explore structural and functional brain changes in insomnia disorder: A PRISMA-compliant whole brain ALE meta-analysis for multimodal MRI. Medicine. 2020;99:14(e19151).
Y.W. and Y.Z. contributed equally to the manuscript.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- [2].Ohayon MM, Reynolds CF., 3RD Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD). Sleep Med 2009;10:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wittchen HU, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011;21:655–79. [DOI] [PubMed] [Google Scholar]
- [4].Riemann D, Nissen C, Palagini L, et al. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol 2015;14:547–58. [DOI] [PubMed] [Google Scholar]
- [5].Farras-Permanyer L, Guardia-Olmos J, Pero-Cebollero M. Mild cognitive impairment and fMRI studies of brain functional connectivity: the state of the art. Front Psychol 2015;6:1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Laird AR, Robinson JL, Mcmillan KM, et al. Comparison of the disparity between Talairach and MNI coordinates in functional neuroimaging data: validation of the Lancaster transform. Neuroimage 2010;51:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lancaster JL, Tordesillas-Gutierrez D, Martinez M, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 2007;28:1194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Turkeltaub PE, Eickhoff SB, Laird AR, et al. Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp 2012;33:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li G, Zhang X, Zhang J, et al. Magnetic resonance study on the brain structure and resting-state brain functional connectivity in primary insomnia patients. Medicine 2018;97:e11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhao L, Wang E, Zhang X, et al. Cortical structural connectivity alterations in primary insomnia: insights from MRI-based morphometric correlation analysis. BioMed Res Int 2015;2015:817595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Joo EY, Noh HJ, Kim JS, et al. Brain gray matter deficits in patients with chronic primary insomnia. Sleep 2013;36:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kay DB, Karim HT, Soehner AM, et al. Sleep-wake differences in relative regional cerebral metabolic rate for glucose among patients with insomnia compared with good sleepers. Sleep 2016;39:1779–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nofzinger EA, Buysse DJ, Germain A, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 2004;161:2126–8. [DOI] [PubMed] [Google Scholar]
- [15].Li S, Tian J, Bauer A, et al. Reduced integrity of right lateralized white matter in patients with primary insomnia: a Diffusion-Tensor Imaging Study. Radiology 2016;280:520–8. [DOI] [PubMed] [Google Scholar]
- [16].Spiegelhalder K, Regen W, Prem M, et al. Reduced anterior internal capsule white matter integrity in primary insomnia. Hum Brain Mapp 2014;35:3431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dai XJ, Nie X, Liu X, et al. Gender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI study. J Clin Sleep Med 2016;12:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dai XJ, Peng DC, Gong HH, et al. Altered intrinsic regional brain spontaneous activity and subjective sleep quality in patients with chronic primary insomnia: a resting-state fMRI study. Neuropsychiatr Dis Treat 2014;10:2163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huang S, Zhou F, Jiang J, et al. Regional impairment of intrinsic functional connectivity strength in patients with chronic primary insomnia. Neuropsychiatr Dis Treat 2017;13:1449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ran Q, Chen J, Li C, et al. Abnormal amplitude of low-frequency fluctuations associated with rapid-eye movement in chronic primary insomnia patients. Oncotarget 2017;8:84877–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang T, Li S, Jiang G, et al. Regional homogeneity changes in patients with primary insomnia. Eur Radiol 2016;26:1292–300. [DOI] [PubMed] [Google Scholar]
- [22].Zhou F, Huang S, Zhuang Y, et al. Frequency-dependent changes in local intrinsic oscillations in chronic primary insomnia: a study of the amplitude of low-frequency fluctuations in the resting state. Neuroimage Clin 2017;15:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Altena E, Van Der Werf YD, Sanz-Arigita EJ, et al. Prefrontal hypoactivation and recovery in insomnia. Sleep 2008;31:1271–6. [PMC free article] [PubMed] [Google Scholar]
- [24].Kim SJ, Lee YJ, Kim N, et al. Exploration of changes in the brain response to sleep-related pictures after cognitive-behavioral therapy for psychophysiological insomnia. Sci Rep 2017;7:12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li Y, Liu L, Wang E, et al. Abnormal neural network of primary insomnia: evidence from spatial working memory task fMRI. Eur Neurol 2016;75:48–57. [DOI] [PubMed] [Google Scholar]
- [26]. Son Y D, Kang J M, Cho S J, et al. fMRI brain activation in patients with insomnia disorder during a working memory task [J]. 2018, 22(2): 487-93. [DOI] [PubMed] [Google Scholar]
- [27].Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res 2000;9:335–52. [DOI] [PubMed] [Google Scholar]
- [28].Gao L, Bai L, Zhang Y, et al. Frequency-dependent changes of local resting oscillations in sleep-deprived brain. PLoS One 2015;10:e0120323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bellesi M, Tononi G, Cirelli C, Serra PA. Region-specific dissociation between cortical noradrenaline levels and the sleep/wake cycle. Sleep 2016;39:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Naya Y, Chen H, Yang C, et al. Contributions of primate prefrontal cortex and medial temporal lobe to temporal-order memory. Proc Natl Acad Sci U S A 2017;114:13555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Porter VR, Buxton WG, Avidan AY. Sleep, cognition and dementia. Curr Psychiatry Rep 2015;17:97. [DOI] [PubMed] [Google Scholar]
- [32].Cellini N. Memory consolidation in sleep disorders. Sleep Med Rev 2017;35:101–12. [DOI] [PubMed] [Google Scholar]
- [33].Sacher J, Neumann J, Funfstuck T, et al. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J Affect Disord 2012;140:142–8. [DOI] [PubMed] [Google Scholar]
- [34].Kuhn S, Gallinat J. Resting-state brain activity in schizophrenia and major depression: a quantitative meta-analysis. Schizophr Bull 2013;39:358–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


