Abstract
Patients with chronic ankle instability (CAI) have postural-control deficits during center-of-pressure excursions than do healthy individuals. While an external analysis of center-of-pressure excursions in CAI has been performed, a quantitative analysis of center-of-gravity movements, to detect the balance deficits associated with CAI, has yet to be performed. Therefore, the aim of the study is to quantify the balance deficits in patients with unilateral CAI.
Forty-four patients with unilateral CAI (24 men; age, 31.7 ± 5.5 years) and 26 uninjured volunteers (12 men; age, 28.6 ± 5.9 years) underwent Neurocom Balance Manager assessments of dynamic and static balance responses in limits of stability, unilateral stance, and forward lunge tests.
In the limits of stability test, there were no significant group differences in the forward direction; however, reaction times were longer in the CAI group than in the control group in the backward (P = .037, effect size [ES] = 0.49) and rightward directions (P = .032, ES = 0.47). Furthermore, the CAI group showed more excursions in the rightward (P = .046, ES = 0.50) and leftward directions (P = .002, ES = 0.80), and less directional control in the leftward direction (P = .036, ES = 0.59). In the unilateral stance test, the center of gravity sway velocity was faster in the CAI group than in the control group, whether eyes were opened or closed (P < .05). There were no significant group differences in forward lunge-test outcomes.
Patients with CAI have poor static and dynamic balance performance compared to that in healthy counterparts. Thus, balance retraining should be an essential component of rehabilitation programs for patients with CAI.
Keywords: ankle, deficits, postural balance, sprains
1. Introduction
The ankle joint is particularly susceptible to injury in athletics; the lateral ankle sprain is among the most common injuries of the ankle joint.[1] A lateral ankle sprain damages not only the integrity of the lateral ligaments but also the mechanoreceptors surrounding the ankle joint.[1,2] These receptors allow a sense of the joint position and movement via joint tension and pressure sensory inputs. The visual and vestibular sensory systems integrate within the sensorimotor system, forming a complex system that controls posture and balance.[3] Consequently, impairment of the musculotendinous receptors may result in recurrent ankle instability and/or sprains after an acute ankle sprain.[2,3] This pathology has been defined as chronic ankle instability (CAI), which is characterized by feelings of recurrent instability and “giving way.”[1]
The mechanisms contributing to CAI are traditionally separated into mechanical and functional impairments.[4] As most patients have no clinical evidence supporting mechanical impairments, the study of functional instability is more relevant to clinical practice.[3] The incidence of longstanding residual symptoms after ankle sprains has been reported to be as high as 70%, and the development of CAI affects 31% to 40% of individuals who have suffered a single ankle sprain.[1] Worse still, prolonged instability and repetitive sprains gradually damage the structure of the ankle joint, causing early-onset osteoarthritis in 68% to 78% of patients with CAI.[5] Even with regular physiotherapy, some patients with sprained ankles fail to return to their previous level of physical activity.[5] Furthermore, conventional rehabilitation may be insufficient to reduce the incidence of CAI.[6]
Previous studies and meta-analyses have frequently reported postural-control deficits in patients with CAI.[3] Furthermore, changes in proprioception and neuromuscular control could result in the development of CAI.[4] Postural stability control, maintained by hip and ankle strategies, maybe undermined when proper movements are not performed under a coordinated control system.[3] Various measurements and techniques have been utilized to evaluate postural-control deficits in CAI, such as the single-limb-stance test, time-to-boundary test,[7] Star Excursion Balance Test, and Y-balance test.[8] Instrumented devices, such as force-plates, have been used to quantify postural control by measuring the center-of-pressure (COP) path. However, the accuracy and sensitivity of the measuring methods used in previous studies have been questioned.[9] Furthermore, the single-limb-stance test is not adequately sensitive to evaluate the balance deficits associated with CAI.[9] To maintain balance, continuous body movement and adjustments are performed to keep the body's center-of-gravity (COG) over the specified base of support.[10] The quantification of the ability to maintain balance has been mainly based on the measurement of COP excursions. Thus, until recently, the relationship between COG sway and balance deficits in patients with CAI remained unclear. However, balance is the result of the interaction between postural stability and visual control. Previous studies are limited as they focused primarily on postural control and ignored the function of visual control.[11]
Novel computerized posturography devices have been developed for a more objective assessment of static and dynamic balance responses. Among these, the Neurocom Balance Manager (Neurocom International, LIGENG, USA)[12] is a versatile and useful tool that can perform a wide range of advanced computerized tests, such as limits of stability (LOS), unilateral stance (US), and forward lunge (FL) tests.[12,13] These tests are best described as functional tests that can be used to quantify balance deficits in patients with CAI. Depending on the contextual analysis of COG excursions, researchers can determine the degree to which a person has the ability to make postural corrections and maintain balance.[3] Patients with CAI have fewer strategies and show more difficulty in making postural corrections than do healthy individuals, indicating the presence of impaired balance response and postural control.[5] Furthermore, several studies have shown that patients with CAI display more rigid control strategies during COP excursions than do healthy individuals.[14] While an external analysis of COP excursions in CAI has been performed, a quantitative analysis of COG movements to detect the balance deficits associated with CAI has yet to be performed. Therefore, the primary purpose of this study was to detect balance impairments in individuals with unilateral CAI by comparing static and dynamic balance performance parameters between patients with CAI and healthy counterparts. Based on previous work,[3–6,14] it was hypothesized that alterations in COG movements would further diminish balance response in CAI patients. The findings of this study could further the field's understanding of the functional restrictions related to CAI, and facilitate improvements in rehabilitation, especially in terms of the appropriate application of physiotherapy in individuals with CAI.
2. Methods
2.1. Participants
Forty-four patients with unilateral CAI (24 men, 20 women; age = 31.7 ± 5.5 years; height = 170.7 ± 7.0 cm; mass = 65.9 ± 9.8 kg) and 26 uninjured volunteers (12 men, 14 women; age = 28.6 ± 5.9 years; height = 172.1 ± 5.9 cm; mass = 65.4 ± 8.1 kg) were recruited from the Department of Rehabilitation Medicine, Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine during September 17th to 28th, 2019. For this study, CAI was operationally defined as recurrent episodes of ankle instability (ie, the ankle “giving way”) subsequent to a history of at least 1 ankle sprain.[15] The inclusion criteria for the CAI group were as follows:
-
(1)
a history of acute LAS, but not within the past 6 weeks;
-
(2)
multiple episodes of the ankle giving way or recurrent sprains within the past 12 months;
-
(3)
a Cumberland ankle instability tool (CAIT) score <24; and
-
(4)
no prior balance training.
The exclusion criteria were as follows: a history of ankle injury within the last 3 months, other lower extremity injuries or surgeries within the last 12 months, a vestibular disorder, any peripheral neuropathies, and a history of concussion within the last 3 months.
The inclusion criteria for the control group were as follows:
-
(1)
no history of injury to either ankle;
-
(2)
no history of the ankle giving away;
-
(3)
no lower extremity injuries, vestibular disorders, or cerebral concussions within the last 3 months;
-
(4)
a CAIT score ≥29; and
-
(5)
no prior balance training.
Participants in the control group were matched to those in the CAI group in terms of age, height, and weight.
All participants read and signed an informed consent form, and the study was approved by the Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine Institutional Review Board (approval #SH9H-2019-T54-2).
2.2. Instrumentation
The Neurocom Balance Manager can assess dynamic and static balance performance in LOS, US, and FL tests. The device comprised a computer processing system, force platform, and other auxiliary instruments, and sampled at a frequency of 100 Hz, based on the whole force-plate structure, with 1 transducer oriented horizontally and 4 transducers oriented vertically. Changeable pressure information was conveyed to the host every 10 seconds. Variation in COG movement parameters was calculated and converted to computerized posturography, which was displayed on the screen. By using this visual feedback, participants could make postural corrections to maintain maximum balance. During the testing procedure, the Neurocom force plate remained fixed.
2.3. Procedures
Demographic data were collected before testing. At the beginning of the testing session, an examiner (JLL) presented a verbal and visual demonstration of the testing procedure, and the participant was allowed 3 to 5 minutes of practice before testing. All participants were barefoot during testing. Participants performed 3 trials (separated by 30 seconds) of each test condition, with an approximately 5-minute rest between test conditions to minimize fatigue effects.[11] Participants rarely had discarded trials, and no one reported fatigue during or after the testing session.
2.4. LOS
The LOS test required participants to transfer their COG toward 8 targets at intervals of 45° around the body's COG (presented on a computer monitor) while standing in the standard position. These targets were set by the manufacturer at 100% of the body's LOS, based on the participant's height. Participants were instructed to use their ankle joints as the primary axis of motion to move toward each target as quickly and directly as possible, maintain the target position for 10 seconds, and then return to the initial position. The participants were required to maintain their bodies in a straight line, in the standard standing position, during the test. The computer signaled the participant to move to each target sequentially, as separate subtests (8 seconds each). The 4 dependent LOS outcome measures provided by Neurocom were as follows: the reaction time (RT; the time required to react to each target, measured in seconds), movement velocity (the average speed of the COG movements, measured in degrees per second), max excursions (the percentage of the maximum distance of leaning relative to the linear movement), and directional control (DC; the percentage of the average angular motion of leaning relative to the linear movement). Trials were discarded and repeated if participants changed their foot position relative to the platform, lifted their heel from the platform, or lost their balance at any point in the trial.
2.5. US
Participants were instructed to adopt a single-limb stance on the testing platform, using the involved limb in the CAI group and the dominant leg in the control group. Participants were instructed to hold their unsupported leg at approximately 90 ° of knee flexion and 30 ° of hip flexion, and to look forward, resting their hands on their hips. The participants were required to maintain their balance for 10 seconds, with their body erect. The test was performed under 2 conditions, with eyes open and closed, in this order and with 3 trials each. Mean COG sway velocity (measured in degrees per second) was calculated as an outcome measure assessing static balance. Trials were discarded and repeated if the unsupported leg touched down on the opposite leg, or was braced against the testing platform.
2.6. FL
In the FL test, patients with CAI were instructed to flex the hip and knee of the involved limb, with the goal of stepping forward as quickly and as far as possible while maintaining the tiptoes of the uninvolved limb firmly planted on the platform. The controls flexed the hip and knee of the dominant limb to step forward too. Participants started in a standard standing position with their feet at shoulder width. At the end of the task, the uninvolved limb or nondominant limb was positioned behind the involved limb or dominant limb in a tandem stance. Participants completed 3 trials, with a 30-second rest period between trials. The 4 dependent FL outcome measures provided by Neurocom were as follows: distance (the percentage of the distance stepped forward relative to the participant's height), impact index (the percentage of the force delivered to the force plate relative to the participant's weight), contact time (the time at which the involved foot contacted the force plate, measured in seconds), and impulse index (the percentage of the impact index relative to the contact time). Trials were discarded and repeated if the tiptoes of the uninvolved limb left the platform or balance was lost at any point in the trial.
2.7. Statistical analysis
The average of the 3 trials in each testing condition was calculated and submitted to analysis. The normality of all data from both groups was evaluated using Shapiro–Wilks and Levene tests. Group differences were evaluated using the independent-samples t test for demographic and COG data with a normal distribution and the Mann–Whitney U nonparametric test for COG data not normally distributed. Kolmogorov–Smirnov follow-up tests were conducted for significant results. Between-group effect sizes (ES) are presented. Additionally, in the CAI group, Spearman correlation coefficients (q) were computed to assess the relationships between CAIT scores (ie, self-reported ankle function) and COG parameters (ie, balance responses). The correlational analysis was only performed in the CAI group, as a sufficient spread in the data was lacking in the control group. Correlational coefficients were interpreted as weak (<0.3), moderate (0.3–0.5), or strong (>0.5). The significance level was set at .05, and all statistical analyses were conducted using SPSS (version 22.0; IBM Corp, Armonk, NY).
3. Results
There were no significant differences between the groups in age, mass, and height (P > .05); however, as expected the CAIT scores significantly differed between the control and CAI groups (P < .001) (Table 1).
Table 1.
Demographic data.
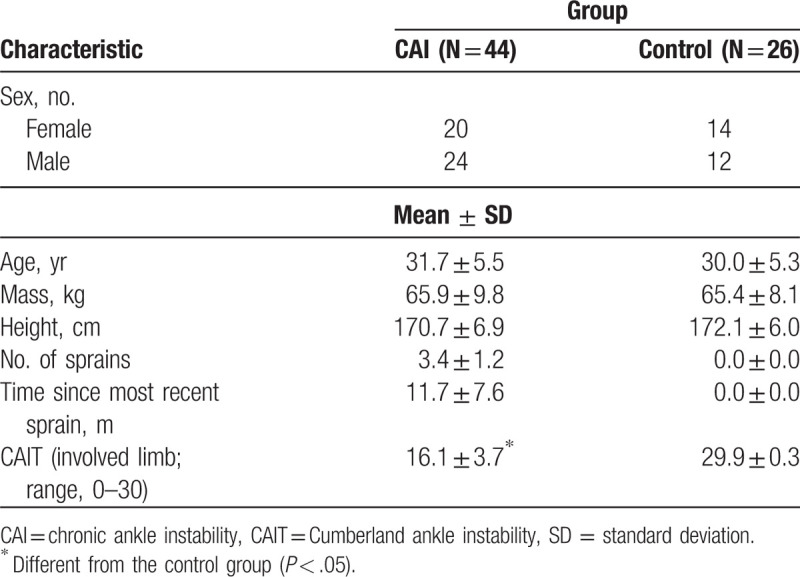
The COG parameters of the LOS test are shown according to the groups in Table 2. There were no significant differences in COG parameters between the groups in the forward direction. However, the CAI group had slower RTs than did the control group in the backward (P = .037, ES = 0.49) and rightward directions (P = .032, ES = 0.47; Fig. 1). Additionally, the CAI group had more excursions in the rightward (P = .046, ES = 0.50) and leftward directions (P = .002, ES = 0.80; Fig. 2), and less DC in the leftward direction (P = .036, ES = 0.59; Fig. 3), compared to those in the control group.
Table 2.
Mean and standard deviation of center of gravity parameters for LOS.
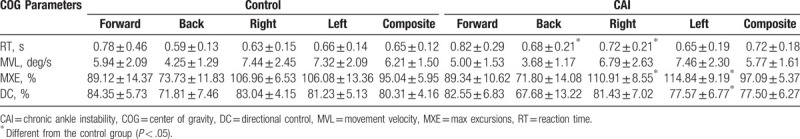
Figure 1.
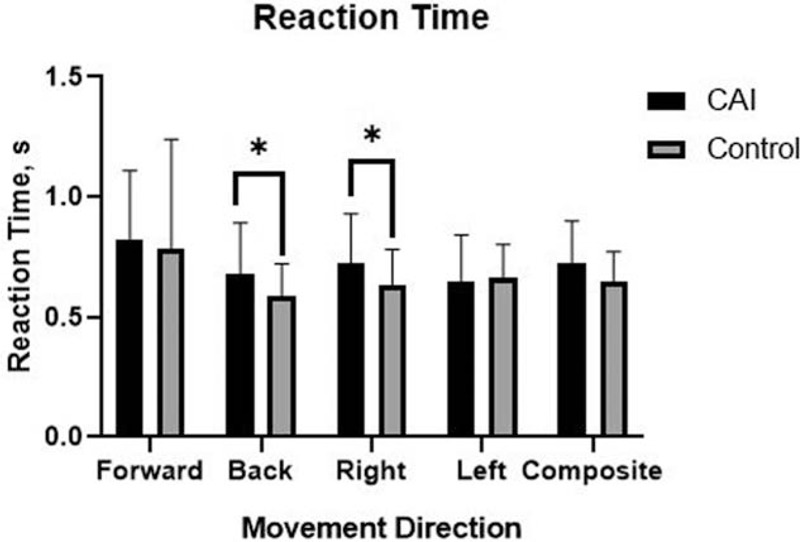
Bar graph of reaction time representing the average ± standard deviation in each group. ∗Different from the control group (P < .05).
Figure 2.
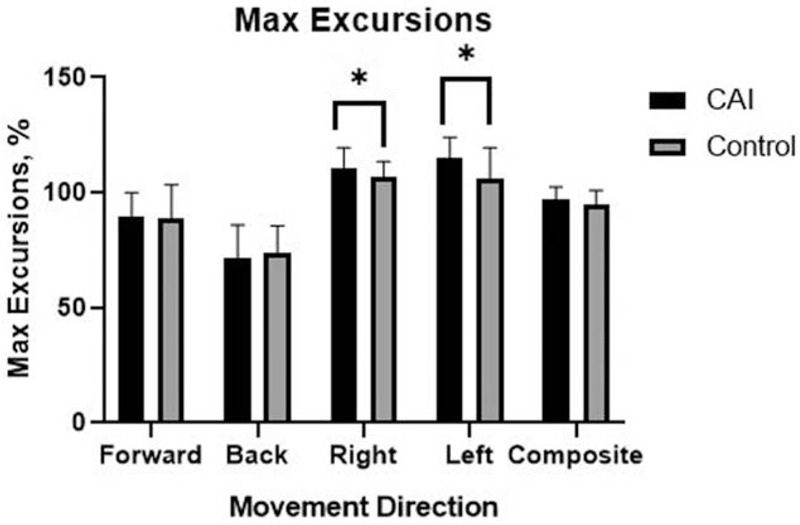
Bar graph of max excursions representing the average ± standard deviation in each group. ∗Different from the control group (P < .05).
Figure 3.
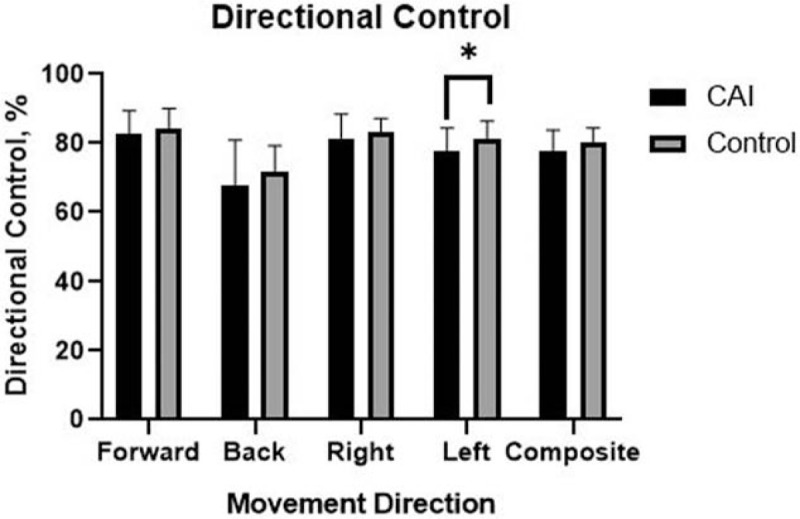
Bar graph of directional control representing the average ± standard deviation in each group. ∗Different from the control group (P < .05).
In the US test, the COG sway velocity (measured in degrees per second) was faster in the CAI group than in the control group during both eyes opened and closed conditions (1.27 ± 0.19 vs 0.87 ± 0.04 with eyes opened; 4.88 ± 0.53 vs 2.15 ± 0.20 with eyes closed) (P < .05, ES = 0.16 with eyes opened; ES = 0.48 with eyes closed). However, there were no differences between the groups in the outcomes of the FL test.
4. Discussion
The extents of both loss and recovery of balance performance are important in determining the prospective outcomes in patients with CAI; therefore, an objective measurement of balance performance impairments associated with CAI is essential. The Neurocom Balance Manager uses COG parameters to quantitate a participant's ability to maintain balance. In the present pilot study, this device was applied to objectively quantify the balance deficits associated with CAI. Our study found that COG parameters varied substantially across the different directions on the LOS. The CAI group had slower RTs than did the control group in the backward and rightward directions. Since the disrupted sensorimotor pathways are associated with diminished postural reflex responses, the CAI group needs more time to react to the targets. Bączkowicz et al[3] reported reducing posterior musculoskeletal activation in CAI patients, patients need more time to react to the target in the backward direction. Twenty-eight CAI patients had instability of the right ankle, which may explain slower RTs in the rightward direction. Further researches need to explore the relationships between the injured limbs and functional performance on the same side. DC is defined based on 100% being a straight line from the participant's COG to the intended target. The CAI group had less DC than those in the control group in the leftward direction. Since the proprioceptive deficit was reported,[7] CAI patients cannot sense corrective joint movement and position, and subsequently, they cannot control the COG movement properly. The alternation of DC in the leftward direction is more difficult to explain. A possible reason for this result could be the right dominant leg in most participants. A participant's COG is more difficult to control while moving to the left. The NeuroCom max excursions variable quantifies the maximum displacement of COG away from targets. We observed that the CAI group had more excursions in the rightward and leftward directions, describing the poor dynamic balance process in the frontal plane. Since inaccurate predictions of movement and DC may result in more excursions, the patient's COG is moved away from the targets. Besides impaired proprioception and neuromuscular control, other factors, such as muscle strength and joint range of motion (ROM), result in balance deficits.[7–9] Dynamic balance tasks place relatively great strength demands on the lower extremity muscles. Decreased strength of lower extremity closed kinetic chain muscles, especially of the peroneus longus and brevis, and restricted motion in the subtalar or talocrural joint affect performance on the LOS in the rightward and leftward directions. The study results indicate that the balance performance in the anterior- and posterior-reach directions of the LOS test was the least affected, while the balance performance in the medial- and lateral-reach directions showed significant differences between CAI and control groups. Thus, patients with CAI showed poor dynamic balance performance during the LOS test.
In the US test, patients with CAI showed faster COG sway velocity, with eyes opened or closed, suggesting impairment in static balance performance. Static balance performance is the ability to limit the movement of the COG while maintaining balance over a stable base of support. CAI is a complex syndrome with various impairments, such as proprioceptive deficit, neuromuscular impairment, muscle weakness, and decreased joint ROM.[8] These impairments may increase the linear COG movement and lead to faster US postural sway velocity. These findings, obtained during functional testing, are consistent with previous studies that reported poor balance response and postural-control deficits in patients with CAI.[3,7,8–10]
According to a recent systematic review with a meta-analysis, poor balance performance and high postural sway in patients with CAI are reported.[16] Poor balance performance in patients with CAI may be related to deficits in proprioception, which are associated with impaired mechanoreceptors in the joints and soft tissues of the ankle region.[3,8] The reductions in joint movement and position sense are partially due to inadequate signals from damaged mechanoreceptors.[17] Furthermore, Garn et al[18] reported that patients with CAI are less able to sense the joint position and detect joint movement for the previously sprained ankle, consequently resulting in impaired neuromuscular responses and poor balance performance. Under these conditions, foot biomechanics may be changing upon loading, but the ankle joint is unable to adapt to alternations in the surface, leading to incorrect foot placement and predisposing the body to be more unstable.[16–18] Vestibular and visual inputs are also important for appropriate postural control and balance response.[12]
Static and dynamic balance assessments not only provide clinicians with an analysis of the patient's ability to maintain balance while incorporating movement but can also determine the effectiveness of physical therapy procedures in CAI.[16] Since the therapeutic strategies of CAI patients are varied based on their functional deficits, clinicians assess patients’ outcomes and recognize deficits to make appropriate treatment decisions. The current standards of clinical practice rely on self-reported questionnaires or noninstrumented clinical tests for clinicians and researchers to determine the loss and recovery of balance performance in CAI.[3,12] Although these tools have proven useful, they have limitations related to their subjectivity and patient apprehension. The quantification of balance responses and postural control is important to the development of rehabilitation interventions in CAI. Thus, the LOS, US, and FL tests have great clinical relevance and should be employed to detect functional performance deficits in patients with CAI, as these tests are associated with the joint ROM, lower-limb strength, proprioception, and neuromuscular response.[16]
Identifying the functional impairments is critical to the development of effective prevention and treatment strategies for patients with CAI. Both surgical and conventional interventions have been used to treat CAI. The efficacy of surgery is not definite, and a consensus on the optimal surgical techniques for patients with CAI is lacking.[19] In contrast, nonsurgical treatments, including taping, joint mobilization, and functional training, have been reported to reduce the occurrence of recurrent ankle injuries.[20] In the present study, balance deficits were observed in patients with CAI, suggesting that balance retraining should be an essential consideration in rehabilitation. Various balance-retraining programs can improve balance responses and postural control in patients with CAI, indicating possible benefits in proprioception and neuromotor control.[21] Balance-retraining programs may also have benefits in preventing recurrent injury and improving sports performance.[22] Although the optimal type of balance-training programs remains controversial, 4 weeks of traditional balance training can effectively improve the self-reported function and dynamic and static postural control during physical activities.[23] A traditional balance training program usually consists of functional tasks and single-leg balance activities.[21] McKeon et al[24] reported on the effectiveness of a progressive dynamic balance-training program, which was designed to challenge motor and landing deficits in patients with CAI. A recent study evaluated the effectiveness of 2 clinically-based 4-week programs, a dynamic balance training program and a traditional balance-training program; both programs produced equally improved outcomes.[24] Moreover, joint mobilizations may be of great benefit in improving dynamic balance responses in patients with CAI.[8] A systematic review conducted by Han et al[25] suggested that a single application of manual therapy is inadequate to reverse the alternations associated with CAI, while 6 sessions of manual therapy can produce positive effects. Thus, to achieve the desired functional performance improvements in clinical practice, multiple sessions and the use of balance-retraining programs combined with manual therapy are essential.
The present study has several limitations, including the relatively small number of participants. Additionally, there were some variables that could not be controlled. For example, the degree of ankle instability and age varied among participants. Thus, demonstration of the significance of these findings requires further study. We did not explore whether balance deficits are the risks of the development of CAI, so we could not determine the relationship between balance deficits and recurrent ankle sprains in patients with CAI. The future studies will focus on exploring the risk factors of recurrent ankle sprains in CAI.
5. Conclusions
The results of the present study provide new information regarding functional restrictions in patients with CAI. Overall, these findings demonstrate that patients with CAI have poor static and dynamic balance performance than the healthy counterparts. Therefore, it is suggested that the quantification of balance may be a helpful tool to provide symptomatic treatment and monitor the effectiveness of specific rehabilitation in CAI patients. The future studies will focus on exploring the risk factors of recurrent ankle sprains in CAI. Since compensatory movement patterns can increase the risk of an ankle injury, future studies are needed to examine the potential neurological and biomechanical mechanisms that relate to poor balance performance in CAI.
Author contributions
Formal analysis: Ling Zhang.
Investigation: Ling Zhang and Junlan Lu.
Methodology: Ling Zhang, Junlan Lu, and Shuai Fan.
Project administration: Shuai Fan and Xing Jiang.
Supervision: Bin Cai.
Writing – original draft: Ling Zhang.
Ling Zhang orcid: 0000-0002-8573-9044.
Footnotes
Abbreviations: CAI = chronic ankle instability, CAIT = Cumberland ankle instability tool, COG = center-of-gravity, COP = center-of-pressure, DC = directional control, FL = forward lunge, LOS = limits of stability, ROM = range of motion, RT = reaction time, US = unilateral stance.
How to cite this article: Zhang L, Lu J, Cai B, Fan S, Jiang X. Quantitative assessments of static and dynamic balance performance in patients with chronic ankle instability. Medicine. 2020;99:17(e19775).
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
LZ and JL contributed equally to this work.
This work was supported by the (China) State's Key Project of Research and Development Plan [grant numbers 2018YFF 0300504].
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- [1].Hershkovich O, Tenenbaum S, Gordon B, et al. A large-scale study on epidemiology and risk factors for chronic ankle instability in young adults. J Foot Ankle Surg 2015;54:183–7. [DOI] [PubMed] [Google Scholar]
- [2].Brand RL, Black HM, Cox JS. The natural history of inadequately treated ankle sprain. Am J of Sports Med 1977;5:248–9. [DOI] [PubMed] [Google Scholar]
- [3].Bączkowicz D, Majorczyk E. Assessment of relationships between joint motion quality and postural control in patients with chronic ankle joint instability. J Orthop Sports Phys Ther 2017;47:570–7. [DOI] [PubMed] [Google Scholar]
- [4].Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train 2002;37:364–75. [PMC free article] [PubMed] [Google Scholar]
- [5].Anandacoomarasamy A, Barnsley L. Long term outcomes of inversion ankle injuries. Br J Sports Med 2005;39:114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Donovan L, Hertel J. A new paradigm for rehabilitation of patients with chronic ankle instability. Phys Sports Med 2012;40:41–51. [DOI] [PubMed] [Google Scholar]
- [7].Arnold BL, Ross SE, Linens S, et al. Ankle instability is associated with balance impairments: a meta-analysis. Med Sci Sports Exer 2009;41:1048–62. [DOI] [PubMed] [Google Scholar]
- [8].Munn J, Sullivan SJ. Evidence of sensorimotor deficits in functional ankle instability: a systematic review with meta-analysis. J Sci Med Sport 2010;13:2–12. [DOI] [PubMed] [Google Scholar]
- [9].Kobayashi T, Tanaka M, Shida M. Intrinsic risk factors of lateral ankle sprain: a systematic review and meta-analysis. Sports Health 2016;8:190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rosen AB, Yentes JM. Alterations in cortical activation among individuals with chronic ankle instability during single-limb postural control. J Athl Train 2019;54:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hadadi M, Abbasi F. Comparison of the effect of the combined mechanism ankle support on static and dynamic postural control of chronic ankle instability patients. Foot Ankle Int 2019;40:702–9. [DOI] [PubMed] [Google Scholar]
- [12].Pickerill ML, Harter RA. Validity and reliability of limits-of-stability testing: a comparison of 2 postural stability evaluation devices. J Athl Train 2011;46:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Feger MA, Donovan L. Lower extremity muscle activation during functional exercises in patients with and without chronic ankle instability. PM R 2014;6:602–11. [DOI] [PubMed] [Google Scholar]
- [14].McKeon PO, Booi MJ, Mattacola CG, et al. Lateral ankle ligament anesthesia significantly alters single limb postural control. Gait Posture 2010;32:374–7. [DOI] [PubMed] [Google Scholar]
- [15].Gribble PA, Delahunt E, Bleakley C, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the international ankle consortium. Br J Sports Med 2014;48:1014–8. [DOI] [PubMed] [Google Scholar]
- [16].Witchalls J, Adams R, Blanch P, et al. Intrinsic functional deficits associated with increased risk of ankle injuries: a systematic review with meta-analysis. Br J Sports Med 2012;46:515–9. [DOI] [PubMed] [Google Scholar]
- [17].Sandrey MA, Kent TE. The effects of eversion fatigue on frontal plane joint position sense in the ankle. J Sport Rehab 2008;17:257–63. [DOI] [PubMed] [Google Scholar]
- [18].Garn SN, Newton RA. Kinesthetic awareness in subjects with multiple ankle sprains. Phys Ther 1988;68:1667–71. [DOI] [PubMed] [Google Scholar]
- [19].Doherty C, Bleakley C. Treatment and prevention of acute and recurrent ankle sprain: an overview of systematic reviews with meta-analysis. Br J Sports Med 2017;51:113–25. [DOI] [PubMed] [Google Scholar]
- [20].Krips R, Sierevelt IN. Interventions for treating chronic ankle instability. Cochrane Database Syst Rev 2011;4:124–8. [DOI] [PubMed] [Google Scholar]
- [21].Burcal CJ, Jeon H. Cortical measures of motor planning and balance training in patients with chronic ankle instability. J Athl Train 2019;54:727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Saugri B, Nargi R. Corrective exercises improve movement efficiency and sensorimotor function but not fatigue sensitivity in chronic ankle instability patients: a randomized controlled trial. Clin Sport Med 2019;29:193–202. [DOI] [PubMed] [Google Scholar]
- [23].Hale SA, Hertel J. The effect of a 4-week comprehensive rehabilitation program on postural control and lower extremity function in individuals with chronic ankle instability. J Orthop Sports Phys Ther 2007;37:303–11. [DOI] [PubMed] [Google Scholar]
- [24].McKeon PO, Ingersoll CD. Balance training improves function and postural control in those with chronic ankle instability. Med Sci Sports Exerc 2008;40:1810–9. [DOI] [PubMed] [Google Scholar]
- [25].Shi X, Han J, Witchalls J, et al. Does treatment duration of manual therapy influence functional outcomes for individuals with chronic ankle instability: a systematic review with meta-analysis? Musculoskelet Sci Pract 2019;40:87–95. [DOI] [PubMed] [Google Scholar]


