Abstract
Background:
Previous studies have demonstrated that the C-reactive protein to albumin ratio (CAR) is correlated with the clinical outcomes of solid tumors. However, the available data have not been systematically evaluated. The objective of the present meta-analysis was to explore the prognostic value of the CAR in solid tumors.
Methods:
Eligible studies were identified from the PubMed, EMBASE and Web of Science electronic databases. The clinical characteristics, disease -free survival (DFS) /progression-free survival (PFS) and overall survival (OS) were extracted from the eligible studies. The pooled hazard ratios (HRs) and 95% confidence intervals were calculated with STATA 12.0 software. We also performed subgroup, meta-regression and sensitivity analyses.
Results:
In total, twenty-seven eligible studies including 10556 patients were enrolled in the present meta-analysis. The pooled HRs with 95% confidence intervals showed that the CAR was significantly associated with poor OS (HR = 1.95, 95% CI: 1.71–2.22) and DFS/PFS (HR = 1.82, 95% CI: 1.61–2.07) in patients with solid tumors. Although publication bias was found in the studies with regard to OS, a further trim and fill analysis revealed that the adjusted HR was 1.82 (95% CI: 1.69–1.96), which was close to the original HR. Subgroup analysis confirmed the CAR as a strong prognostic marker in patients with solid tumors, regardless of the tumor type, detection time, cut-off value, sample size and area.
Conclusion:
Our meta-analysis indicated that a high CAR might be an unfavorable prognostic marker for OS and DFS/PFS in patients with solid tumors.
Keywords: C-reactive protein to albumin ratio, meta-analysis, prognosis, solid tumors
1. Introduction
Cancer remains the root cause of global mortality and has seriously threatened human health. Based on estimates form the American Cancer Society, 1,735,350 new cancer cases and 609,640 cancer deaths were projected to occur in the United States in 2018.[1] Despite great progress made in therapy during the past decades, the survival time of cancer patients is still unfavorable. It is now thought that early diagnosis and treatment monitoring can improve the prognosis of cancer patients. Therefore, there is an urgent need to identify a novel biomarker that can effectively monitor progression and predict prognosis in cancer patients.
Currently, it is known that inflammatory cells and factors may affect the progression of cancer, such as accelerating its growth and inhibiting the apoptosis of transformed cells.[2] Chronic inflammation develops through the action of various inflammatory mediators, including TNF-α, interleukins (IL)-6, and IL-17, leading to eradication of antitumor immunity and accelerated tumor progression.[3] It is estimated that as many as 20% of cancers are instigated by chronic inflammation or persistent infections.[4] During chronic inflammation processes, cytokines, and other related species are found in the blood circulation. Research efforts are now aimed at identifying and measuring these species to inform therapeutic strategy or identify patients who are at risk of develop cancer. These inflammatory biomarkers may help oncologists to determine patient relapses after antitumor therapy. There is a wealth of evidence showing that inflammation-related biomarkers in plasma have been found to be associated with a higher risk of developing cancer: C-reactive protein (CRP), serum amyloid A and soluble tumor necrosis factor receptor-2 were more prevalent in the blood of patients who went on to develop cancer.[5–7] Other biomarker approaches are simpler and easily implemented. Inflammation-based scores, including the Glasgow Prognosis Score (GPS), neutrophil to lymphocyte ratio, platelet to lymphocyte ratio (PLR) and lymphocyte to monocyte ratio have attracted attention as valuable prognosticators and predictors of survival outcomes in many cancers.[8–11] However, the commonly measured inflammatory maker in cancer patients is CRP, and after being combined with albumin to form a scale, it can identify patients who will likely develop cachexia and those who have poor survival. As a familiar inflammation-and-nutrition-based score, the CRP to albumin ratio (CAR) is a combination of CRP and albumin. Recently, 1 published meta-analysis reported that the pretreatment CAR may serve as a prognostic marker in several solid tumors, excluding gynecologic cancer.[12] Due to the lack of a thorough analysis on the reliability and degree of the prognostic significance of the CAR in solid tumors, we performed the present meta-analysis to further elucidate this relationship.
2. Method
2.1. Search strategy
A comprehensive search was employed in the PubMed, EMBASE and Web of Science databases up to April 2019 without applying a start date limit. The following items were applied to research: (“tumor” OR “cancer” OR “neoplasms” OR “carcinoma”) AND (“C-reactive protein/Albumin ratio” OR “CRP/Alb ratio” OR “CAR”) AND (“prognosis” OR “outcome”) AND “cohort studies”. Additionally, possible missing papers were also searched in reference lists of selected papers and related articles.
2.2. Inclusion and exclusion criteria
Studies were selected for the meta-analysis if they satisfied the following criteria:
-
(1)
patients were pathologically diagnosed with any type of solid tumors.
-
(2)
CAR was measured based on the CRP and albumin levels in serum.
-
(3)
The study was designed as a cohort study.
-
(4)
The hazard ratios (HRs) with 95% confidence intervals (CIs) for survival outcomes were reported or could be calculated from the available data in the study.
Articles were excluded according to the following criteria:
-
(1)
the study did not report the prognostic value of the CAR in solid tumors.
-
(2)
The study had a small sample size, with fewer than 30 patients.
-
(3)
The study was a case report, letter, conference abstract, review, duplicate article or animal experiment. (4) The article was not written in English.
2.3. Literature quality assessment
The Newcastle–Ottawa Quality Assessment Scale was used for the quality estimation of the literature by 2 independent investigators. It included 3 aspects, namely, patient selection, study comparability and study endpoints, resulting in a maximum score of 9. Studies that earned scores ≥5 were considered to be of high quality, otherwise, they were considered low quality and removed. Disagreements were resolved by discussion.
2.4. Data extraction
Two investigators read the full texts and collected data independently, including the surname of the first author, year of publication, study country, tumor types, clinical stage, sample size, cut-off value, detection time, follow-up time and survival data. Furthermore, we extracted the HRs and 95% CIs, which were used to assess the association of the CAR with the survival of patients with the solid tumors, including overall survival (OS), disease-free survival (DFS), and progression-free survival (PFS). If the HRs for survival data were not presented directly in the study, they were calculated using established methods provided by Tierney et al.[13] Any inconsistencies between the two investigators with respect to the data extraction were resolved by discussion.
In the present meta-analysis all statistical analyses were conducted using STATA 12.0 software. The pooled HRs and theirs 95%CIs were used to evaluate the relation between the CAR and the survival of patients with solid tumors. If the pooled HRs were greater than 1, we concluded that a high CAR was an unfavorable prognostic factor for patients. Statistical heterogeneity among the studies was evaluated with Cochran Q test and the I2 statistic, with the significance level set at P < .1 or I2 > 50%. A random effects model was used to investigate the pooled HR when significant heterogeneity existed. Otherwise, a fixed effects model was applied. To explore the sources of heterogeneity, subgroup analysis and meta-regression were performed according to the tumor type, detection time, cut-off value, sample size and area. We evaluated the publication bias using a funnel plot and Begg and Egger tests. When the funnel plot was symmetrical and the P value of Begg and Egger tests were >.05, no significant publication bias was considered to exist in this meta-analysis. If publication bias was found, a trim and fill analysis was used to evaluate the number of missing studies and recalculate the pooled risk estimate with the addition of those missing studies.[14] In addition, Sensitivity analysis was also conducted by sequentially omitting individual studies to evaluate the stability of the results.
3. Result
3.1. Study selection
A total of 1275 articles were identified from a search of the PubMed, EMBASE and Web of Science electronic databases. By reading the titles and author details, 880 articles were excluded because they were not relevant to the CAR or were duplicate articles. The remaining 395 articles were further excluded by inspecting the abstracts, and 318 articles were excluded because they were irrelevant types of articles, including letters, reviews and so on. In total, 77 studies were further assessed by reading the full text, and 50 studies were excluded because HR or the full text could not be obtained. In the selection process, 27 eligible articles were finally enrolled for analysis of the prognostic value of the CAR in solid tumors (Fig. 1).
Figure 1.
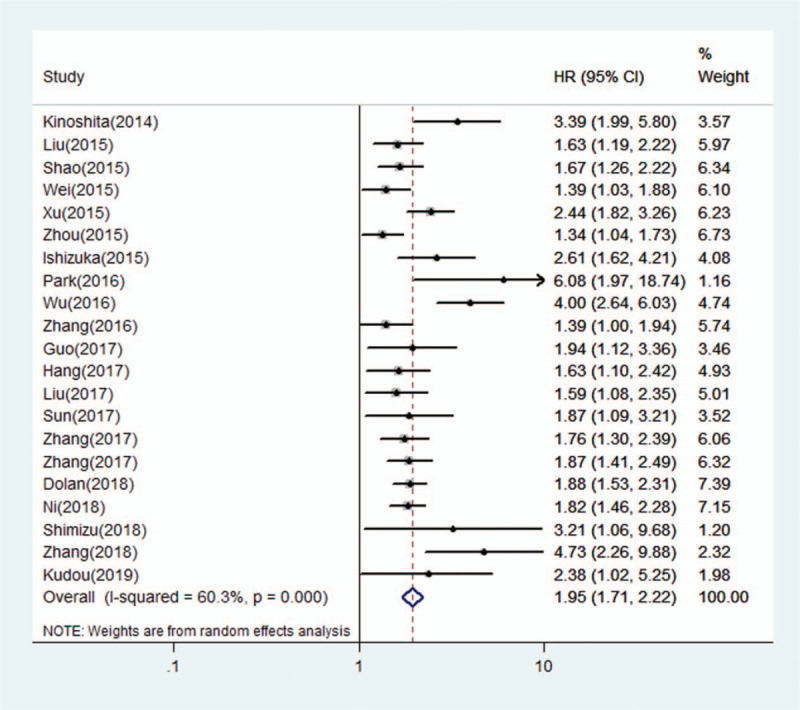
The flow chart of the study selection process in this meta-analysis.
3.2. Characteristics of eligible studies
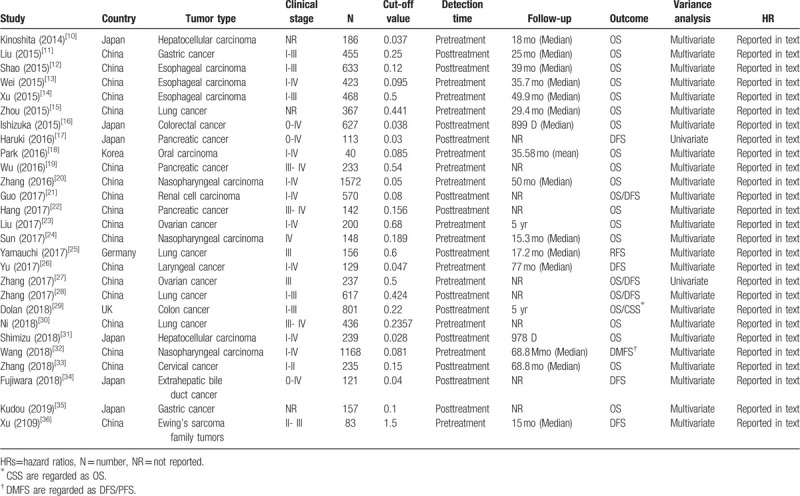
In total, twenty-seven eligible studies with 10556 patients published between 2014 and 2019 were included in the present meta-analysis.[15–41] Eighteen studies were performed in China, and 9 studies were conducted in other countries (6 in Japan, and 1 each in Korea, Germany and the UK). Among the 27 eligible studies, 8 studies focused on head and neck cancers (3 each on esophageal carcinoma and nasopharyngeal carcinoma, 1 each on oral carcinoma and laryngeal cancer), 11 studies focused on abdominal cancer (3 on pancreatic cancer, 2 each on hepatocellular carcinoma, colorectal cancer and gastric cancer, 1 each on bile duct cancer and renal cell carcinoma). 3 studies focused on gynecologic cancer (2 on ovarian cancer, 1 on cervical cancer), 5 on other cancers (4 on lung cancer, 1 on Ewing's sarcoma family tumors). The study sample sizes ranged from 40 to 1572, with a median size of 197. The cut-off values for the CAR ranged from 0.028 to 1.5, with a median cut-off value of 0.156. The level of the CAR in these studies was measured in pretreatment or posttreatment. Among the included studies, OS was reported in 21 studies, and DFS/PFS was reported in 10 studies. The results of the studies were mostly analyzed by multivariate methods, while univariate methods were also utilized. The HRs and 95% CIs for both OS and DFS/PFS were directly reported in all studies. The quality assessment scores of the included studies ranged from 5 to 9, so all studies were regarded as having high quality (Table 2). More detailed information about the main on characteristics was summarized is in Table 1.
Table 2.
Newcastle-Ottawa Scale (NOS) scores for the quality assessment of the studies included in this meta-analysis.
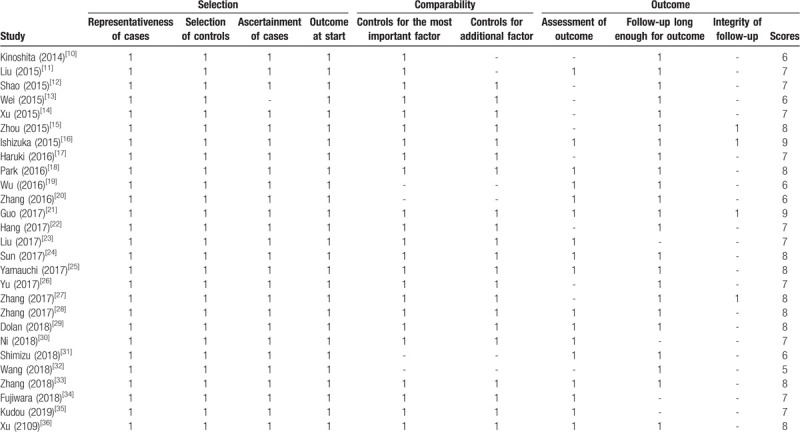
Table 1.
The main characteristics of the studies included in this meta-analysis.
3.3. Correlation between the CAR and survival outcomes in solid tumors
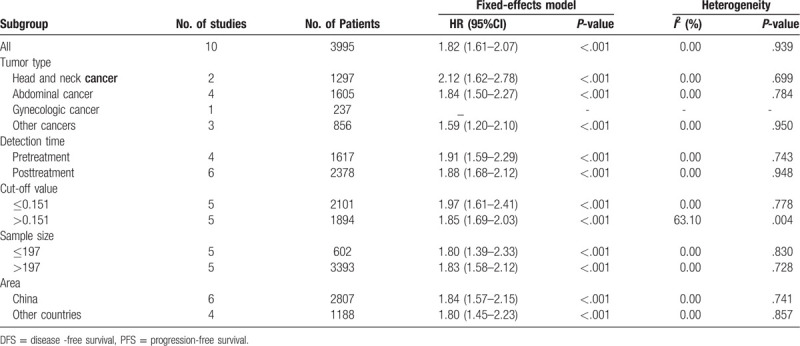
In total, 21 studies with 8786 patients reported a relationship between a high CAR and OS. For OS, we calculated pooled HR using a random-effects model because significant heterogeneity was observed in this meta-analysis (I2 = 60.3%, P < .001). The pooled HR for OS was 1.95 (95% CI: 1.71–2.22, P < .001), which suggested that a high CAR was significantly associated with poor OS in cancer patients (Fig. 2). Ten studies with 3995 patients reported the relationship between the CAR and DFS/PFS. For DFS/PFS, we calculated the pooled HR using a fixed effects model because no heterogeneity was observed in this meta-analysis (I2 = 0.0%, P = .939). The pooled HR for DFS/PFS was 1.82 (95% CI: 1.61–2.07, P < .001), which also suggested that a high CAR was significantly associated with poor DFS/PFS in cancer patients (Fig. 3).
Figure 2.
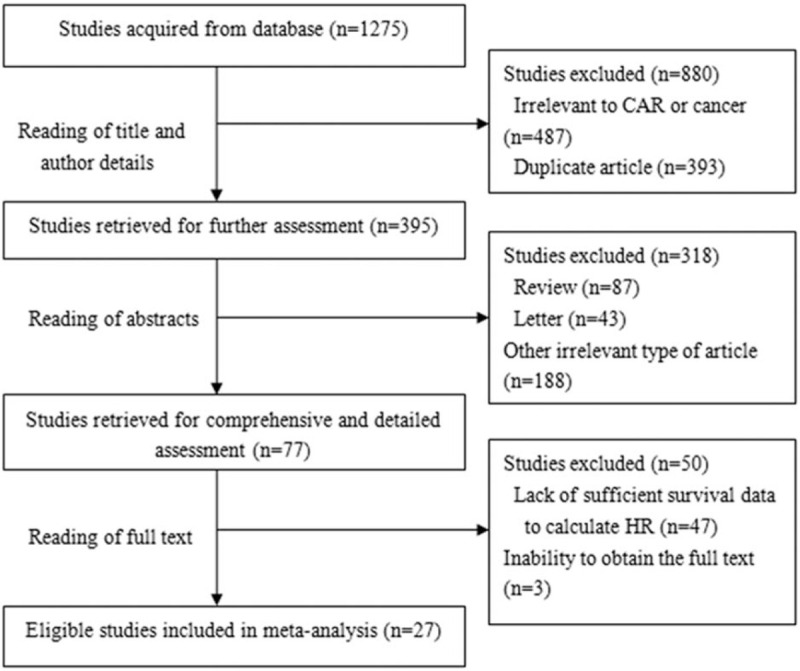
Forest plots of pooled HR of the relationship between a high CAR and OS. CAR = C-reactive protein to albumin ratio, OS=overall survival.
Figure 3.
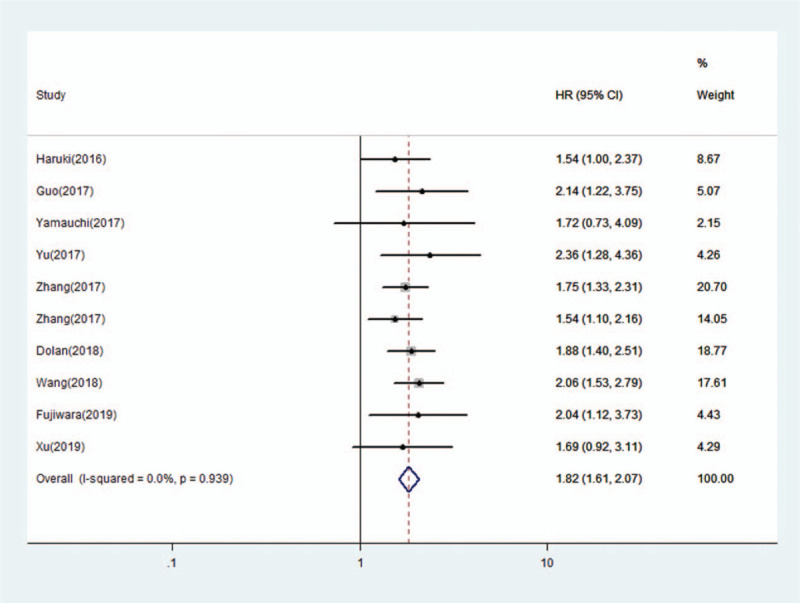
Forest plots of pooled HR of the relationship between a high CAR and DFS/PFS. CAR=C-reactive protein to albumin ratio, DFS=disease -free survival, PFS=progression-free survival.
3.4. Publication bias and sensitivity analysis
In this meta-analysis, a funnel plot and Begg and Egger tests were used to check for potential publication bias. For OS, the funnel plot showed significant asymmetry (Fig. 4A), which combined with the results of Begg test (P = .024) and Egger test (P = .013), suggested that there was statistically significant publication bias in the included studies when combining the HRs of OS. Based on the trim and fill analysis for OS, missing studies were imputed in the contour-enhanced funnel plots (Fig. 5). The analysis indicated that the imputed HR was 1.82 (95% CI: 1.69–1.96), which had no influence on the overall effect of a high CAR on OS. Publication bias was not found in the meta-analysis with DFS/PFS, as shown by asymmetrical funnel plot (Fig. 4B), and the results of Egger test (P = .592) and Begg test (P = .652). To assess whether the results were reliable, it was necessary to conduct further sensitivity analysis. From the results, no substantial fluctuation of pooled HRs for OS (Fig. 6) and DFS/PFS (Fig. 7) was observed when omitting any individual study, which suggested that the pooled HRs for OS and DFS/PFS were robust in our study.
Figure 4.
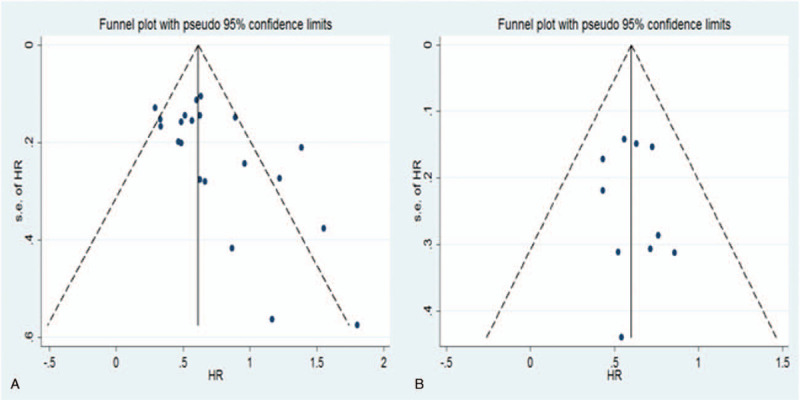
Funnel plots of publication bias of the relationship between a high CAR and OS and DFS/PFS. CAR=C-reactive protein to albumin ratio, DFS=disease -free survival, OS=overall survival, PFS=progression-free survival.
Figure 5.
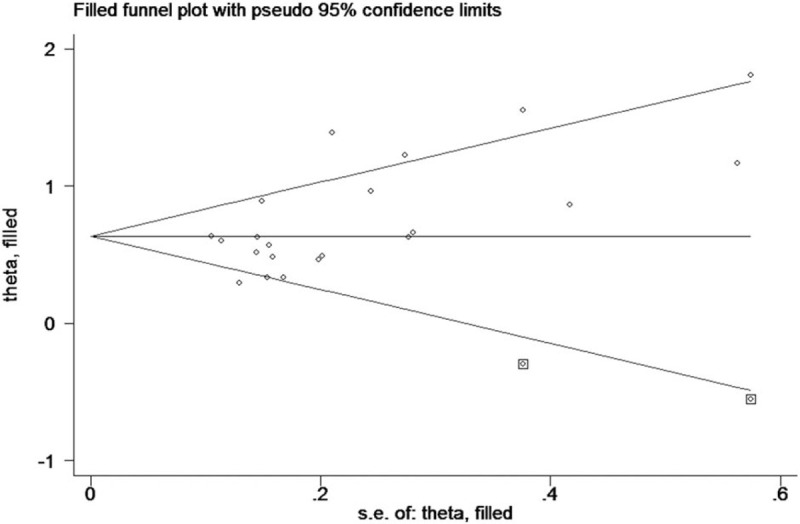
Funnel plots of trim and fill analysis of the relationship between a high CAR and OS. CAR = C-reactive protein to albumin ratio, OS=overall survival.
Figure 6.
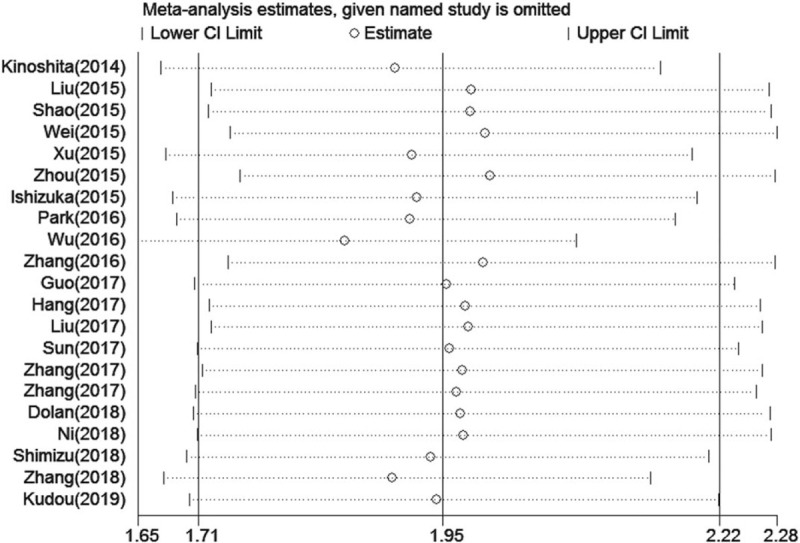
Sensitivity analysis of the relationship between a high CAR and OS. CAR = C-reactive protein to albumin ratio, OS=overall survival.
Figure 7.
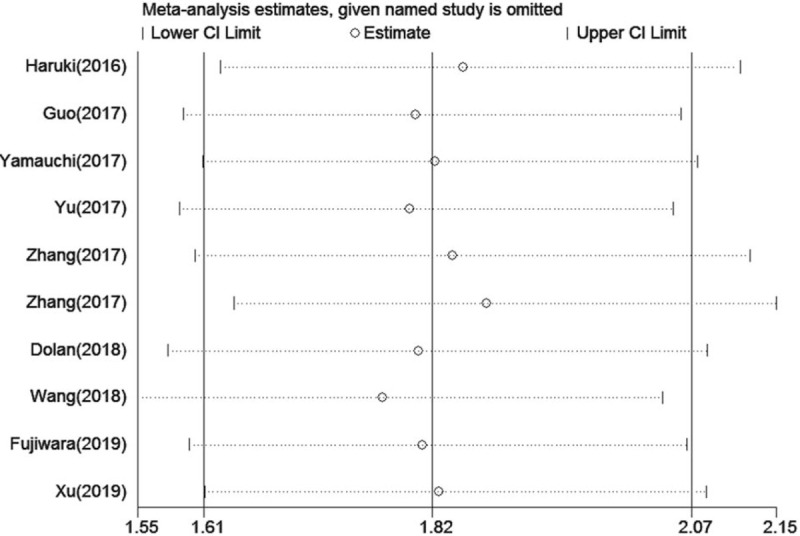
Sensitivity analysis of the relationship between a high CAR and DFS/PFS. CAR=C-reactive protein to albumin ratio, DFS=disease -free survival, PFS=progression-free survival.
3.5. Subgroup analyses
To identify the potential sources of heterogeneity, we performed a subgroup analysis based on the tumor type, detection time, cut-off value, sample size, and area. In the subgroup analysis, we found that a high CAR was closely related to worse OS in patients with head and neck cancer (HR = 1.81; 95% CI: 1.39–2.34; P < 0.001), abdominal cancer (HR = 2.26; 95% CI: 1.80–2.85; P < .001), gynecologic cancer (HR = 1.87; 95% CI: 1.49–2.35; P < .001), and other cancers (HR = 1.66; 95% CI: 1.44–1.95; P < .001) (Table 3). In addition, the subgroup analysis of detection time showed that a high CAR was significantly related to poorer OS both in the pretreatment group (HR = 1.82; 95% CI: 1.64–2.01; P < .001) and the posttreatment group (HR = 1.88; 95% CI: 1.68–2.11; P < .001). The subgroup analysis by cut-off value showed that a high CAR was significantly linked to worse OS regardless of the low cut-off value (≤ 0.156 mg/L) or high cut-off values (>0.156 mg/L). Subgroup analysis on other factors comprising sample size and areas did not alter the significant prognostic impact of a high CAR on OS in patients with solid tumors. In general, the results showed that the pooled HR for OS was stable and reliable, suggesting that the above factors analyzed in the subgroup analysis are not the main source of heterogeneity. Additionally, we performed meta-regression analysis to further determine whether the above factors could account for the majority of the heterogeneity (Table 3). The results showed that all the P values were greater than .05 when we performed meta-regression using any 1 of the above factors as the covariate, which further confirmed that the above factors were not the main the source of heterogeneity. Although no heterogeneity was observed in this meta-analysis for DFS/PFS, the tumor type, detection time, cut-off value, sample size and area may impact the relationship between a high CAR and the outcomes of cancer patients. The results of the subgroup analysis on above factors did not alter the significant prognostic impact of a high CAR on DFS/PFS in patients with solid tumors (Table 4). Therefore, high CAR is significantly associated with poor OS and DFS/PFS despite potential confounding factors.
Table 3.
Subgroup analysis for OS in this meta-analysis.
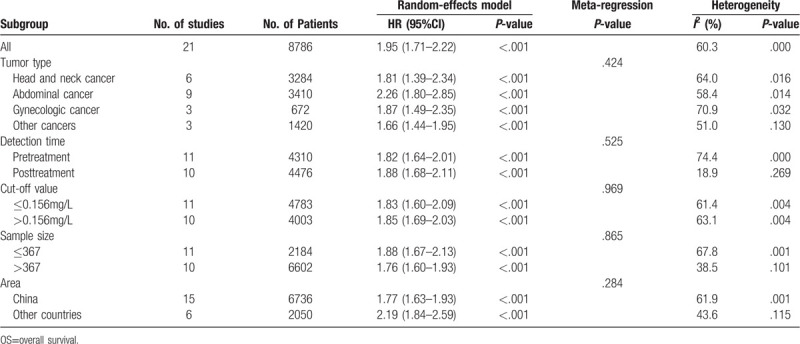
Table 4.
Subgroup analysis for DFS/PFS in this meta-analysis.
4. Discussion
The relationship between inflammation and cancer development has been widely studies.[2] Various types of immune and inflammatory cells are frequently present in the tumor microenvironment.[42] For example, the numbers of circulating blood cells including neutrophils, lymphocytes, and platelets, and the levels of circulating proteins including CRP and ILs that are associated with inflammatory responses, are key factors in the recognition of pathways for tumorigenesis and growth.[43] Recently, inflammation-based prognostic scores, including the NLR, PLR, and GPS have been reported to have prognostic value in patients with various types of cancers.[44–46] However, some studies revealed that the CAR was superior to other inflammation-based prognostic scores, including the NLR, PLR, GPS, and other systemic immune-inflammation indexes, because the CAR had a higher AUC value than other markers.[16,18]
The possible prognostic value of the CAR has been shown in solid tumors. Two meta-analyses published in 2017 both revealed that a high CAR was associated with poor prognosis in solid tumors. In their studies, the impact of the pretreatment CAR on prognosis in human cancers was analyzed, but they overlooked the possibility that the posttreatment CAR might also be an unfavorable prognostic marker for outcomes in patients with solid tumors. In addition, a recent meta-analysis seemed to provide a comprehensive report on the relation between pretreatment CAR and clinical outcomes in several solid tumors, but it did not include gynecologic cancer.
For this reason, we conducted this updated meta-analysis to comprehensively investigate the prognostic value of the CAR in cancer patients. In the present paper, we included 27 studies with 10556 patients with solid tumors. The pooled HR showed that a high CAR was associated with poor OS and DFS/PFS. Although publication bias was found in the studies with regard to OS, a further trim and fill analysis revealed that the adjusted HR was 1.82 (95% CI: 1.69–1.96), which was close to the original HR. We further conducted the subgroup analysis to confirm the above results and exploit more information for clinical application value in the future. When stratified by the detection time, the pooled HR of the pretreatment subgroup was similar to that of the posttreatment subgroup for both OS and DFS/PFS, which suggested that a high CAR played a significant prognostic role in both in pre-treatment and post-treatment patients. Furthermore, our subgroup meta-analysis showed that a high CAR was significantly associated with poor OS in patients with head and neck cancer, abdominal cancer and gynecologic cancer. However, subgroup analysis by other factors, such as the cut-off values, sample sizes and areas did not significantly influence the pooled results of OS and DFS/PFS. Finally, sensitivity analysis demonstrated that the predictive value of a high CAR for patients with solid cancer was reliable. Compared with previously published meta-analyses, our study results enable a more comprehensive understanding of the predictive role of a high CAR in several solid tumors.
However, there were several limitations in our meta-analysis. First, there was some heterogeneity for OS in our meta-analysis and our subgroup and meta-regression analyses failed to identify the source of heterogeneity. Second, 2 studies provided HRs and 95% CIs from univariable analyses, which could lead to bias towards the overestimation of the prognostic role of the CAR, as the HR in multivariable analyses may not be statistically significant after the consideration of other elements. Third, we were not able to conduct a subgroup analysis according to gynecologic cancer for DFS/PFS due to a lack of sufficient data. Finally, the cut-off value for the CAR ranged from 0.028 to 1.5, which varied greatly in the eligible studies and may have some influence on the pooled results.
5. Conclusion
In conclusion, the current meta-analysis suggested that a high CAR is significantly associated with poor OS and DFS/PFS in patients with solid tumors. Thus, the CAR might be used as a novel biomarker to predict the prognosis of patients with solid tumors. Certainly, more high quality studies are needed to further confirm our conclusions.
Author contributions
Conceptualization: Xinhua Cui, Zhiqiang Jia.
Data analysis: Xinhua Cui, Zhiqiang Jia, Dingchao Chen, Chunwei Xu, Peng Yang.
Original draft writing: Xinhua Cui, Zhiqiang Jia.
Review & editing: Xinhua Cui, Zhiqiang Jia, Dingchao Chen.
Footnotes
Abbreviations: CAR = C-reactive protein to albumin ratio, CIs = confidence intervals, CRP = C-reactive protein, DFS = disease -free survival, GPS = glasgow prognosis score, HRs = hazard ratios, ILs = interleukins, OS = overall survival, PFS = progression-free survival, PLR = platelet to lymphocyte ratio.
How to cite this article: Cui X, Jia Z, Chen D, Xu C, Yang P. The prognostic value of the C-reactive protein to albumin ratio in cancer: an updated meta-analysis. Medicine. 2020;99:14(e19165).
The present meta-analysis was based on previous published articles, and no experiments involving humans and animal. Therefore, no ethical approval is required.
The present study was not supported by any fund.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Korniluk A, Koper O, Kemona H, et al. From inflammation to cancer. Ir J Med Sci 2017;186:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 2007;117:1175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wang K, Karin M. Tumor-elicited inflammation and colorectal cancer. Adv Cancer Res 2015;128:173–96. [DOI] [PubMed] [Google Scholar]
- [5].Shrotriya S, Walsh D, Bennani-Baiti N, et al. C-Reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS One 2015;10:e0143080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang JY, Zheng YZ, Yang J, et al. Elevated levels of serum amyloid A indicate poor prognosis in patients with esophageal squamous cell carcinoma. BMC Cancer 2012;12:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang F, Zhao Z, Zhao N. Clinical implications of tumor necrosis factor receptor 2 in breast cancer. Oncol Lett 2017;14:2393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. [DOI] [PubMed] [Google Scholar]
- [9].Zhou X, Du Y, Huang Z, et al. Prognostic value of PLR in various cancers: a meta-analysis. PloS One 2014;9:e101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [11].Nishijima TF, Muss HB, Shachar SS, et al. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev 2015;41:971–8. [DOI] [PubMed] [Google Scholar]
- [12].Xu HJ, Ma Y, Deng F, et al. The prognostic value of C-reactive protein/albumin ratio in human malignancies: an updated meta-analysis. OncoTargets and therapy 2017;10:3059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [15].Kinoshita A, Onoda H, Imai N, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 2015;22:803–10. [DOI] [PubMed] [Google Scholar]
- [16].Liu X, Sun X, Liu J, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol 2015;8:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shao Y, Ning Z, Chen J, et al. Prognostic nomogram integrated systemic inflammation score for patients with esophageal squamous cell carcinoma undergoing radical esophagectomy. Scientific Rep 2015;5:18811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wei XL, Wang FH, Zhang DS, et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: the C-reactive protein/albumin ratio. BMC cancer 2015;15:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu XL, Yu HQ, Hu W, et al. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PloS One 2015;10:e0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhou T, Zhan J, Hong S, et al. Ratio of C-Reactive Protein/Albumin is An Inflammatory Prognostic Score for Predicting Overall Survival of Patients with Small-cell Lung Cancer. Sci Rep 2015;5:10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ishizuka M, Nagata H, Takagi K, et al. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol 2016;23:900–7. [DOI] [PubMed] [Google Scholar]
- [22].Haruki K, Shiba H, Shirai Y, et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg 2016;40:2254–60. [DOI] [PubMed] [Google Scholar]
- [23].Park HC, Kim MY, Kim CH. C-reactive protein/albumin ratio as prognostic score in oral squamous cell carcinoma. J Korean Assoc Oral Maxillofac Surg 2016;42:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wu M, Guo J, Guo L, et al. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol 2016;37:12525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhang Y, Zhou GQ, Liu X, et al. Exploration and validation of C-reactive protein/albumin ratio as a novel inflammation-based prognostic marker in nasopharyngeal carcinoma. J Cancer 2016;7:1406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guo S, He X, Chen Q, et al. The C-reactive protein/albumin ratio, a validated prognostic score, predicts outcome of surgical renal cell carcinoma patients. BMC cancer 2017;17:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hang J, Xue P, Yang H, et al. Pretreatment C-reactive protein to albumin ratio for predicting overall survival in advanced pancreatic cancer patients. Sci Rep 2017;7:2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liu Y, Chen S, Zheng C, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC cancer 2017;17:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sun P, Chen C, Xia Y, et al. The ratio of C-reactive protein/albumin is a novel inflammatory predictor of overall survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. Dis Markers 2017;2017:6570808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yamauchi Y, Safi S, Muley T, et al. C-reactive protein-albumin ratio is an independent prognostic predictor of tumor recurrence in stage IIIA-N2 lung adenocarcinoma patients. Lung cancer (Amsterdam, Netherlands) 2017;114:62–7. [DOI] [PubMed] [Google Scholar]
- [31].Yu ST, Zhou Z, Cai Q, et al. Prognostic value of the C-reactive protein/albumin ratio in patients with laryngeal squamous cell carcinoma. Onco Targets Ther 2017;10:879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang W, Ye B, Liang W, et al. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer. Sci Rep 2017;7:9548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang F, Ying L, Jin J, et al. The C-reactive protein/albumin ratio predicts long-term outcomes of patients with operable non-small cell lung cancer. Oncotarget 2017;8:8835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer 2018;119:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ni XF, Wu J, Ji M, et al. Effect of C-reactive protein/albumin ratio on prognosis in advanced non–small-cell lung cancer. Asia Pac J Clin Oncol 2018;14:402–9. [DOI] [PubMed] [Google Scholar]
- [36].Shimizu T, Ishizuka M, Suzuki T, et al. The value of the C-reactive protein-to-albumin ratio is useful for predicting survival of patients with child-pugh class a undergoing liver resection for hepatocellular carcinoma. World J Surg 2018;42:2218–26. [DOI] [PubMed] [Google Scholar]
- [37].Wang Y, Yang L, Xia LP, et al. High C-reactive protein/albumin ratio predicts unfavorable distant metastasis-free survival in nasopharyngeal carcinoma: a propensity score-matched analysis. Cancer Manag Res 2018;10:371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang WW, Liu KJ, Ye B, et al. Pretreatment C-reactive protein/albumin ratio is associated with poor survival in patients with stage IB-IIA cervical cancer. Cancer Med 2018;7:105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fujiwara Y, Haruki K, Shiba H, et al. The comparison of inflammation-based prognostic scores in patients with extrahepatic bile duct cancer after pancreaticoduodenectomy. J Surg Res 2019;238:102–12. [DOI] [PubMed] [Google Scholar]
- [40].Kudou K, Saeki H, Nakashima Y, et al. C-reactive protein/albumin ratio is a poor prognostic factor of esophagogastric junction and upper gastric cancer. J Gastroenterol Hepatol 2019;34:355–63. [DOI] [PubMed] [Google Scholar]
- [41].Xu KH, Lou Y, Sun R, et al. Establishment of a nomogram-based model for predicting the prognostic value of inflammatory biomarkers and preoperative D-Dimer level in Spinal Ewing's Sarcoma Family Tumors: a retrospective study of 83 patients. World Neurosurg 2019;121:E96–104. [DOI] [PubMed] [Google Scholar]
- [42].Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [44].Yamanaka T, Matsumoto S, Teramukai S, et al. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 2007;73:215–20. [DOI] [PubMed] [Google Scholar]
- [45].Melling N, Gruning A, Tachezy M, et al. Glasgow Prognostic Score may be a prognostic index for overall and perioperative survival in gastric cancer without perioperative treatment. Surgery 2016;159:1548–56. [DOI] [PubMed] [Google Scholar]
- [46].Yu J, Ding Z, Yang Y, et al. Increased platelet-to-lymphocytes ratio is associated with poor long-term prognosis in patients with pancreatic cancer after surgery. Medicine 2018;97:e11002. [DOI] [PMC free article] [PubMed] [Google Scholar]


