Supplemental Digital Content is available in the text
Keywords: Bayesian analysis, connective tissue disease, diagnosis, interstitial lung disease, Krebs von den Lungen-6, surfactant protein D
Abstract
Purpose:
The aim of the study was to estimate and compare the diagnostic accuracy of serum Krebs von den Lungen-6 (KL-6) and surfactant protein D (SP-D) for identifying interstitial lung disease (ILD) from non-ILD among connective tissue disease (CTD) patients.
Materials and Methods:
Original articles on the diagnostic accuracy of serum KL-6 and SP-D in differentiating CTD-ILD from CTD-nonILD were identified from three public databases. The overall quality of evidence and methodologic quality of each eligible study were assessed by the Grading of Recommendations, Assessment, Development and Evaluation approach and Quality Assessment of Diagnostic Accuracy Studies, respectively. We used the bivariate model to calculate random-effect sensitivity, specificity, likelihood ratios, and area under curve. Furthermore, trial sequential analysis (TSA) was used to determine whether sample sizes incorporated in the meta-analysis were powerful for evaluating the diagnostic utility. Bayesian network analysis was performed to compare the diagnostic accuracy of 2 serum biomarkers in differentiating ILD among CTD patients and various subgroups.
Results:
Twenty-nine studies were included in the quantitative synthesis. No threshold effects were observed (all P values >.05). For diagnosis of ILD among CTD patients, overall sensitivity and specificity of serum KL-6 were 0.76 (95% confidence interval [CI]: 0.68–0.82) and 0.89 (95% CI: 0.83–0.93), whereas those for serum SP-D were 0.65 (95% CI: 0.45–0.80) and 0.88 (95% CI: 0.80–0.93). Comprehensive comparison of 2 circulating biomarkers using back-calculated likelihood ratio (LR) demonstrated that serum KL-6 corresponded to a higher LR+ and a lower LR– in comparison to serum SP-D, as well as in SSc-ILD. TSA indicated that evidence for serum KL-6 and SP-D in identifying CTD-ILD is powerful; nonetheless, more trials were needed for validation of serum KL-6 and SP-D in differentiating CTD-ILD subtypes, including different CTD and ethnicities.
Conclusions:
This meta-analysis suggested that serum KL-6 had superior diagnostic accuracy to SP-D for differentiating ILD from non-ILD among CTD patients, providing a convenient and non-invasive approach for screening and management of ILD among CTD patients.
1. Introduction
Interstitial lung disease (ILD) is one of the most common manifestations of systemic connective tissue disease (CTD) with prevalence varying from 10% to 30%, leading to remarkable morbidity and mortality in patients with CTD.[1–3] ILD often complicates the course of known CTD including systemic sclerosis (SSc), rheumatoid arthritis (RA), idiopathic inflammatory myopathy (IIM), systemic lupus erythematous (SLE), primary Sjögren syndrome, mixed CTD (MCTD), and undifferentiated CTD (UCTD). In addition, it may also be manifested as interstitial pneumonia with autoimmune features (IPAF), the first and only manifestation of CTD with incidence of 15% approximately.[4] Although many patients with ILD may progress slowly or stay steady, some of those may develop life-threatening respiratory failure caused by extensive fibrosis. Therefore, it is imperative to precisely diagnose and treat the patients with CTD-associated ILD (CTD-ILD) early in case of the poor prognosis.
The ascertainment and evaluation of ILD mainly depend on pulmonary function tests, chest radiography, conventional thoracic computed tomography, high-resolution chest computed tomography (HRCT), and lung biopsy as needed.[5–7] Although these techniques have great advantages in identifying ILD in CTD patients, their limitations restrict the widespread use in monitoring these patients either due to the high-dose radiation, or intra- and interrater bias in CT scanning, or the unstable cooperation between patients and spirometer. Furthermore, lung biopsy is not conventional as well because of its invasive manner. Thus, serum biomarkers which can identify the existence of ILD and to follow the progression are more preferred, comparing to frequently repeated pulmonary function tests or radiographic examination.
Krebs von den Lungen-6 (KL-6) and surfactant protein D (SP-D) are important component of the pulmonary innate immune system, which can be used as biomarkers for pulmonary disease states when they are translocated on the extrapulmonary epithelial surfaces or in serum. KL-6, which was first described by Kohno et al in 1985, is classified as one of the human MUC 1 antigen and preferentially expressed on type II pneumocytes in normal lungs.[8,9] During the pathological process, KL-6 is released from the regenerated type II pneumocytes into blood through respiratory bronchiolar epithelial cells and basement membrane which has been injured by the recruited inflammatory cells.[10] The elevated level of KL-6 may trigger transforming growth factor-β (TGF-β) signaling, leading to pro-fibrotic and anti-apoptotic effects on lung fibroblasts.[11] KL-6 elevation has been reported in different benign and malignant pulmonary diseases, including idiopathic pulmonary fibrosis, CTD-ILD, hypersensitivity pneumonia, radiation pneumonia, pulmonary tuberculosis, drug-induced ILDs, acute respiratory distress syndrome, lung cancer, and pulmonary sarcoidosis[12,13–15] SP-D, a collectin of the CC-type lectin superfamily, participates in the innate immune response by opsonization or lysis of inhaled pulmonary pathogens.[13] SP-D is produced constitutively by Clara cells and alveolar type II cells, but the baseline level of circulating SP-D increases when patients suffer allergic bronchopulmonary aspergillosis, community-acquired pneumonia, interstitial lung diseases, acute lung injury, and idiopathic pulmonary fibrosis.[14] Recent studies have recommended KL-6[15–17] and SP-D[18–20] as serological biomarkers for the diagnosis of CTD-ILD. Herein, we performed this meta-analysis to evaluate the diagnostic utility of serum KL-6 and SP-D in differentiating ILD from non-ILD among CTD patients.
2. Methods
This study was conducted in accordance with the framework recommended by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.[21] The study does not require ethical approval since all data involved in this study were publicly available and no patients were enrolled.
2.1. Literature search
A systematic literature search of Pubmed, Embase and Web of Sciences was performed to identify relevant studies published by February 10th, 2020 without restrictions to publication types, regions, or languages. Terms on CTD were not included in the primary search due to the wide spectrum of the definition which made it difficult to perform. The following MeSH terms were searched in [Title/Abstract]: (“Krebs von den Lungen-6” or “KL-6” or “MUC1”), (“Pulmonary Surfactant-Associated Protein D”[Mesh] OR pulmonary surfactant-associated protein D[Title/Abstract] OR surfactant associated protein D[Title/Abstract] OR surfactant protein D[Title/Abstract] OR pulmonary surfactant protein D[Title/Abstract] OR surfactant-associated glycoprotein D[Title/Abstract] OR surfactant associated glycoprotein D[Title/Abstract] OR lung protein D[Title/Abstract] OR SP-D[Title/Abstract]), with the combination of terms——(“Lung Diseases, Interstitial”[Mesh] or “interstitial lung disease∗” or “ILD∗” or “interstitial pneumonia” or “interstitial pneumonitis” or “diffuse parenchymal lung diseases” or “interstitial pneumonitides”), respectively.
2.2. Study selection
Studies were included in the meta-analysis when they meet the following criteria: studies focused on patients with CTDs or collagen vascular diseases including SSc, IIM, RA, SLE, Sjögren syndrome, MCTD, UCTD, systemic vasculitis or other systematic autoimmune diseases; the diagnosis of ILD (also known as pulmonary fibrosis) was established according to the results of chest radiography, conventional thoracic computed tomography, HRCT, or pulmonary function tests (either FVC% or DLco% <80%); patients with CTD but without ILD were included as the controls; studies that provided sufficient data to complete cross-tabulations (2 × 2 tables) for evaluating the diagnostic accuracy of serum KL-6 or SP-D in differentiating CTD-ILD from CTD-nonILD. When the same population were published in different reports, the most recent or complete report was included. Case reports or case series, reviews, letters, conference abstracts were excluded since their limitation in the assessment or analysis of data. Studies with healthy controls merely or insufficient data for completing cross-tabulations were excluded as well.
2.3. Data extraction and quality assessment
Data from the eligible studies were extracted and summarized independently by 2 investigators. Any disagreement was resolved by consensus. The following information from each study were collected: first author's name, year of publication, disease subtypes, affiliation of study, ethnicity of population, assay method, cutoff value, sample size, number of case group, number of control group, and frequency exceeding or within cutoff point of serum KL-6. Data from 2 × 2 tables including values of true-positive (TP), false-positive (FP), false-negative (FN), and true-negative (TN) were extracted to calculate pooled sensitivity and specificity for the assessment of diagnostic capacity of KL-6.
The revised version of Quality Assessment of Diagnostic Accuracy Studies (namely QUADAS-2)[22] was used to evaluate the bias of methodologic quality (including risk of bias and concerns regarding applicability) of each included study by 2 independent investigators. Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework was conducted to assess the quality of evidence of outcomes according to 5 domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias.[23] In addition, Deeks funnel plot asymmetry test was performed with STATA15.0 (College Station, TX) to determine the presence of publication bias.[24]
To determine the optimal information size, trial sequence analysis (TSA, version 0.9 beta, http://www.ctu.dk/tsa) was introduced to quantify the statistical reliability of data in the cumulative meta-analysis.[25] The required sample size was calculated with a predestined type I error of 5% and type II error of 20% (power of 80%). The control event rates were calculated from the unweighted mean of the event proportions in all control groups of the included studies, and a relative risk reduction of 35% was assumed for required sample size. A diversity adjusted information size was calculated with the eligible studies. The cumulative Z curve was calculated with a random-effects (DJ) model.
2.4. Data synthesis and analysis
We used the bivariate model to calculate random-effects sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio and diagnostic odds ratio (DOR), together with their 95% confidence intervals (CIs). Hierarchical summary receiver-operating characteristic (SROC) plots were constructed as well. Threshold effects were quantified by the Spearman correlation coefficient between sensitivity and 1– specificity. Significant statistical heterogeneity between studies was considered by the χ2 test when P < .05 or I2 statistic >50%. Potential sources of heterogeneity were explored by subgroup analyses, according to several clinically relevant covariates: diseases subtypes, ethnicity, detective methods, and number of participants (<50 or ≥50). Bayesian analysis was conducted to compare the diagnostic accuracy of serum KL-6 and SP-D in CTD-ILD.[26]
3. Results
3.1. Characteristics of eligible studies
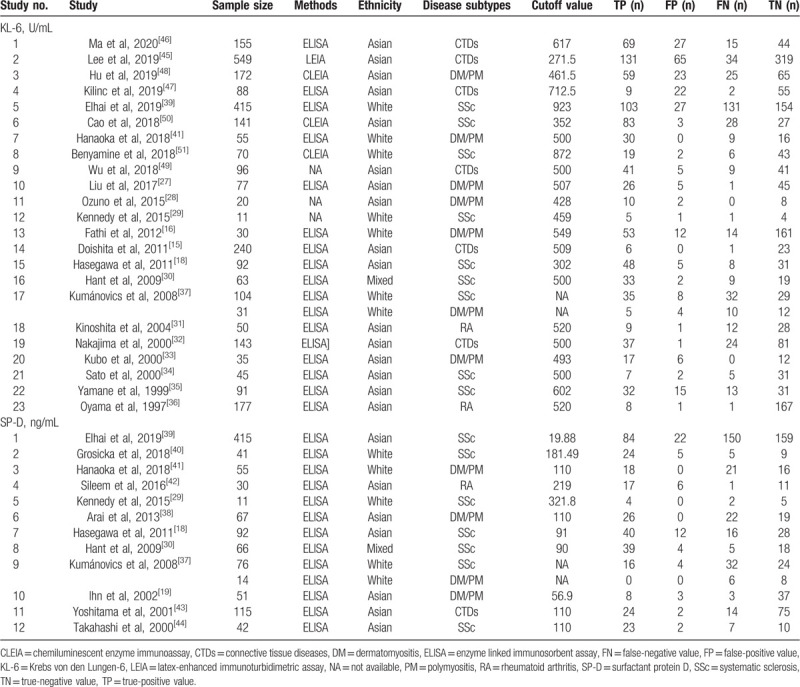
Twenty-three articles[15,16,18,27–37] on serum KL-6 comprising 2950 cases (1265 patients with CTD-ILD and 1685 patients with CTD-nonILD), 12 studies[18,19,29,30,37–44] on serum SP-D including 1083 participants (583 patients with CTD-ILD and 497 CTD-non-ILD patients) fulfilled the predefined inclusion criteria and were included in the final analysis (Fig. 1). Double data from 2 × 2 tables were extracted from one[37] of the publications since the article gave diagnostic details on 2 kinds of CTD—dermatomyositis (DM)/polymyositis (PM) and SSc, whereas 3 articles[15,32,43,45–47] with a combination data of various CTD were not included in the subgroup analyses with coefficient of disease subtypes. The characteristics of eligible studies are summarized in Table 1,[15,16,18,19,27–51] which included 11 publications were about SSc, 9 publications on adult DM/PM,[16,19,27,28,33,37,38,41,48] 3 publications[31,36,42] on RA, and 7 publications[15,32,43,45–47,49] on different CTDs whose data could not extracted to complete cross tabulations for disease subsets. Sixteen studies described the detective method for serum KL-6 as enzyme-linked immunosorbent assay (ELISA), and 3 publications used chemiluminescent enzyme immunoassay (CLEIA),[48,50,51] and 1 publication used latex-enhanced immunoturbidimetric assay[45] for the detection of serum KL-6, respectively,[50] whereas 3 publications did not give details about assay methods.[28,29,49] The cut-off value of serum KL-6 in the eligible studies ranged from 250 U/ml to 600 U/ml whereas two studies[39,51] with cutoff value approximate to 900 U/mL, whereas the major ranges of cutoff value for serum SP-D varied from 90 to 200 ng/mL except 2 studies[29,39]
Figure 1.
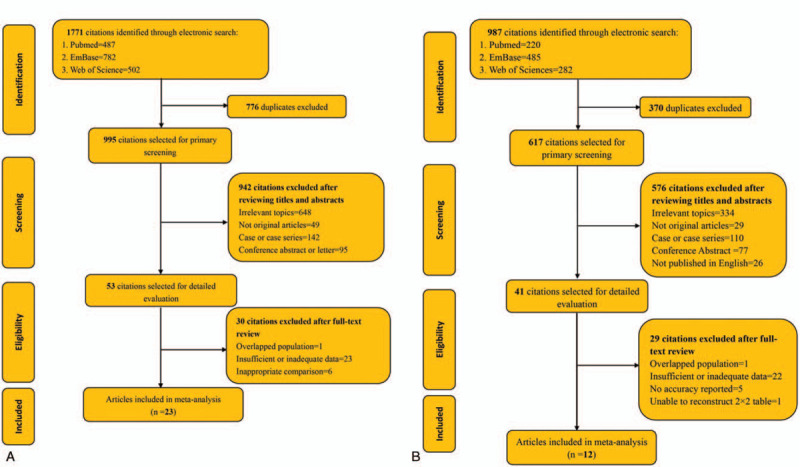
Schematic graph of studies selection. For the schematic screening for serum KL-6 (A): 1771 citations were identified from 3 database: PubMed (487), Embase (782) and Web of science (502). After removing 776 duplicates, 995 publications were selected for primary screening by reviewing titles and abstracts. A total of 648 articles were excluded since they had little correlation with KL-6 nor connective tissue diseases, and 49 studies were excluded because the types of articles were reviews or meta-analysis. In addition, 142 anecdotal reports and 95 conference abstracts were excluded as well since their limitation in the assessment or analysis of data. Fifty-three citations were selected for further evaluation by full-text review. One study with overlapped population, 23 articles without powerful data for the complement of cross-tabulations, and 6 articles with healthy controls or no controls were excluded. At last, 23 articles were included in our meta-analysis. The similar schematic screening for serum SP-D was presented in panel B with a total of 12 articles were enrolled in the quantitative analysis for SP-D. KL-6 = Krebs von den Lungen-6, SP-D = surfactant protein D.
Table 1.
Characteristics of each eligible study.
3.2. Quality assessment
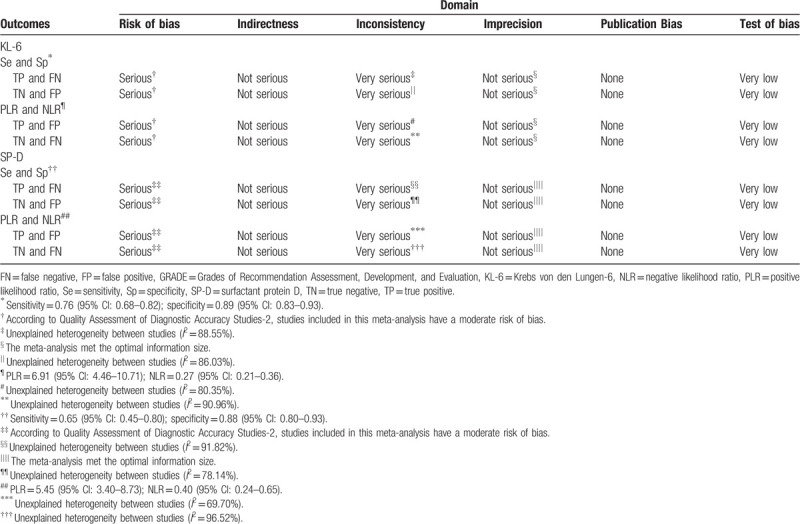
The results of QUADAS-2 scores of every eligible study were presented in Figure 2. All studies were included in the further analysis. The overall quality of evidence was considered low according to the GRADE assessment; downgrading was due mainly to risk of bias and inconsistency, and imprecision (Table 2). Therefore, our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. The result of Deeks funnel plot asymmetry test performed with STATA revealed no evidence of publication bias[23] (Supplementary Figure 1), with P values of .06 for serum KL-6 and 0.10 for serum SP-D (both P values >.05). 3.3 Data synthesis
Figure 2.
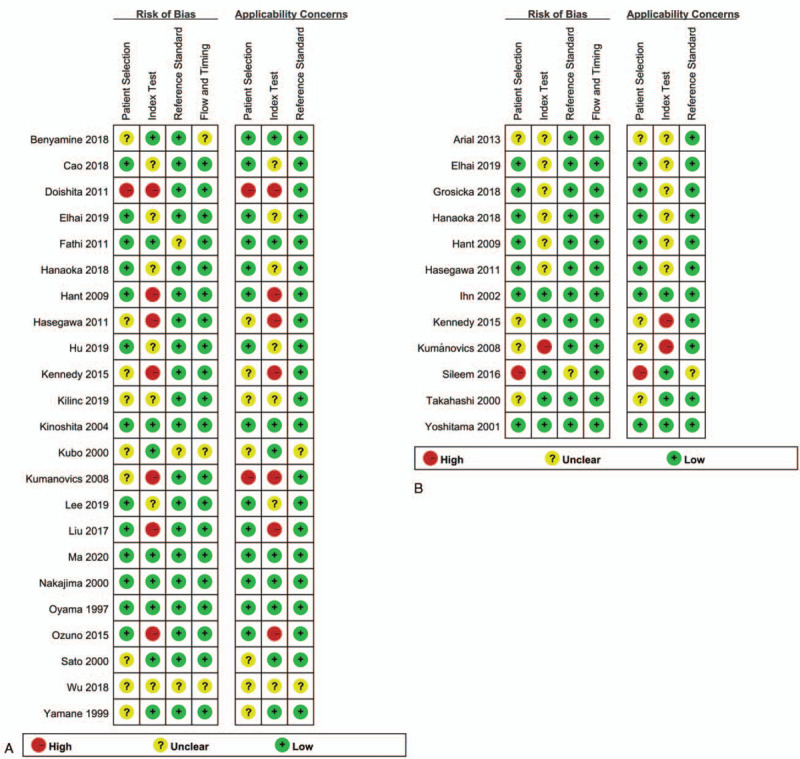
Stacked bar charts of QUADAS-2 scores for studies on KL-6 (A) and SP-D (B). KL-6 = Krebs von den Lungen-6, SP-D = surfactant protein D.
Table 2.
Study quality of evidence based on GRADE guidelines.
No threshold effects were observed when pooling the data (all P > .05, summarized in Table 3). The forest plots indicate the presence of substantial heterogeneity with I2 values exceeding 50% for all diagnostic assessment, including pooled sensitivity, specificity, likelihood ratios, and DOR; thus, a random-effects model was applied to combine these estimates. The primary analysis showed that the pooled sensitivity, specificity, PLR, NLR, and DOR for diagnostic accuracy of KL-6 in distinguishing CTD-ILD from CTD-non-ILD were 0.76 (95% confidence interval [CI]: 0.68–0.82), 0.89 (95% CI: 0.83–0.93), 9.55 (95% CI: 6.91–10.71), 0.27 (95% CI: 0.21–0.36), and 25.16 (95% CI: 13.84–45.75), respectively, whereas those of serum SP-D were 0.65 (95% CI: 0.45–0.80), 0.88 (95% CI: 0.80–0.93), 5.45 (95% CI: 3.40–8.73), 0.40 (95% CI: 0.24–0.65), and 13.63 (95% CI: 6.48–28.65). (Fig. 3). The area under the hierarchical summary receiver-operating characteristic curve (HSROC) was 0.88, with 95% credible interval of 0.83–0.93 for serum KL-6, and 0.88 (95%CI: 0.81–0.95) for serum SP-D (Fig. 4). Table 3 summarized the sensitivity analyses performed according to several clinically relevant covariates: diseases subtypes, ethnicity, detective methods and number of participants (<50 or ≥50). Table 3 showed all results from subgroup analyses. In detail, pooled sensitivity of serum KL-6 was 0.88 (95% CI: 0.62–0.97) for DM/PM-ILD, and 0.70 (95% CI: 0.59–0.79) for SSc-ILD, respectively, whereas pooled specificity of KL-6 was 0.87 (95% CI: 0.73–0.94) for DM/PM-ILD, and 0.87 (95% CI: 0.79–0.92) for SSc-ILD; pooled sensitivity and specificity of serum SP-D were 0.66 (95% CI: 0.44–0.83) and 0.83 (95% CI: 0.75–0.89) for SSc-ILD. Sixteen studies involving Asian population indicated that serum KL-6 had pooled sensitivity of 0.78 (95% CI: 0.71–0.84), along with pooled specificity of 0.88 (95% CI: 0.80–0.93), whereas 7 publications concerned with white population revealed that pooled sensitivity and pooled specificity of serum KL-6 were 0.63 (95% CI: 0.46–0.77) and 0.92 (95% CI: 0.82–0.97), respectively; pooled sensitivity and specificity of serum SP-D among Asian were 0.57 (95% CI: 0.34–0.77) and 0.90 (95% CI: 0.81–0.95). Excluding studies without ELISA technique, 17 publications showed pooled sensitivity of serum KL-6 were 0.75 (95% CI: 0.63–0.84) and pooled specificity of 0.93 (95% CI: 0.86–0.97).
Table 3.
Sensitivity analyses performed for subgroups of studies.
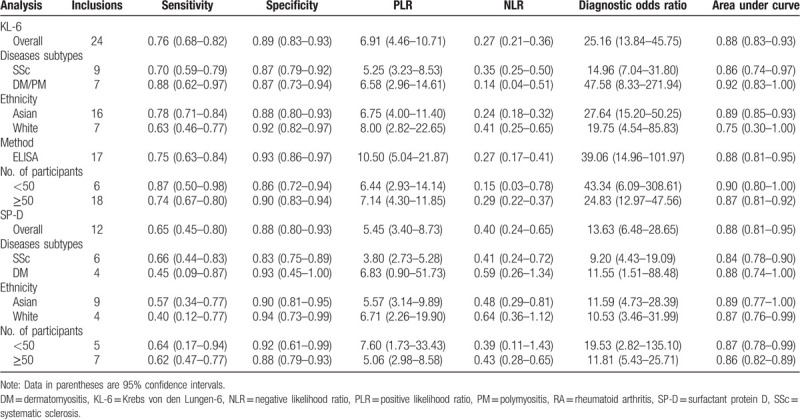
Figure 3.
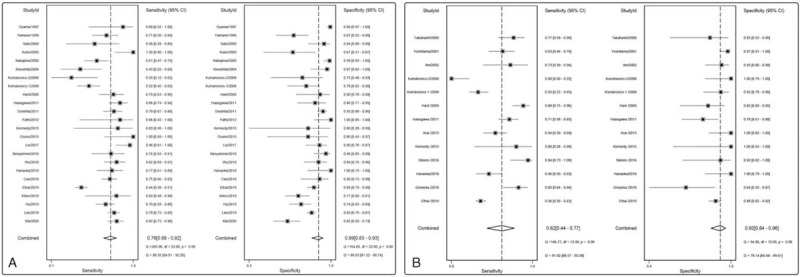
Forest plots of the diagnostic capacity estimates of serum KL-6 (A) and SP-D (B) in CTD-ILD: sensitivity and specificity. Substantial heterogeneity was observed in the diagnostic parameters across studies since all I2 exceeded than 50%. CTD-ILD = connective tissue disease-interstitial lung disease, KL-6 = Krebs von den Lungen-6, SP-D = surfactant protein D.
Figure 4.
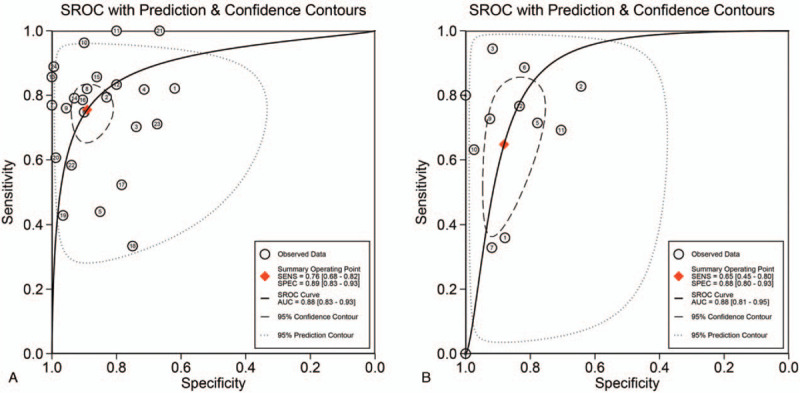
Hierarchical summary receiver operating characteristic (SROC) plots of KL-6 (A) and SP-D (B) for differentiating CTD with ILD from CTD without ILD. Each circle indicates an eligible study. CTD = connective tissue diseases, ILD = interstitial lung disease, KL-6 = Krebs von den Lungen-6, SP-D = surfactant protein D.
3.3. Bayesian analysis
We evaluated the pre-test probability of 30%, corresponding post-test probabilities following a “positive” or “negative” of each circulating biomarker, based on the summary sensitivity and specificity using Fagan plot analysis. The Fagan plot analysis in Figure 5 suggested that the diagnostic efficacy of serum KL-6 in CTD-ILD is superior to SP-D in both diagnosis (rule in) and exclusion (rule-out), with 75% versus 70% post-test probability of CTD-ILD following a “positive” result and 11% versus 15% post-test probability following a “negative” result when pre-test probability was 30%. Furthermore, Bayesian analysis was performed to compare efficacies between serum KL-6 and SP-D in the diagnosis of ILD among Asian/white population, SSc subsets and DM/PM subsets (Supplementary Figures 2–3). In subgroup analyses, Bayesian analysis demonstrated that serum KL-6 is more useful than SP-D in ruling out ILD among Asian population with 9% versus 13% post-test probability following a “negative” result, whereas SP-D is more informative to rule in ILD than KL-6 with 77% versus 74% post-test probability following a “positive” result. Serum SP-D is superior in differentiating ILD among whites than KL-6 with 77% versus 74% post-test probability following a “positive” result and 15% versus 21% post-test probability following a “negative” result (Supplementary Figure 2). Serum KL-6 has better diagnostic utility for both in rule in and rule out ILD among CTD patients in DM than in SSc, with 69% versus 62% post-test probability of CTD-ILD following a “positive” result, and 13% versus 15% post-test probability following a “negative” result (Supplementary Figure 3A-3B). The result in DM/PM subset showed that SP-D is more powerful in ruling in than KL-6 with 94% versus 74% post-test probability of CTD-ILD following a “positive” result, while KL-6 is more useful to rule out ILD than SP-D with 6% versus 19% post-test probability (Supplementary Figure 3C-3D). These results suggest that, in the absence of clinical and/or radiological suspicion, serum KL-6 may provide solid evidence to rule in and rule out ILD among CTD patients and SSc subset in comparison with serum SP-D, whereas SP-D is more informative to rule in ILD among DM/PM patients.
Figure 5.
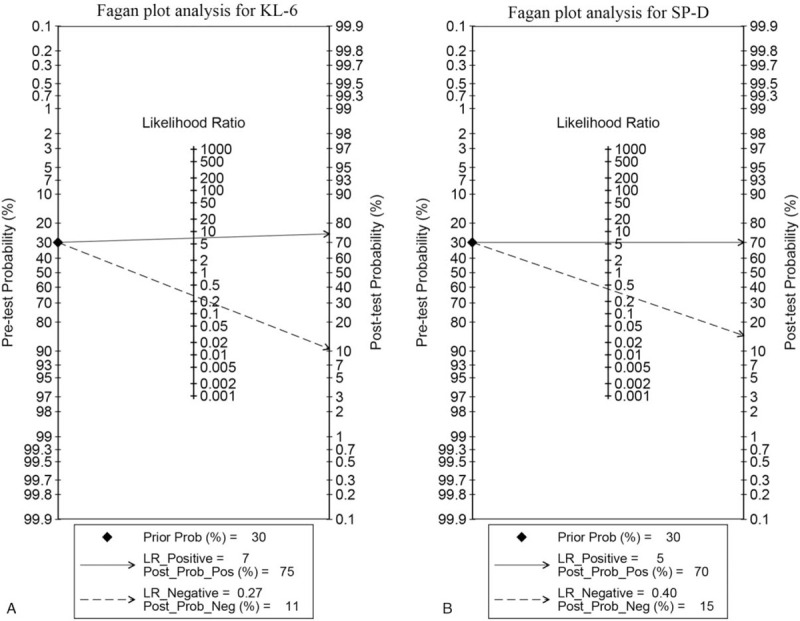
Fagan plot analysis to evaluate the clinical utility of serum KL-6 and serum SP-D for diagnosing CTD-ILD. The Fagan plot consists of a vertical axis on the left with the pre-test probability, an axis in the middle representing the likelihood ratio, and a vertical axis on the right representing the post-test probability. With pre-test probability of CTD-ILD of 30%, the post-test probability of CTD-ILD was obtained from positive and negative results of both circulating biomarkers. CTD-ILD = connective tissue disease-interstitial lung disease, KL-6 = Krebs von den Lungen-6, SP-D = surfactant protein D.
3.4. TSA
Supplementary Figure 4 shows TSA for the association between serum KL-6 and CTD-ILD (Supplementary Figure 4A), SP-D and CTD-ILD (Supplementary Figure 4B). In this analysis, the required information size was calculated with a control event proportion of 15%. The result for the correlation of serum KL-6 and CTD-ILD showed that the cumulative Z curve crossed both the conventional boundary and the trial sequential monitoring boundary. Thus, the evidence is solid enough to confirm that there is a strong association between serum KL-6 and CTD-ILD, and no further trials were needed. Regarding the analysis for the correlation of SP-D to CTD-ILD, the cumulative Z curve crossed the conventional boundary, indicating powerful sample sizes.
Similarly, TSA was performed to assess the correlation of serum KL-6/SP-D with SSc-ILD, DM/PM-ILD, and RA-ILD, and the required information size was calculated assuming a control event proportion of 0.15 and 0.19, respectively. The results demonstrated that the cumulative evidence is insufficient to draw a conclusion. Consequently, more trials are necessary to answer these questions.
4. Discussion
ILD is a life-threatening complication among CTD patients with substantial morbidity and mortality. Early diagnosis and aggressive treatment are necessary for patients with CTD-ILD to improve poor prognosis. Comparing to frequent exposure to radiology imaging and pulmonary tests, a reliable serology biomarker is more preferred. It has been reported that serum KL-6 and SP-D can develop as invasive biomarkers to distinguish the existence of CTD-ILD and to follow the progression of CTD-ILD. Nonetheless, the diagnostic accuracy of serum KL-6 and SP-D varied greatly. To the best of our knowledge, this is the first meta-analysis to estimate the diagnostic utility of serum KL-6 and SP-D in differentiating patients with CTD-ILD from those without ILD, suggesting they are both useful in CTD patients suspect of ILD, and serum KL-6 has higher diagnostic utility than SP-D in differentiating ILD from non-ILD among CTD patients. Generally, we identified 29 studies, together including >2000 participants in the quantitative synthesis. Bivariate models were performed to explore the diagnostic capacity of serum KL-6 and SP-D in distinguishing CTD-ILD, and Bayesian analysis was introduced to compare the diagnostic accuracy between serum KL-6 and serum SP-D among overall population, different ethnicities and various disease subsets. The results revealed that serum KL-6 may become a sound biomarker for discriminating ILD from non-ILD in CTD patients with high diagnostic accuracy with sensitivity of 76%, specificity of 89%, DOR of 25.16, and area under curve (AUC) of 0.88, in comparison with radiology imaging or pulmonary function tests, whereas serum SP-D has a sensitivity of 65%, specificity of 88%, DOR of 213.63, and AUC of 0.88. In Bayesian analysis, serum KL-6 shows superior diagnostic efficacy in ruling in a true diagnosis and ruling out a false diagnosis when comparing to serum SP-D. In addition, serum KL-6 shows higher diagnostic utility to rule in and rule out ILD than serum SP-D among white population. In Asian population, serum KL-6 is superior to SP-D in ruling out ILD, but the latter is more informative to the former in ruling in ILD. Regarding to disease subsets, the result indicated that serum KL-6 played a crucial role in discriminating ILD in SSc subtypes than SP-D. In summary, the diagnostic efficacy of KL-6 is superior to SP-D in differentiating ILD. The accrued evidence is powerful to support the conclusion for the association between serum KL-6 and CTD-ILD as well as SP-D and CTD-ILD, as both their cumulative Z curve crossed conventional boundary assessed by TSA approach. Otherwise, the literature published until now showed insufficient evidence to confirm or refute the association between serum KL-6/SP-D and subtypes of CTD-ILD.
To investigate all potential sources of heterogeneity, we have performed subgroup analyses to rule out the presence of heterogeneity among these diagnostic estimates. Subgroup analyses present the diagnostic accuracy of serum KL-6 and SP-D in various ethnicities and disease subsets, and covariates including ethnicities, disease subsets, and sample size are not the sources of heterogeneity. When stratified with sample size, the result indicated that pooled DOR estimate was a little higher in studies with <50 participants than in those with ≥50 participants (19.53 vs 11.81). However, the presence of heterogeneity cannot be explained by these covariates. It is possible that more confounding factors including the different standard used for the diagnosis of ILD and severity of ILD contributed a lot to the substantial heterogeneity, which cannot be extracted from the primary studies. Apart from 2 studies with cutoff values approximate to 900 U/mL,[39,51] the cutoff value of serum KL-6 from the remained studies approached to 500 U/mL, whereas all eligible studies adopted ELISA to detect serum levels of SP-D with cutoff value approximately to 110 ng/mL. Intriguingly, it has been reported that reference range of serum KL-6 and SP-D level may be affected by polymorphism in different ethnic groups,[14,52] whereas both serum KL-6 and SP-D levels increased with age. However, no evidence was obtained to further analyze the confounding factor because of scarce data. Thus, further observation is needed to understand the difference of serum KL-6 and SP-D cutoff value among different ethnic population as well as with various detective techniques.
HRCT provides many details for the diagnosis of ILD with sensitivity of 95% and a specificity approaching 100%; however, the use of CT for screening and follow-up is considered carefully in the clinic in case of the accumulating radiation dose.[53] Thus, a method, that balances between the high diagnostic sensitivity of CT and the radiation exposure for daily routine, should be established and validated. Our study indicated that serum KL-6 may become a promising objective diagnostic tool to discriminate ILD from non-ILD among CTD patients with high diagnostic accuracy, especially to increase the proportion of non-ILD among CTD patients when they were misdiagnosed by radiography or pulmonary function tests. The sensitivity of serum KL-6 is hopefully higher than its specificity in comparison to HRCT since serum KL-6 can be detected in the course of ILD[12]; nonetheless, the specificity of KL-6 seems to be higher than its sensitivity for the detection of ILD among CTD patients. The reasons can be ascribed to the following aspects: most studies enrolled in our meta-analysis selected the radiography or pulmonary function tests as “criterion standard” rather than HRCT only to identify ILD. The subgroup analyses on HRCT as “criterion standard,” however, could not be performed due to scarce data; interobserver agreement on the diagnosis ILD is usually influenced by motion artifact and respiratory misregistration (gaps or overlap in breathing cycles due to variation in the depth of inspiration), leading to low diagnostic kappa values of ILD, even based on HRCT scanning.[54] Therefore, serum KL-6 may become a promising biomarker which can balance between the accumulating radiation and diagnostic accuracy.
Our meta-analysis had some limitations and it should be cautious when interpreting these results. First, the limited information of the eligible articles restrained us from investigating all potential causes of heterogeneity. These heterogeneities can be partially explained; however, it could not be markedly diminished by subgroup analyses. It is noted that some aspects may restrain this analysis: The detection of KL-6 or SP-D in enrolled studies are not the same; various morphological phenotypes and stages of ILDs may affect the level of serum KL-6 and SP-D; various disease subtypes have accounted for the heterogeneity; except disease subtypes, ethnicity, adoptive method, and sample size, other characteristics including the selection of “criterion standard,” the exposure of smoking history and types of study design may account for part of the heterogeneity, which are not available from the studies. This means that these diagnostic estimates are somewhat unstable with wide confidence intervals. Meanwhile, the scarcity of data made analysis of heterogeneity underpowered. Therefore, more studies are necessary to allow the identification of the possible confounding factors. Second, the methodological quality and overall quality of evidence are relatively low, which is the major concern in this meta-analysis. Third, in our meta-analysis, subgroup analyses for both serum KL-6 and SP-D were not performed in patients with RA, SLE, and primary Sjögren syndrome due to insufficient data obtained from eligible studies, whereas subgroup analyses for SP-D were not conducted in white population and DM/PM-ILD subsets due to scarce data for synthesis. Therefore, additional studies were necessary to determine the significance of these circulating biomarkers in various subsets. Finally, variable thresholds in these studies limited estimation of pooled sensitivities and specificities at each threshold, which restrained us from discovering a best cutoff value of serum KL-6.
5. Conclusions
In summary, our meta-analysis suggested that serum KL-6 had superior diagnostic accuracy to SP-D for differentiating ILD from non-ILD among CTD patients, which may provide a convenient and noninvasive approach for screening and managing ILD among CTD patients. However, the quality of evidence assessed by GRADE was considered low, and it should be cautious when interpretation with these results.
Author contributions
Conceptualization: Danli Zhong, Qian Wang.
Data curation: Danli Zhong, Dong Xu, Chaojun Hu.
Formal analysis: Danli Zhong, Chanyuan Wu, Jingjing Bai.
Investigation: Qian Wang, Xiaofeng Zeng.
Writing – original draft: Danli Zhong.
Writing – review & editing: Qian Wang.
Danli Zhong orcid: 0000-0003-4084-4067.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AUC = area under curve, CI = confidence intervals, CTDs = connective tissue diseases, DM = dermatomyositis, DOR = diagnostic odds ratio, GRADE = Grading of Recommendations, Assessment, Development and Evaluation, HRCT = high-resolution chest computed tomography, ILDs = interstitial lung diseases, KL-6 = Krebs von den Lungen-6, LR = likelihood ratio, NLR = negative likelihood ratio, PLR = positive likelihood ratio, PM = polymyositis, QUADAS = Quality Assessment of Diagnostic Accuracy Studies, RA = rheumatoid arthritis, SP-D = surfactant protein D, SROC = summary receiver-operating characteristic, SSc = systemic scleroderma.
How to cite this article: Zhong D, Wu C, Bai J, Hu C, Xu D, Wang Q, Zeng X. Comparative diagnostic efficacy of serum Krebs von den Lungen-6 and surfactant D for connective tissue disease-associated interstitial lung diseases: a meta-analysis. Medicine. 2020;99:16(e19695).
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The authors report no conflicts of interest.
The study received grants from the Chinese National Key Technology R&D Program of Ministry of Science and Technology (2017YFC0907604), National Science and Technology Major Project of the Ministry of Science and Technology of China (2019ZX09734001–002–004), Medical and health science and technology innovation project of Chinese Academy of Medical Sciences (2019-I2M-2-008) and the National Natural Science Foundation of China (81601430, 81471615).
Supplemental Digital Content is available for this article.
References
- [1].Wallace B, Vummidi D, Khanna D. Management of connective tissue diseases associated interstitial lung disease: a review of the published literature. Curr Opin Rheumatol 2016;28:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ (Clinical research ed) 2016;352:h6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet (London, England) 2012;380:689–98. [DOI] [PubMed] [Google Scholar]
- [4].Mittoo S, Gelber AC, Christopher-Stine L, et al. Ascertainment of collagen vascular disease in patients presenting with interstitial lung disease. Respiratory medicine 2009;103:1152–8. [DOI] [PubMed] [Google Scholar]
- [5].Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008;58:15–25. [DOI] [PubMed] [Google Scholar]
- [6].Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum 1998;41:778–99. [DOI] [PubMed] [Google Scholar]
- [7].Cortet B, Perez T, Roux N, et al. Pulmonary function tests and high resolution computed tomography of the lungs in patients with rheumatoid arthritis. Annals of the rheumatic diseases 1997;56:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kohno N, Akiyama M, Kyoizumi S, et al. A novel method for screening monoclonal antibodies reacting with antigenic determinants on soluble antigens; a reversed indirect-enzyme linked immunosorbent assay (RI-ELISA). Hiroshima journal of medical sciences 1987;36:319–23. [PubMed] [Google Scholar]
- [9].Kohno N, Akiyama M, Kyoizumi S, et al. Detection of soluble tumor-associated antigens in sera and effusions using novel monoclonal antibodies, KL-3 and KL-6, against lung adenocarcinoma. Jpn J Clin Oncol 1988;18:203–16. [PubMed] [Google Scholar]
- [10].Inoue Y, Barker E, Daniloff E, et al. Pulmonary epithelial cell injury and alveolar-capillary permeability in berylliosis. Am J Respir Crit Care Med 1997;156:109–15. [DOI] [PubMed] [Google Scholar]
- [11].Ohshimo S, Yokoyama A, Hattori N, et al. KL-6, a human MUC1 mucin, promotes proliferation and survival of lung fibroblasts. Biochem Biophys Res Commun 2005;338:1845–52. [DOI] [PubMed] [Google Scholar]
- [12].Ishikawa N, Hattori N, Yokoyama A, et al. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. Respir Investig 2012;50:3–13. [DOI] [PubMed] [Google Scholar]
- [13].Wu H, Kuzmenko A, Wan S, et al. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Investig 2003;111:1589–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sorensen GL, Hjelmborg J, Kyvik KO, et al. Genetic and environmental influences of surfactant protein D serum levels. Am J Physiol Lung Cell Mol Physiol 2006;290:L1010–7. [DOI] [PubMed] [Google Scholar]
- [15].Doishita S, Inokuma S, Asashima H, et al. Serum KL-6 level as an indicator of active or inactive interstitial pneumonitis associated with connective tissue diseases. Intern Med (Tokyo, Japan) 2011;50:2889–92. [published Online First: 2011/12/02]. [DOI] [PubMed] [Google Scholar]
- [16].Fathi M, Barbasso Helmers S, Lundberg IE. KL-6: a serological biomarker for interstitial lung disease in patients with polymyositis and dermatomyositis. Journal of internal medicine 2012;271:589–97. [DOI] [PubMed] [Google Scholar]
- [17].Oguz EO, Kucuksahin O, Turgay M, et al. Association of serum KL-6 levels with interstitial lung disease in patients with connective tissue disease: a cross-sectional study. Clin Rheumatol 2016;35:663–6. [DOI] [PubMed] [Google Scholar]
- [18].Hasegawa M, Fujimoto M, Hamaguchi Y, et al. Use of serum clara cell 16-kDa (CC16) levels as a potential indicator of active pulmonary fibrosis in systemic sclerosis. J Rheumatol 2011;38:877–84. [DOI] [PubMed] [Google Scholar]
- [19].Ihn H, Asano Y, Kubo M, et al. Clinical significance of serum surfactant protein D (SP-D) in patients with polymyositis/dermatomyositis: correlation with interstitial lung disease. Rheumatology 2002;41:1268–72. [DOI] [PubMed] [Google Scholar]
- [20].Chen F, Shu X, Lu X, et al. A comprehensive study of novel serum markers of ILD associated with inflammatory myopathies. Arthritis Rheum 2012;64:S823. [Google Scholar]
- [21].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;3:e123–30. e1000097 6. [PMC free article] [PubMed] [Google Scholar]
- [22].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [23].Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- [24].Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93. [DOI] [PubMed] [Google Scholar]
- [25].Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- [26].Carlin JB. Meta-analysis for 2 x 2 tables: a Bayesian approach. Stat Med 1992;11:141–58. [DOI] [PubMed] [Google Scholar]
- [27].Liu Y, Zhao Y, Xu A. Clinical value of serum KL-6 for lung diseases in patients with polymyositis and dermatomyositis. Int J Clin Exp Pathol 2017;10:3310–4. [Google Scholar]
- [28].Ozuno NT, Akamatsu H, Takahashi H, et al. Quantitative diagnosis of connective tissue disease-associated interstitial pneumonia using thoracic computed tomography images. Clin Rheumatol 2015;34:2113–8. [DOI] [PubMed] [Google Scholar]
- [29].Kennedy B, Branagan P, Moloney F, et al. Biomarkers to identify ILD and predict lung function decline in scleroderma lung disease or idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2015;32:228–36. [PubMed] [Google Scholar]
- [30].Hant FN, Ludwicka-Bradley A, Wang HJ, et al. Surfactant protein D and KL-6 as serum biomarkers of interstitial lung disease in patients with scleroderma. J Rheumatol 2009;36:773–80. [DOI] [PubMed] [Google Scholar]
- [31].Kinoshita F, Hamano H, Harada H, et al. Role of KL-6 in evaluating the disease severity of rheumatoid lung disease: comparison with HRCT. Respir Med 2004;98:1131–7. [DOI] [PubMed] [Google Scholar]
- [32].Nakajima H, Harigai M, Hara M, et al. KL-6 as a novel serum marker for interstitial pneumonia associated with collagen diseases. J Rheumatol 2000;27:1164–70. [PubMed] [Google Scholar]
- [33].Kubo M, Ihn H, Yamane K, et al. Serum KL-6 in adult patients with polymyositis and dermatomyositis. Rheumatology (Oxford, England) 2000;39:632–6. [DOI] [PubMed] [Google Scholar]
- [34].Sato S, Nagaoka T, Hasegawa M, et al. Elevated serum KL-6 levels in patients with systemic sclerosis: association with the severity of pulmonary fibrosis. Dermatology (Basel, Switzerland) 2000;200:196–201. [DOI] [PubMed] [Google Scholar]
- [35].Yamane K, Ihn H, Kubo M, et al. Serum levels of KL-6 as a useful marker for evaluating pulmonary fibrosis in patients with systemic sclerosis. J Rheumatol 2000;27:930–4. [PubMed] [Google Scholar]
- [36].Oyama T, Kohno N, Yokoyama A, et al. Detection of interstitial pneumonitis in patients with rheumatoid arthritis by measuring circulating levels of KL-6, a human MUC1 mucin. Lung 1997;175:379–85. [DOI] [PubMed] [Google Scholar]
- [37].Kumanovics G, Minier T, Radics J, et al. Comprehensive investigation of novel serum markers of pulmonary fibrosis associated with systemic sclerosis and dermato/polymyositis. Clin Exp Rheumatol 2008;26:414–20. [PubMed] [Google Scholar]
- [38].Arai S, Kurasawa K, Maezawa R, et al. Marked increase in serum KL-6 and SP-D levels during 1st 4 weeks after tratment predicts poor prognosis in patients with active interstitial pneumonia associated with polymyositis/dermatomyositis. Ann Rheum Dis 2013;71. [DOI] [PubMed] [Google Scholar]
- [39].Elhai M, Hoffmann-Vold AM, Avouac J, et al. Performance of candidate serum biomarkers for systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol 2019;71:972–82. [DOI] [PubMed] [Google Scholar]
- [40].Grosicka A, Manasar A, Kucharz EJ, et al. Serum concentration of surfactant protein D in patients with systemic sclerosis: the potential marker of the interstitial lung disease severity. Best Pract Res Clin Rheumatol 2018;32:541–9. [DOI] [PubMed] [Google Scholar]
- [41].Hanaoka M, Katsumata Y, Kawasumi H, et al. KL-6 is a long-term disease-activity biomarker for interstitial lung disease associated with polymyositis/dermatomyositis, but is not a short-term disease-activity biomarker. Modern Rheumatol 2018;1–20. [DOI] [PubMed] [Google Scholar]
- [42].Sileem AE, Said AM, Alsowey AM, et al. Clinical significance of serum surfactant protein D in patients with rheumatoid arthritis-associated interstitial lung diseases. Egyptian Journal of Chest Diseases and Tuberculosis 2016;65:479–84. [Google Scholar]
- [43].Yoshitama T, Yamaguchi A, Izumihara T, et al. Comparative evaluation of KL-6 and surfactant protein D as serum markers for interstitial pneumonia associated with collagen diseases. Mod Rheumatol 2001;11:121–6. [DOI] [PubMed] [Google Scholar]
- [44].Takahashi H, Kuroki Y, Tanaka H, et al. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am J Respir Crit Care Med 2000;162:258–63. [DOI] [PubMed] [Google Scholar]
- [45].Lee JS, Lee EY, Ha YJ, et al. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther 2019;21:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ma H, Lu J, Song Y, et al. The value of serum Krebs von den lungen-6 as a diagnostic marker in connective tissue disease associated with interstitial lung disease. BMC pulmonary medicine 2020;20:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kilinc AA, Arslan A, Yildiz M, et al. Serum KL-6 level as a biomarker of interstitial lung disease in childhood connective tissue diseases: a pilot study. Rheumatol Int 2019;doi: 10.1007/s00296-019-04485-4. [DOI] [PubMed] [Google Scholar]
- [48].Hu C, Wu C, Yang E, et al. Serum KL-6 is associated with the severity of interstitial lung disease in Chinese patients with polymyositis and dermatomyositis. Clin Rheumatol 2019;38:2181–7. [DOI] [PubMed] [Google Scholar]
- [49].Wu X, Luo CN, Shi YM, et al. The diagnostic value of serum KL-6 in connective tissue disease associated interstitial lung disease. Ann Rheum Dis 2018;77:1710–1. [DOI] [PubMed] [Google Scholar]
- [50].Cao XY, Hu SS, Xu D, et al. Serum levels of Krebs von den Lungen-6 as a promising marker for predicting occurrence and deterioration of systemic sclerosis-associated interstitial lung disease from a Chinese cohort. Int J Rheum Dis 2019;22:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Benyamine A, Heim X, Resseguier N, et al. Elevated serum Krebs von den Lungen-6 in systemic sclerosis: a marker of lung fibrosis and severity of the disease. Rheumatol Int 2018;38:813–9. [DOI] [PubMed] [Google Scholar]
- [52].Horimasu Y, Hattori N, Ishikawa N, et al. Different MUC1 gene polymorphisms in German and Japanese ethnicities affect serum KL-6 levels. Respir Med 2012;106:1756–64. [DOI] [PubMed] [Google Scholar]
- [53].Neroladaki A, Botsikas D, Boudabbous S, et al. Computed tomography of the chest with model-based iterative reconstruction using a radiation exposure similar to chest X-ray examination: preliminary observations. Eur Radiol 2013;23:360–6. [DOI] [PubMed] [Google Scholar]
- [54].Noth I, Martinez FJ. Recent advances in idiopathic pulmonary fibrosis. Chest 2007;132:637–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


