Abstract
Despite the plethora of evidence in support of the use of structural osseous autograft in lumbar spondylodiscitis, attention has recently been turned to the addition of synthetic materials such as polyetheretherketone (PEEK) to restore anterior vertebral column support.
From January 2015 to April 2017, 7 patients with lumbar polymicrobial spondylodiscitis were surgically treated with a minimally invasive oblique retroperitoneal approach to the infected focus. The patients underwent a standard lateral minimally invasive oblique retroperitoneal approach using direct lateral interbody fusion system. The PEEK cages were loaded with autologous bone graft. All the patients underwent posterior fixation with percutaneous pedicle screw instrumentation. Lumbar function was measured using Oswestry Disability Index, and pain was measured with visual analog scale. Fusion and subsidence were also recorded.
The study included 5 female and 2 male patients. The median age was 58.9 years. The duration of follow-up was 31.8 ± 6.1 months (range: 24–47). All patients recovered from the infection without relapse within 24-month follow-up. Visual analog scale significantly declined from 7.57 ± 0.53 before surgery to 1.57 ± 0.53 at 12-month follow-up. Mean Oswestry Disability Index decreased from 72.14 ± 6.82 before surgery to 22.28 ± 2.13 after surgery. All patients had solid fusion at 2-year follow-up. Fusion occurred at 6 to 15 months (mean 9.8 months).
The specific use of PEEK cages in lumbar polymicrobial spondylodiscitis suggests reliable outcome in terms of clinical and imaging outcomes in our limited cases.
Keywords: minimally invasive oblique retroperitoneal approach, mixed infections, polyetheretherketone (PEEK), spondylodiscitis
1. Introduction
For cases of spondylodiscitis requiring surgical debridement of the anterior column and subsequent fusion, the choice of graft material for structural support continues to be a topic of debate.[1] The structural support with trimmed iliac bone/fibula bone got its popularity in the last century.[2] The fusion rate for structural support with trimmed iliac bone was high in a series of researches.[3,4] However, the drawback for the cortical autograft is significant donor site morbidity as well as the ability of sagittal alignment maintenance.[5] The combination of titanium cages and autograft was used in the setting of spondylodiscitis in the past 2 decades.[6] Compared with the cortical autograft, the combination of titanium cages and autograft had much less donor site morbidity and acceptable fusion rate. However, the outcomes of segmental correction and subsidence are inconsistently reported.[7] Since 2009, several studies have examined the use of polyetheretherketone (PEEK) implants in the setting of spondylodiscitis.[8–13] Compared with the titanium cages, the biomechanical properties of PEEK implants close to the vertebral body. However, due to the potential for biofilm formation, there are concerns with using PEEK implants in the setting of infection. Within the current evidence on potential for biofilm formation, the results suggest that PEEK is equal or not lower than other materials such as titanium.[14]
Here, we present a series of patients with lumbar spondylodiscitis who were surgically treated with a minimally invasive oblique retroperitoneal approach to the infected focus. More specifically, this study was planned to investigate the usefulness of PEEK, filled with autogenous bone graft, to reliably reduce and stabilize destructed unstable septic spine at the infection site.
2. Materials and methods
2.1. Patient selection
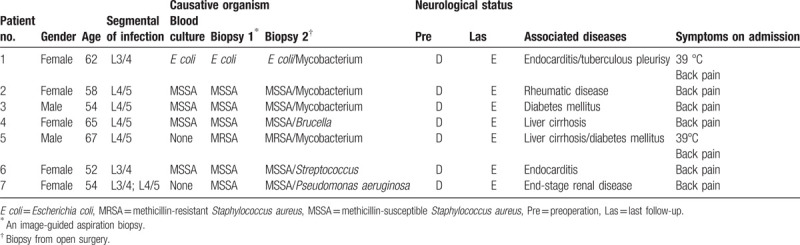
We conducted a retrospective review of patients who had been treated with the minimally invasive oblique retroperitoneal approach for treatment of lumbar osteomyelitis. This study was approved by the Institutional Review Board of The First Affiliated Hospital of Chongqing Medical University. Informed consent was obtained from all individual participants included in the study. Patients were recruited from January 2015 to April 2017, and follow-up was continued until April 2019. All patients were treated by the senior author (Prof Yun-Sheng Ou). The patient demographics are summarized in Table 1.
Table 1.
The patient demographics.
2.2. Surgical procedure
2.2.1. Preoperative management
All patients were clinically diagnosed with polymicrobial spondylodiscitis based on clinical presentation, imaging findings (plain radiographs, computed tomography [CT] scanning, and magnetic resonance imaging [MRI]), and hematological examinations including blood cell count analysis, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level, and biopsy. Six of 7 patients had Staphylococcus aureus and were started on appropriate intravenous antibiotic therapies. Despite adequate medical management, all the patients had remained nonambulatory due to intractable back pain for varying periods of time before surgery. The new microorganisms were detected in the open surgery. Those microorganisms were mycobacterium species, Brucella, Streptococcus, and Pseudomonas aeruginosa (Table 1).
The patients underwent a standard lateral minimally invasive oblique retroperitoneal approach using direct lateral interbody fusion (CLYDESDALE, Medtronic Sofamor Danek, Memphis, TN) system. The patients underwent debridement with discectomy, and either partial or complete corpectomies at the affected levels, which ranged from L3 to L5. The core technology for treating the lumbar spondylodiscitis is a thorough debridement. The anterior approach is useful for ventral decompression, but posterior decompression may be difficult, and it will also be difficult to remove the abscess of the opposite psoas muscle. The posterior approach gave us direct access to posterior decompression. These 2 approaches have different advantages and are used by the different indications. Following debridement, the patients underwent subsequent PEEK cage placement. The cages were loaded with autologous bone graft. All the patients underwent posterior fixation with percutaneous pedicle screw instrumentation (or using the Wiltse paraspinal muscle-splitting approach).
2.2.2. Postoperative management
For the patients who had an identified microbiology, a 6-month antibiotic treatment (intravenous 6 week; oral 5 month) was administered. Reappearance of clinical symptoms and a vertebral lesion meeting the relapse diagnostic criteria were not found in our limited cases.
2.3. Postoperative evaluation
We evaluated functional characteristics using Oswestry Disability Index (ODI).[15] The patient's perception of reasons for the pain was measured with visual analog scale (VAS).[16] To assess deformity, a radiologist measured Cobb angle in the sagittal plane on plain radiographs as the angle between the superior endplate of the L1 and superior endplate S1. Fusion was assessed using the criteria of Lee et al.[17] Subsidence was assessed using the criteria of Pee et al[18] with 5 mm of sinking of graft material under the endplates.
2.4. Statistical analysis
Descriptive statistics were used to explore the mean scores and standard deviations for outcome measures. Differences in categorical variables were assessed with χ2 or Fisher exact tests. t Tests or Wilcoxon rank-sum tests were used to compare parametric and nonparametric continuous data. A P < .05 was considered statistically significant. Data were presented in the form of χ ± s and processed by SPSS (Version 22.0, IBM Corp., New York, NY).
3. Results
The mean age was 58.9 years (52–67) for lumbar spondylodiscitis. All patients had significant back pain; 3 patients complained of brachialgia. Two patients suffered from infection with significant epidural mass and sensorimotor deficits. The most common pathogenic germ was S aureus, less common were mycobacteria species or Streptococcus. The etiology of the infection was known in 2 cases: 1 was spondylodiscitis because of steroid medication and 1 was spondylodiscitis because of an infected wound in a patient with diabetes after lumbar disc operation (Table 1).
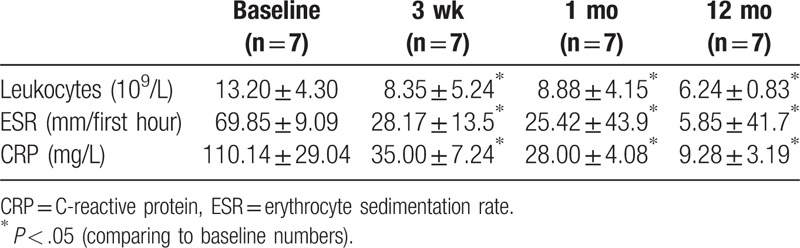
For those patients, an oblique retroperitoneal approach decompression and debridement followed by posterior stabilization using transpedicular screws and intervertebral fusion using PEEK implants was performed. The antibiotic therapy started immediately after surgery and microbiological tests, often included nafcillin/vancomycin and lasted, on average for 2 weeks intravenously, decrease of CRP and relief of complaints and was thereafter continued to take oral antibiotics, on average, for 6 weeks. As for the patients with mycobacteria species, patients continued to take oral HREZ (isoniazid, 300 mg/d; rifampicin, 450 mg/d; ethambutol, 750 mg/d; pyrazinamide, 750 mg/d) chemotherapy postoperatively. Pyrazinamide was discontinued at 6 months. The patients required 9- to 12-month regimens of HRE chemotherapy. CRP significantly declined (baseline: 110.14 ± 29.04; 12-month follow-up: 9.28 ± 3.19). Mean ESR decreased from 69.85 ± 9.09 before surgery to 28.17 ± 13.5 after surgery, and with 5.85 ± 41.7 at 12-month follow-up (Table 2).
Table 2.
The change of inflammation results.
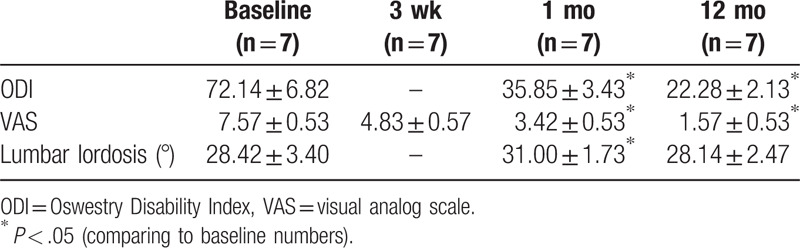
All patients recovered from the infection without relapse. The neurological deficits improved, and the lumbar lordosis was preserved in all patients. Pain significantly declined (baseline: 7.57 ± 0.53; 12-month follow-up: 1.57 ± 0.53). Mean ODI decreased from 72.14 ± 6.82 before surgery to 35.85 ± 3.43 1 months after surgery, and all patients had solid fusion at 2-year follow-up (Table 3). Fusion occurred at 6 to 15 months (mean 9.8 months). Three cases had subsidence.
Table 3.
The change of functional and image results.
3.1. Illustrative cases (case 1 in Table 1)
A patient was a 62-year-old woman with 2 months of severe back pain and paraspinal muscular spasm (VAS: 8; ODI: 80). Routine lateral radiographs showed disc space narrowing at L3/4 with vertebral body height loss (Fig. 1A). Initial MRI showed L3/4 spondylodiscitis and osteitis in both adjacent vertebral bodies. A culture of a biopsy specimen was obtained with Escherichia coli. A 4-week standard antimicrobial treatment was given as initial management. However, the back pain and paraspinal muscle spasm were not relieved (VAS: 8; ODI: 84). Second MRI showed enlarged L3/4 spondylodiscitis and paravertebral abscess (Fig. 1B). During chemotherapy, severe back pain persisted, and new radicular pain occurred (Frankel grade D). Preoperative MRI and CT showed sequestrated bone in L3/L4 body and herniated disc mixed with vertebral abscess, and the Kyphosis angle was 35° (Fig. 1C and D). The patient underwent single-stage debridement via oblique retroperitoneal approach, PEEK cage filled with autologous bone graft, and posterior fixation with percutaneous pedicle screw instrumentation (Fig. 2A). The culture of specimen from the surgery was obtained with E coli and mycobacteria species. After surgery, the patient's radiculopathy alleviated immediately, and the back pain alleviated and disappeared in 1 month. A standard antimicrobial treatment was given. ESR and CRP value returned to normal in 2 months after surgery. One-year follow-up with 3D-CT and lateral radiographic demonstrated strong bony fusion, with no obvious Kyphosis angle lost (Fig. 2B, C, and D). The final functional results were VAS: 1 and ODI: 20.
Figure 1.
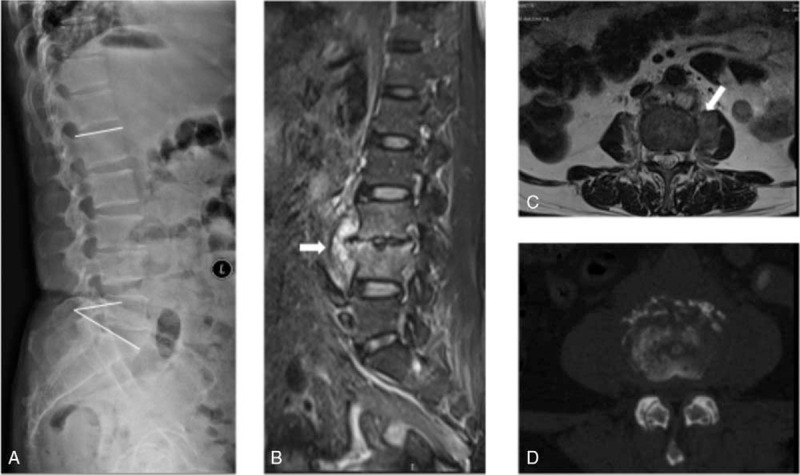
. Preoperative imaging of a 62-year-old woman with vertebral osteomyelitis caused by Escherichia coli and Mycobacterium tuberculosis (case 1 in Table 1). A 62-year-old female patient with L3/4 spondylodiscitis and osteitis. Preoperative neurologic impairment was Frankel grade D. Preoperative X-ray (A) and computed tomography (D) scan show L3/4 bone destruction, and the Kyphosis angle is 35°. MRI (fat-suppressed, gadolinium-enhanced, T2-weighted MRI scan, panels B and C) shows lesions with paravertebral abscess. MRI scan shows bone marrow and disk edema, spinal epidural abscess (the white arrow in panel B), disk enhancement, and a small prevertebral abscess (the white arrow in panels B and C). MRI = magnetic resonance imaging.
Figure 2.
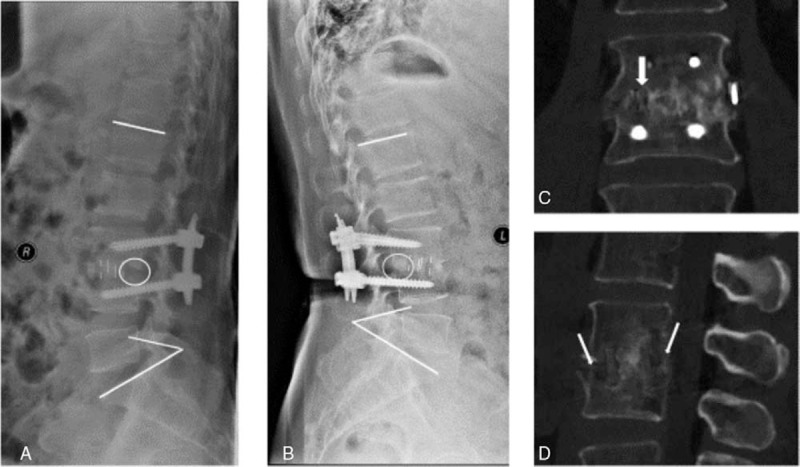
. The follow-up imaging of a 62-year-old woman with vertebral osteomyelitis caused by Escherichia coli and Mycobacterium tuberculosis (case 1 in Table 1). (A) The image was obtained 2 weeks after the surgery, and the Kyphosis angle is 34°. (B) The image was obtained 1 year after the surgery, and the Kyphosis angle is 33°, with no obvious Kyphosis angle lost. The circle in panel A shows no bone formation 2 weeks after the surgery. The circle in panel B shows bone formation 1 year after the surgery. One year after the surgery, CT scan shows strong bony fusion. (C) Coronary CT reconstruction obtained through the central portion of the disc space (the white arrow), demonstrating trabecular bone formation. (D) Sagittal CT reconstruction obtained through the central portion of the disc space, demonstrating trabecular bone formation crossing the disc space (the white arrows). CT = computed tomography.
4. Discussion
4.1. Antibiotic treatment
Optimal management of lumbar spondylodiscitis relies first on the early detection of such patients. Diagnosis of spondylodiscitis should be considered in patients with new or worsening back pain, fever, elevated ESR or CRP, and sometimes with bloodstream infection or infective endocarditis. An image-guided aspiration biopsy in patients with suspected lumbar spondylodiscitis (based on clinical, laboratory, and imaging studies) should be taken when a microbiologic diagnosis has not been established by blood cultures or serologic tests.[19] However, the causative microorganisms are often not identified, and a recent study reported an increasing incidence of spondylodiscitis with no microbiologic diagnosis.[20] Etiologies of cases of microbiologically confirmed spondylodiscitis are summarized in Table 1. Several reasons may attribute to the poor microbiological diagnostic yield in spondylodiscitis. First, empirical antibiotic therapy has been reported in some studies to have a negative effect on microbiologic diagnosis.[15] Second, there is an epidemiologic change for spondylodiscitis as the isolation of less virulent microorganisms among confirmed spondylodiscitis.[20] Third, there are more than 1 kind of microorganisms in a single patient. Here in our limited cases, the reason for unsuccessful treated patient initially was the incomplete microbiological diagnostic. So, bone samples should be cultured for aerobic and anaerobic bacteria and for fungi. Among patients with a suggestive history, cultures should also be performed for mycobacteria and Brucella species.
To our knowledge, there are just few reports for the mixed infection of spondylodiscitis. Giger et al[21] reported Streptococcus mitis and Gemella morbillorum of thoracic vertebrae. The patient was treated with antibiotics and antifungal drugs, and recovered fully. The current results are congruent with prior studies by Issa et al.[22] Increased vertebral instability was found in polymicrobial vertebral osteomyelitis. The lower back pain and neurological dysfunction were also found in our 7 consecutive cases. Those 2 reasons lead to a higher operation rate for polymicrobial vertebral osteomyelitis compared to monomicrobial group. However, the prognosis of polymicrobial vertebral osteomyelitis was even to monomicrobial group. The mean ODI decreased from 77 prior to surgery to 31 after surgery, and all patients had solid fusion at 1-year follow-up in our study.
4.2. PEEK in spondylodiscitis
Most of the patients with spondylodiscitis can be treated with appropriate antibiotic therapy. However, surgical intervention is indicated in the settings of infection refractory to medical management, abscess debridement, correction of significant deformity, as well as decompression of neural elements. The anterior approach to the lumbar spine has been preferred because pathology mainly affects vertebral bodies and disc spaces in some patients. It also allows direct access to the infected focus and is convenient for debriding/reconstructing the defect. With the advancement of modern spinal instrumentations in the past 2 decades, a minimally invasive lateral approach to the lumbar spine was also a choice for the lumbar spondylodiscitis.[23]
Traditionally, human bone graft (the iliac crest, vascularized rib, or the fibula) has been espoused as the gold standard for anterior fusion in the setting of pyogenic spondylodiscitis.[24] However, autograft options have disadvantages in several important ways, namely mechanical stability and donor site morbidity. Later, the usage of titanium cage was also a choice in our early study[25] and other researches.[7] Commonly used synthetic options include metal (titanium mesh or cages) or organic polymers (PEEK or carbon fiber). PEEK cages have been widely used during the past decade.[26] The radiolucency and low elastic modulus make them attractive for spinal fusion. An in vitro study has shown that PEEK implants have a similar or lower rate of biofilm formation as compared with titanium.[14] Here, we treated 7 patients with a minimally invasive oblique retroperitoneal approach to the infected focus. The usefulness of PEEK, filled with autologous bone, to reconstruct anterior columnar defects following debridement had acceptable results within 24-month follow up. There are several advantages of this procedure compared to cortical autograft. First, there is a limited donor site morbidity that is associated with no-cortical autograft. Second, cage size is easily customizable to the size of the defect created following debridement. Third, synthetic grafts provide immediate structural stability, and there is no period of resorption. The results are encouraging in our limited cases.
4.3. Review the literature
In the past 10 years, almost 9 studies have examined the use of PEEK implants in the setting of pyogenic/non-pyogenic spondylodiscitis.[8–13,18,27,28] These studies include 150 patients followed up between 6 months and 6 years. Thereonly one patient underwent early revision secondary to refractory infection with cage subsidence.[33] The fusion rates were well recorded in 4 studies, with 3 studies finding a 100% fusion rate in 34 patients and 1 study finding a 90.5% fusion rate in 21 patients.[10–12] The cage subsidence is a common phenomenon, with 1 study finding a 67% of PEEK implants subsidence.[10] However, the subsidence rate was even higher in the strut group in 1 study. For the functional part, the result looks good. Brase et al[13] reported 9 patients, and the neurological deficits improved in all patients. Pain significantly declined (from 8.1 to 3.2). Mean ODI decreased from 77 before surgery to 31 after surgery, and life quality improved likewise.[13] There was no difference in clinical and imaging outcomes between the strut group and cage group in the study by Pee et al.[18] Collectively, the specific use of PEEK cages in spondylodiscitis showed successful outcome in terms of clinical and imaging outcomes. Nonoperative management of pyogenic/non-pyogenic spondylodiscitis remains the mainstay of treatment. However, in cases where surgical debridement and stabilization is indicated, synthetic graft is a viable option that avoids donor site morbidity, allows for immediate and durable stability, and is easily customizable to the size of the defect. These benefits outweigh what appears to be a low risk of recurrent infection, and thus should be considered by surgeons managing this challenging problem.
5. Limitations
Information on some of the baseline characteristics (eg, race/ethnicity, education, nutritional status) was lacking. Other limitations included a small sample size and relatively short-term follow-up period. More studies are needed to investigate this issue.
6. Conclusion
The specific use of PEEK cages in lumbar polymicrobial spondylodiscitis suggests reliable outcome in terms of clinical and imaging outcomes in our limited cases.
Author contributions
Data curation: Wei Luo.
Formal analysis: Yun-Sheng Ou.
Project administration: Wei Luo, Yun-Sheng Ou.
Supervision: Yong Zhu.
Validation: Zeng-Hui Zhao.
Investigation: Yun-Sheng Ou.
Writing – original draft: Wei Luo.
Writing – review & editing: Yun-Sheng Ou.
Footnotes
Abbreviations: CRP = C-reactive protein, CT = computed tomography, ESR = erythrocyte sedimentation rate, MRI = magnetic resonance imaging, ODI = Oswestry Disability Index, PEEK = polyetheretherketone, VAS = visual analog scale.
How to cite this article: Luo W, Zhu Y, Zhao ZH, Ou YS. Application of polyetheretherketone cages through minimally invasive oblique retroperitoneal approach for the treatment of lumbar polymicrobial spondylodiscitis: a STROBE-compliant retrospective study with 7 cases. Medicine. 2020;99:17(e18594).
The authors did not and will not receive any benefits or funding from any commercial party related directly or indirectly to the subject of this article.
The authors have no conflicts of interest to disclose.
References
- [1].Saxena SK, Grimm PD, Bharmal HM. Is it safe to use synthetic grafts in pyogenic vertebral osteodiskitis when surgical debridement is required? Clin Spine Surg 2018;31:269–73. [DOI] [PubMed] [Google Scholar]
- [2].Chen WH, Jiang LS, Da LY. Surgical treatment of pyogenic vertebral osteomyelitis with spinal instrumentation. Eur Spine J 2007;16:1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Emery SE, Chan DP, Woodward HR. Treatment of hematogenous pyogenic vertebral osteomyelitis with anterior debridement and primary bone grafting. Spine (Phila Pa 1976) 1989;14:284–91. [PubMed] [Google Scholar]
- [4].McGuire RA, Eismont FJ. The fate of autogenous bone graft in surgically treated pyogenic vertebral osteomyelitis. J Spinal Disord 1994;7:206–15. [DOI] [PubMed] [Google Scholar]
- [5].Robertson PA, Wray AC. Natural history of posterior iliac crest bone graft donation for spinal surgery: a prospective analysis of morbidity. Spine (Phila Pa 1976) 2001;26:1473–6. [DOI] [PubMed] [Google Scholar]
- [6].Fayazi AH, Ludwig SC, Dabbah M, et al. Preliminary results of staged anterior debridement and reconstruction using titanium mesh cages in the treatment of thoracolumbar vertebral osteomyelitis. Spine J 2004;4:388–95. [DOI] [PubMed] [Google Scholar]
- [7].Korovessis P, Repantis T, Iliopoulos P, et al. Beneficial influence of titanium mesh cage on infection healing and spinal reconstruction in hematogenous septic spondylitis: a retrospective analysis of surgical outcome of twenty-five consecutive cases and review of literature. Spine (Phila Pa 1976) 2008;33:E759–67. [DOI] [PubMed] [Google Scholar]
- [8].Walter J, Kuhn SA, Reichart R, et al. PEEK cages as a potential alternative in the treatment of cervical spondylodiscitis: a preliminary report on a patient series. Eur Spine J 2010;19:1004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mondorf Y, Gaab MR, Oertel JM. PEEK cage cervical ventral fusion in spondylodiscitis. Acta Neurochir (Wien) 2009;151:1537–41. [DOI] [PubMed] [Google Scholar]
- [10].Schomacher M, Finger T, Koeppen D, et al. Application of titanium and polyetheretherketone cages in the treatment of pyogenic spondylodiscitis. Clin Neurol Neurosurg 2014;127:65–70. [DOI] [PubMed] [Google Scholar]
- [11].Patel NB, Dodd ZH, Voorhies J, et al. Minimally invasive lateral transpsoas approach for spinal discitis and osteomyelitis. J Clin Neurosci 2015;22:1753–7. [DOI] [PubMed] [Google Scholar]
- [12].Tschöke SK, Fuchs H, Schmidt O, et al. Single-stage debridement and spinal fusion using PEEK cages through a posterior approach for eradication of lumbar pyogenic spondylodiscitis: a safe treatment strategy for a detrimental condition. Patient Saf Surg 2015;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brase A, Ringel F, Stuer C, et al. Debridement and fusion with polyetheretherketone implants in purulent spondylodiscitis: a clinical experience with nine patients. Acta Neurochir (Wien) 2010;152:2001–4. [DOI] [PubMed] [Google Scholar]
- [14].Hahnel S, Wieser A, Lang R, et al. Biofilm formation on the surface of modern implant abutment materials. Clin Oral Implants Res 2015;26:1297–301. [DOI] [PubMed] [Google Scholar]
- [15].Fairbank JC, Pynsent PB. The Oswestry disability index. Spine (Phila Pa 1976) 2000;25:2940–52. discussion 2952. [DOI] [PubMed] [Google Scholar]
- [16].Scott J, Huskisson EC. Graphic representation of pain. Pain 1976;2:175–84. [PubMed] [Google Scholar]
- [17].Lee CK, Vessa P, Lee JK. Chronic disabling low back pain syndrome caused by internal disc derangements: the results of disc excision and posterior lumbar interbody fusion. Spine (Phila Pa 1976) 1995;20:356–61. [DOI] [PubMed] [Google Scholar]
- [18].Pee YH, Park JD, Choi YG, et al. Anterior debridement and fusion followed by posterior pedicle screw fixation in pyogenic spondylodiscitis: autologous iliac bone strut versus cage. J Neurosurg Spine 2008;8:405–12. [DOI] [PubMed] [Google Scholar]
- [19].Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother 2010;65: Suppl 3: iii11–24. [DOI] [PubMed] [Google Scholar]
- [20].Lora-Tamayo J, Euba G, Narvaez JA, et al. Changing trends in the epidemiology of pyogenic vertebral osteomyelitis: the impact of cases with no microbiologic diagnosis. Semin Arthritis Rheum 2011;41:247–55. [DOI] [PubMed] [Google Scholar]
- [21].Giger A, Yusuf E, Manuel O, et al. Polymicrobial vertebral osteomyelitis after oesophageal biopsy: a case report. BMC Infect Dis 2016;16:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Issa K, Pourtaheri S, Stewart T, et al. Clinical differences between monomicrobial and polymicrobial vertebral osteomyelitis. Orthopedics 2017;40:e370–3. [DOI] [PubMed] [Google Scholar]
- [23].Lin Y, Li F, Chen W, et al. Single-level lumbar pyogenic spondylodiscitis treated with mini-open anterior debridement and fusion in combination with posterior percutaneous fixation via a modified anterior lumbar interbody fusion approach. J Neurosurg Spine 2015;23:747–53. [DOI] [PubMed] [Google Scholar]
- [24].Tay BK, Deckey J, Hu SS. Spinal infections. J Am Acad Orthop Surg 2002;10:188–97. [DOI] [PubMed] [Google Scholar]
- [25].Yong Z, Peng W, Wei L, et al. Single-stage posterior instrumentation and unilateral transpedicular debridement for the treatment of thoracolumbar tuberculosis: three years of follow-up. World Neurosurg 2019;121:e230–6. [DOI] [PubMed] [Google Scholar]
- [26].Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007;28:4845–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shiban E, Janssen I, da Cunha PR, et al. Safety and efficacy of polyetheretherketone (PEEK) cages in combination with posterior pedicel screw fixation in pyogenic spinal infection. Acta Neurochir (Wien) 2016;158:1851–7. [DOI] [PubMed] [Google Scholar]
- [28].von der Hoeh NH, Voelker A, Hofmann A, et al. Pyogenic spondylodiscitis of the thoracic spine: outcome of 1-stage posterior versus 2-stage posterior and anterior spinal reconstruction in adults. World Neurosurg 2018;120:e297–303. [DOI] [PubMed] [Google Scholar]


