Abstract
Background:
Pentraxin 3 is an acute inflammatory protein of the long pentraxin subfamily. A meta-analysis was performed to assess diagnostic accuracy of pentraxin 3 for respiratory tract infections.
Methods:
We identify studies examining diagnostic value of pentraxin 3 for respiratory tract infections by searching Pubmed, Web of Knowledge, and Cochrane Library. The sensitivity, specificity, negative likelihood ratio (LR), positive LR, and diagnostic odds ratio were pooled. The area under the summary receiver operator characteristic (SROC) curve and Q point value (Q∗) were calculated.
Results:
A total of 8 studies with 961 individuals were eligible for this meta-analysis. The pooled sensitivity of pentraxin 3 in diagnosis of respiratory tract infections was 0.78, the pooled specificity was 0.73, the area under the SROC curve was 0.84, and the Q∗ was 0.77. The area under the SROC curve of serum and bronchoalveolar lavage fluid (BALF) pentraxin 3 was 0.85 and 0.89, respectively. Meta-regression analysis revealed that cutoff value was the source of heterogeneity among the included studies. The Deek funnel plot test suggested no evidence of publication bias. Subgroup analyses showed that the area under the SROC curve of pentraxin 3 in diagnosis of ventilator-associated pneumonia (VAP) was 0.89.
Conclusion:
Pentraxin 3 has a moderate accuracy for diagnosing respiratory tract infections and VAP. The overall diagnostic value of BALF level of pentraxin 3 is superior to its serum concentration.
Keywords: diagnostic test, meta-analysis, pentraxin 3, respiratory tract infections, ventilator-associated pneumonia
1. Introduction
Respiratory tract infections are major causes of morbidity, hospitalization, and mortality worldwide, particularly in elderly and children.[1,2] Early diagnosis of respiratory tract infections and assessment of disease severity are essential for optimal treatment. The use of traditional microbial culture has limitations, including inadequate sensitivity and difficulty in identifying colonization.[3] The absence of reliable tools for diagnosing respiratory tract infections remains a major challenge. Many biomarkers have been developed to improve the diagnostic accuracy such as C-reactive protein, procalcitonin, erythrocyte sedimentation rate (ESR), soluble triggering receptor expressed on myeloid cells-1, soluble urokinase-type plasminogen activator receptor, plasminogen activation inhibitor-1, and pentraxin 3.[3,4]
Pentraxin 3 is an acute inflammatory protein of the long pentraxin subfamily.[5] The classic short pentraxin C-reactive protein is produced in the liver and induced by proinflammatory cytokines such as interleukin 6.[5] Unlike C-reactive protein, pentraxin 3 can be rapidly produced by neutrophils, mononuclear phagocytes, and myeloid dendritic cells in response to pathogens.[4,6] Pentraxin 3 upregulates the tissue factor in monocytes and promotes the recruitment of neutrophils.[4,7] Circulating pentraxin 3 concentrations are low in normal conditions, and rapidly increase during inflammation.[8] Pentraxin 3 is elevated earlier than C-reactive protein in acute lung injury. Increased pentraxin 3 levels are correlated with disease severity and mortality in patients with acute lung injury and sepsis.[9]
Recent studies show that pentraxin 3 has emerged as a promising marker for diagnosing respiratory tract infections.[9] Therefore, we performed a meta-analysis of eligible clinical studies to assess diagnostic value of pentraxin 3 in respiratory tract infections and ventilator-associated pneumonia (VAP).
2. Materials and methods
2.1. Search strategy
We searched PubMed, Web of Knowledge, and Cochrane Library to identify studies examining diagnostic accuracy of pentraxin 3 for respiratory tract infections published up to August 2019. References of retrieved studies and relevant reviews were manually examined. The following keywords were used: “pentraxin 3,” “PTX3 protein,” “respiratory tract infections,” “pneumonia,” “respiratory infections,” and “pulmonary infections.” Ethical approval was not required, as all analyses were based on previous published studies.
2.2. Study selection
Studies were included if they fulfilled the following criteria: (1) original articles published in English; (2) studies limited to human subjects; (3) papers assessing diagnostic accuracy of pentraxin 3 for respiratory tract infections; (4) studies provided sufficient data to construct the 2 × 2 contingency table, and calculate sensitivity and specificity. Studies with fewer than 10 individuals were excluded.
2.3. Data extraction and quality assessment
Two authors (Wu Ye and Tingyu Tang) independently reviewed the included studies and obtained relevant information. If disagreement occurred, 2 authors reexamined discrepancies and resolved by consensus. The following data were extracted: family name of the first author; publication year; region of the study performed; age and number of study population; prevalence and category of respiratory tract infections; sample source; assay method for pentraxin 3; cutoff level; and number of true positive (TP), false positive (FP), false negative (FN), and true negative (TN).
Two reviewers (Wu Ye and Tingyu Tang) independently assessed the methodological quality of included trials using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS).[10] We attributed a score of 1 point for each “yes,” 0.5 point for each “unclear,” and 0 point for each “no.” The maximum score is 14 point.
2.4. Statistical analysis
All statistical analyses were performed using the MetaDisc version 1.4 software (Clinical Biostatistics Team, Ramón y Cajal Hospital, Madrid, Spain) and Stata version 16.0 software (StataCorp, College Station, TX). The sensitivity, specificity, negative likelihood ratio (LR), positive LR, and diagnostic odds ratio (DOR) were pooled, the summary receiver operator characteristic (SROC) curves were constructed, and the area under the SROC curve and Q point value (Q∗) were calculated. The heterogeneity among included studies was evaluated by the Chi-square test. If heterogeneity was present, meta-regression analyses were performed to explore the sources of heterogeneity. The presence of publication bias was tested using the Deek funnel plot. A P value < .05 was considered statistically significant.
3. Results
3.1. Study characteristics
Our initial literature search yielded 349 studies (Fig. 1). Three hundred twenty-six citations were excluded after review of title and abstract. Of 23 studies selected for full-text assessment, 11 did not meet inclusion criteria, and 4 could not generate the 2 × 2 contingency table. Ultimately, a total of 8 studies with 961 individuals were eligible for this meta-analysis.[9,11–17]
Figure 1.
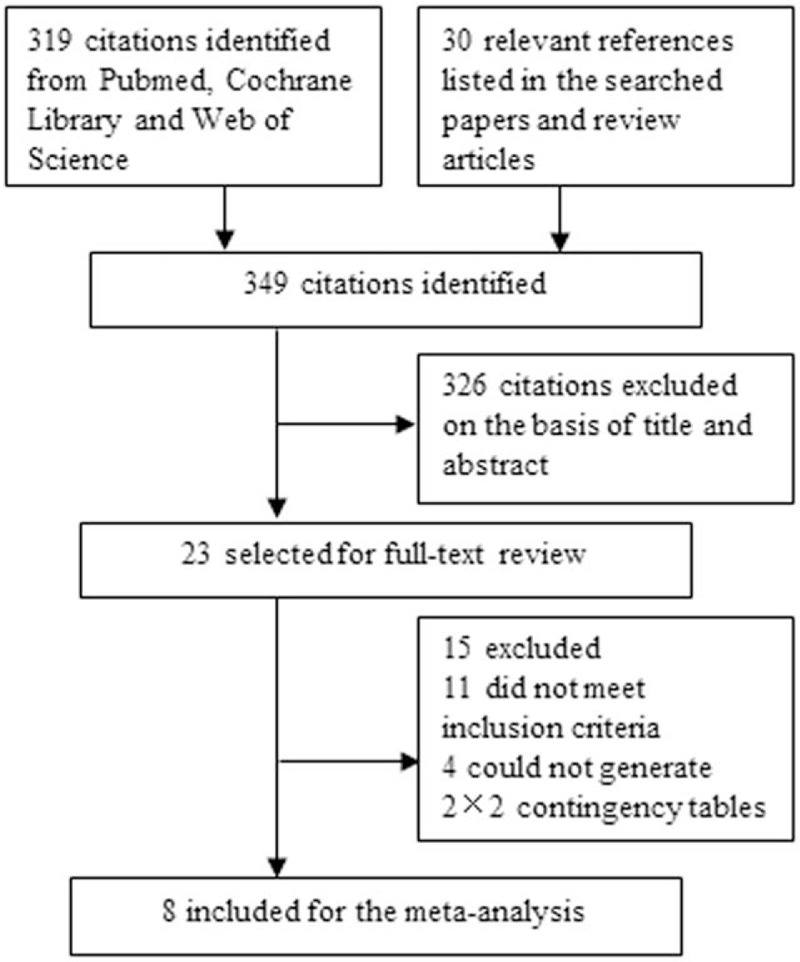
Flowchart of the study selection process.
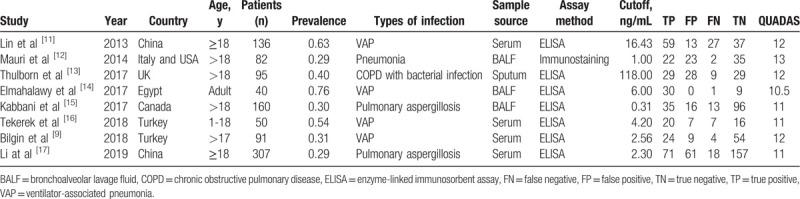
The clinical characteristics of included studies are presented in Table 1. Serum specimens were collected in 5 studies, sputum was for 1 trial and bronchoalveolar lavage fluid (BALF) was gathered in the other 4 studies. Two studies tested both BALF and serum samples,[14,17] 3 trials only collected serum, 2 studies only detected BALF, and the other study obtained sputum. The concentration of pentraxin 3 was measured by the enzyme-linked immunosorbent assay (ELISA) in 7 studies, while immunostaining was performed in only 1 study. Commercial ELISA kits were used to measure pentraxin 3 levels according to the manufacturer's instructions. The cutoff levels of pentraxin 3 among the included studies were ranged from 0.312 ng/mL to 118 ng/mL. In our meta-analysis, the QUADAS scores for included studies were all above 10, indicating that all studies were of high quality.
Table 1.
Clinical characteristics of included studies.
3.2. Diagnostic accuracy for respiratory tract infections
The forest plot for sensitivity and specificity of pentraxin 3 in diagnosis of respiratory tract infections is presented in Fig. 2. The pooled sensitivity was 0.78 [95% confidence interval (CI), 0.74–0.82] and the pooled specificity was 0.73 (95% CI, 0.70–0.77). The positive LR was 2.94 (95% CI, 2.11–2.10), the negative LR was 0.30 (95% CI, 0.22–0.41), and the DOR was 10.84 (95% CI, 6.02–19.51). As shown in Fig. 3, the area under the SROC curve was 0.84 and the Q∗ was 0.77, indicating a moderate diagnostic accuracy.
Figure 2.
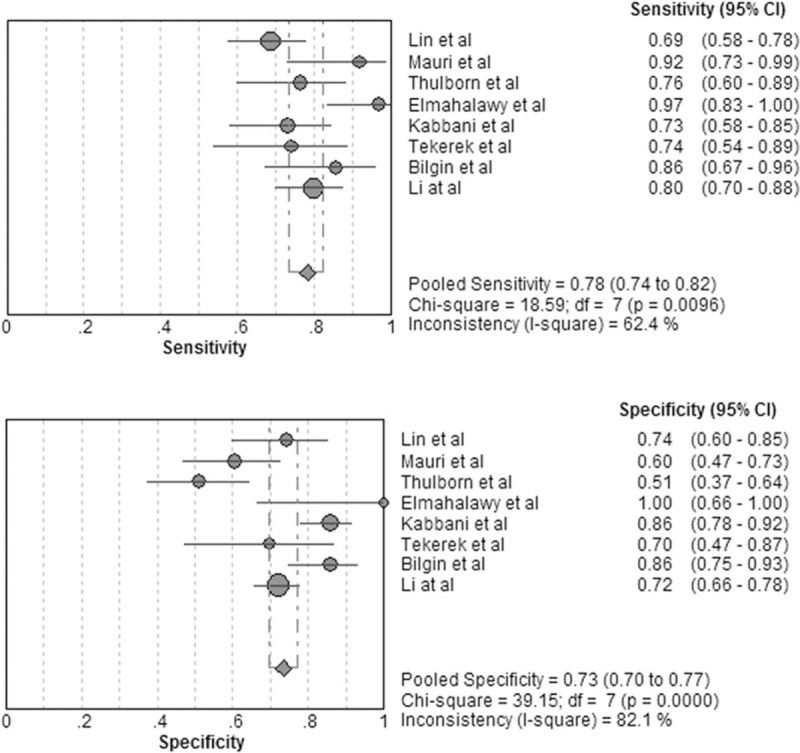
Forest plot of sensitivity and specificity for pentraxin 3 in diagnosis of respiratory tract infections. The pooled sensitivity was 0.78 (95% CI, 0.74–0.82) and the pooled specificity was 0.73 (95% CI, 0.70–0.77).
Figure 3.
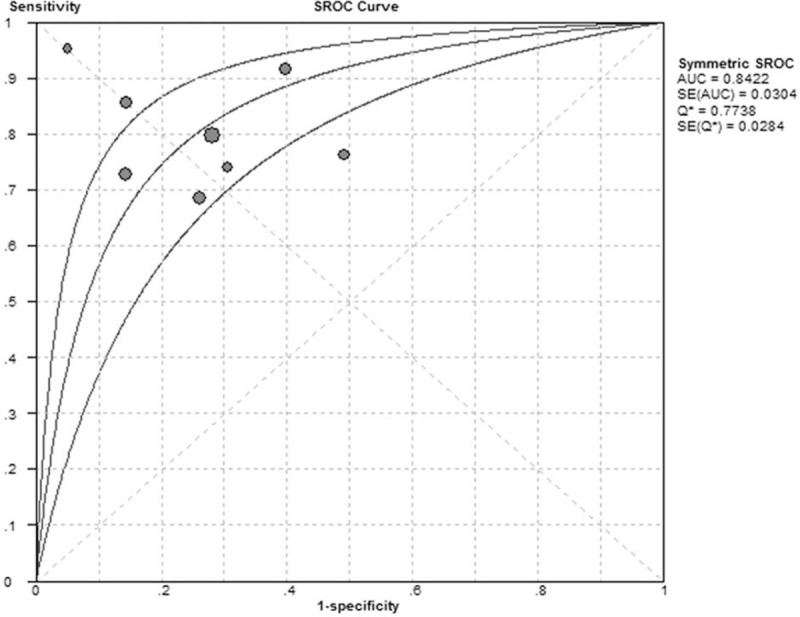
Summary receiver operating characteristic (SROC) curve of pentraxin 3 in diagnosis of respiratory tract infections. The area under the SROC curve was 0.84 and the Q∗ was 0.77.
3.3. Heterogeneity assessment and meta-regression analysis
The I2 for pooled sensitivity, specificity, and DOR was 62.4%, 82.1%, and 60.7%, respectively. The results indicated substantial heterogeneity among the included studies. We performed meta-regression analysis to explore the sources of potential heterogeneity. The relevant covariates included age (≥18 years vs <18 years), sample source (serum, sputum vs BALF), assay method (immunostaining vs ELISA), cutoff value (≥10 ng/mL vs <10 ng/mL). The meta-regression analysis showed that the source of heterogeneity among studies was related to the cutoff value (P = .04).
3.4. Publication bias
The Deek funnel plot test suggested no evidence of publication bias among the included studies (P = .25, Fig. 4).
Figure 4.
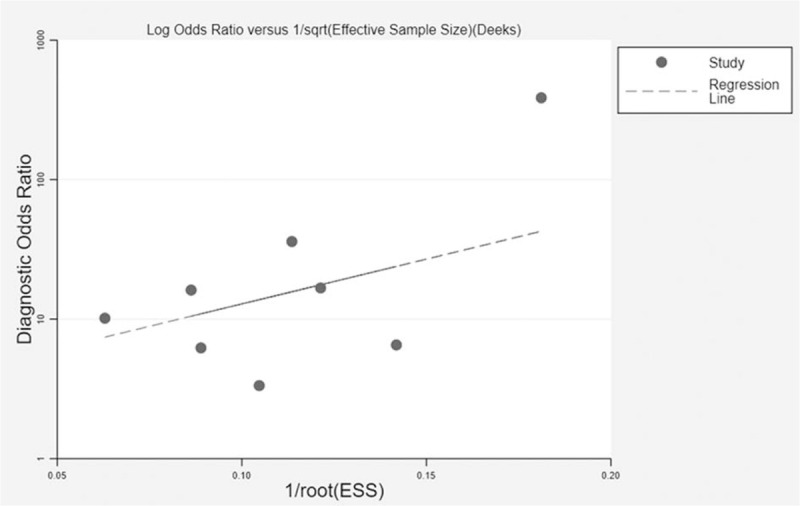
The Deek funnel plot for assessment of publication bias. No publication bias was found among the included studies.
3.5. Subgroup analysis
The pooled sensitivity of pentraxin 3 in VAP diagnosis was 0.77 (95% CI, 0.70–0.83) and the pooled specificity was 0.80 (95% CI, 0.73–0.86; Fig. 5). The positive LR was 3.59 (95% CI, 2.04–6.34), the negative LR was 0.24 (95% CI, 0.12–0.51), the DOR was 15.92 (95% CI, 4.51–56.15), the area under the SROC curve was 0.89, and the Q∗ was 0.82.
Figure 5.
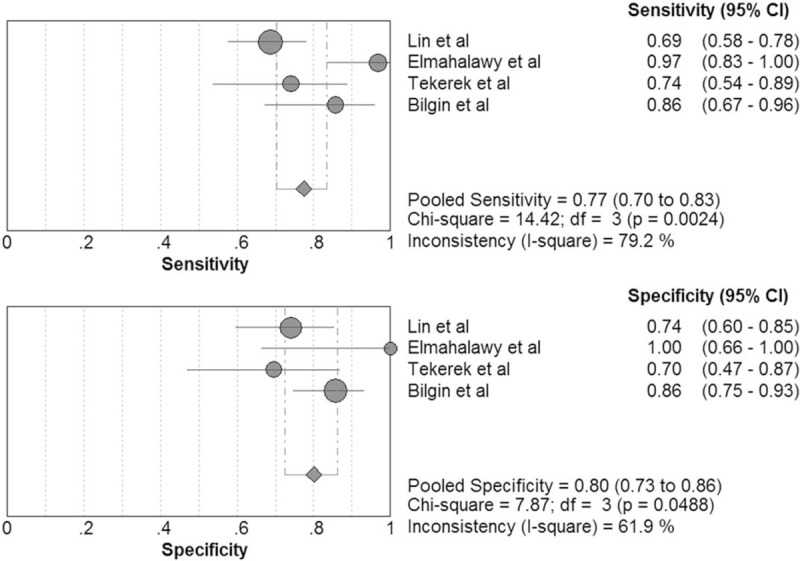
Forest plot of sensitivity and specificity for pentraxin 3 in diagnosis of ventilator-associated pneumonia (VAP). The pooled sensitivity was 0.77 (95% CI, 0.70–0.83) and the pooled specificity was 0.80 (95% CI, 0.73–0.86).
The pooled sensitivity of pentraxin 3 in diagnosis of bacterial respiratory tract infections was 0.77 (95% CI, 0.71–0.83) and the pooled specificity was 0.72 (95% CI, 0.65–0.78; Fig. 6). The positive LR, negative LR, DOR, area under the SROC curve, and Q∗ was 2.95 (95% CI, 1.59–5.47), 0.30 (95% CI, 0.18–0.51), 10.60 (95% CI, 3.76–29.87), 0.89, and 0.80, respectively.
Figure 6.
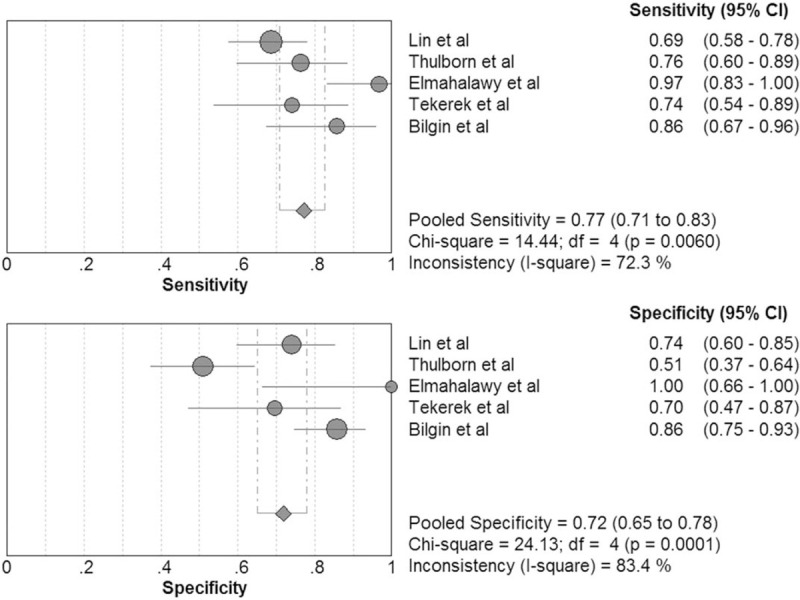
Forest plot of sensitivity and specificity for pentraxin 3 in diagnosis of bacterial respiratory tract infections. The pooled sensitivity was 0.77 (95% CI, 0.71–0.83) and the pooled specificity was 0.72 (95% CI, 0.65–0.78).
The pooled sensitivity and specificity of serum pentraxin 3 for diagnosing respiratory tract infections was 0.77 (95% CI, 0.71–0.82) and 0.75 (95% CI, 0.70–0.79; Fig. 7), respectively. The positive LR was 3.19 (95% CI, 2.33–4.38), the negative LR was 0.29 (95% CI, 0.20–0.43), and the DOR was 11.35 (95% CI, 5.91–21.78). The area under the SROC curve was 0.85 and the Q∗ was 0.78.
Figure 7.
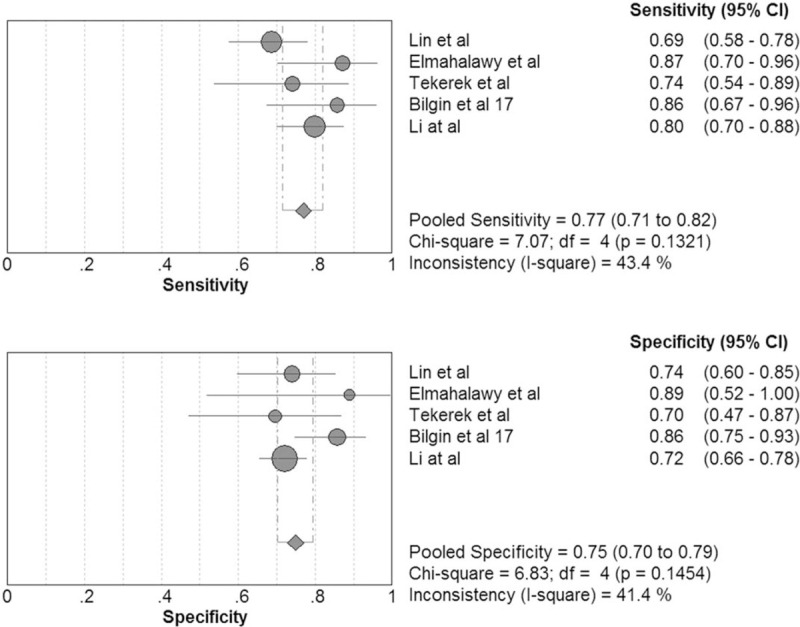
Forest plot of sensitivity and specificity for serum pentraxin 3 in diagnosis of respiratory tract infections. The pooled sensitivity was 0.77 (95% CI, 0.71–0.82) and the pooled specificity 0.75 (95% CI, 0.70–0.79).
The pooled sensitivity of BALF pentraxin 3 for diagnosis of respiratory tract infections was 0.85 (95% CI, 0.78–0.90) and the pooled specificity was 0.80 (95% CI, 0.76–0.84; Fig. 8). The positive LR, negative LR, and DOR was 4.09 (95% CI, 2.29–7.31), 0.18 (95% CI, 0.09–0.35), and 23.41 (95% CI, 11.64–47.09), respectively. The area under the SROC curve was 0.89 and the Q∗ was 0.83.
Figure 8.
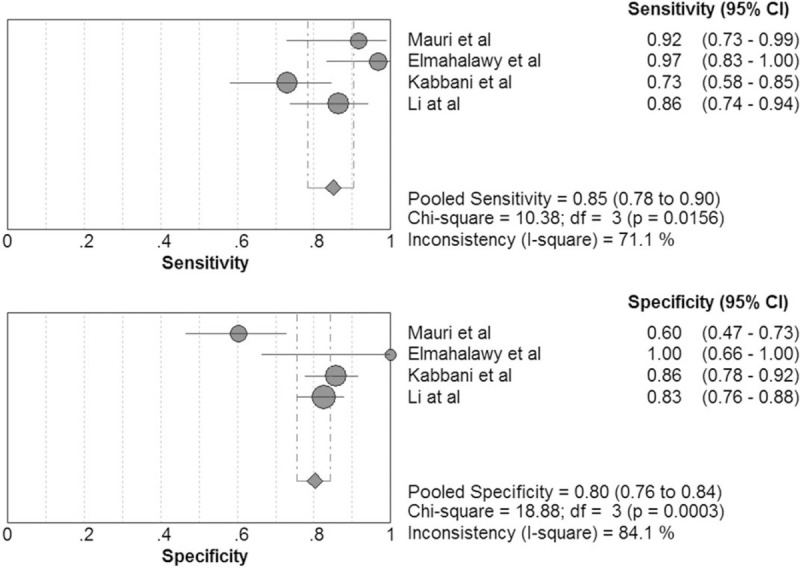
Forest plot of sensitivity and specificity for BALF pentraxin 3 in diagnosis of respiratory tract infections. The pooled sensitivity was 0.85 (95% CI, 0.78–0.90) and the pooled specificity was 0.80 (95% CI, 0.76–0.84).
4. Discussion
Respiratory tract infections remain the most common reason for patients to seek medical service.[18,19] A rapid and accurate approach to diagnose respiratory tract infections is crucial for starting appropriate treatment.[18,19] Pentraxin 3, the first identified member of the long pentraxin subfamily, is a vital component of innate immunity related to sepsis.[4,6,20] Pentraxin 3 plays an important role in the early stages of inflammation by recognizing microorganisms, promoting pathogen recognition and regulating complement activation.[5,21,22] To our knowledge, no meta-analysis to date has assessed diagnostic value of pentraxin 3 in respiratory tract infections. In the present study, we included 8 eligible trials with 961 patients to estimate the test value of pentraxin 3 for respiratory tract infections and performed subgroup analyses to explore diagnostic accuracy for VAP and bacterial respiratory tract infections.
Our meta-analysis shows that the pooled sensitivity of pentraxin 3 in diagnosis of respiratory tract infections was 0.78(CI, 0.74–0.82) and the pooled specificity was 0.73 (95% CI, 0.70–0.77). In forest plot, most included studies agreed on the pooled sensitivity and specificity except 2 studies.[13,14] The reported sensitivity and specificity in the study by Elmahalawy et al[14] was 96.8% and 100%, respectively. The results were based on a small population with 40 patients. In the study by Thulborn et al,[13] the diagnostic specificity of pentraxin 3 was 50.9%. Of the 8 included studies, only this study[13] measured pentraxin 3 concentrations in sputum. In the present meta-analysis, QUADAS scores of included studies are all above 10, which suggested that the qualities of all trials are high.
The SROC curve illustrates a summary of overall test accuracy. The area under the SROC curve ranging 0.50 to 0.70 represented low accuracy, 0.70 to 0.90 suggested moderate accuracy, and >0.90 revealed high accuracy.[23,24] In the present study, the area under the SROC curve was 0.84 and the Q∗ was 0.77, indicating that pentraxin 3 had a moderate accuracy for diagnosing respiratory tract infections. Our subgroup analyses suggested that overall diagnostic accuracy of pentraxin 3 was similar for VAP and bacterial respiratory tract infections.
BALF is a common source of samples for diagnosing lung infections. The main advantage of BALF is that it is close to the site of lung infections and can be a good indicator of the local lung environment.[25,26] BALF and serum biomarkers may provide different diagnostic values. In the present meta-analysis, the pooled sensitivity and specificity of serum pentraxin 3 in diagnosis of respiratory tract infections was 0.77 and 0.75, respectively. The pooled sensitivity of BALF pentraxin 3 was 0.85 and the specificity was 0.80. Among the included studies, 2 studies[14,17] examined both BALF and serum samples, and both found that BALF pentraxin 3 has a diagnostic value superior to serum pentraxin 3. These results indicated that the overall diagnostic accuracy of pentraxin 3 in BALF was better than that in serum. BALF biomarkers may more accurately reflect lung inflammation.
Heterogeneity among the included studies was evaluated in the current study. The I2 for pooled sensitivity, specificity, and DOR are all >50%, indicating substantial heterogeneity among included trails. The meta-regression analysis showed that cutoff value was the source of heterogeneity. The cutoff levels of pentraxin 3 were ranged from 0.312 ng/mL to 118 ng/mL in the present meta-analysis. Two studies used cut-off values above 10 ng/mL.[11,13] Samples were collected at different time points in the included studies, which is also a possible source of heterogeneity. Diagnostic studies may have publication bias. Studies with negative results are not easily published, whereas those with positive data are more likely to be published. In the present study, the Deek funnel plot test showed no potential publication bias.
The present study has some limitations. First, pentraxin 3 was calculated by different methods, and its cutoff value varied across included studies, which made it difficult to determine the real diagnostic value. Our meta-regression analysis suggested that cutoff value contributed to heterogeneity. Second, included studies were limited to those in English, which may cause publication bias. Third, respiratory tract infections are made up of many different diseases, which may result in heterogeneity.
In summary, the available evidence suggests that pentraxin 3 has a moderate accuracy for diagnosing respiratory tract infections, VAP, and bacterial respiratory tract infections. BALF level of pentraxin 3 is superior to its serum concentration in diagnosis of respiratory tract infections.
Author contributions
Conceptualization: Wu Ye, Qing-Dong Huang.
Data curation: Wu Ye, Ting-Yu Tang.
Formal analysis: Wu Ye, Ting-Yu Tang.
Methodology: Wu Ye, Ting-Yu Tang.
Software: Wu Ye, Ting-Yu Tang.
Supervision: Guang-Yue Qin.
Validation: Ting-Yu Tang, Guang-Yue Qin.
Writing – original draft: Wu Ye, Qing-Dong Huang.
Writing – review & editing: Ting-Yu Tang, Guang-Yue Qin.
Footnotes
Abbreviations: BALF = bronchoalveolar lavage fluid, COPD = chronic obstructive pulmonary disease, DOR = diagnostic odds ratio, ELISA = enzyme-linked immunosorbent assay, ESR = erythrocyte sedimentation rate, FN = false negative, FP = false positive, LR = likelihood ratio, Q∗ = Q point value, QUADAS = Quality Assessment of Diagnostic Accuracy Studies, SROC = summary receiver operator characteristic, TN = true negative, TP = true positive, VAP = ventilator-associated pneumonia.
How to cite this article: Ye W, Huang QD, Tang TY, Qin GY. Diagnostic value of pentraxin 3 in respiratory tract infections: a meta-analysis. Medicine. 2020;99:14(e19532).
This study was supported by grants from medical health science and technology plan projects of Zhejiang province (2019RC090).
The authors have no conflicts of interest.
References
- [1].Malosh RE, Martin ET, Ortiz JR, et al. The risk of lower respiratory tract infection following influenza virus infection: a systematic and narrative review. Vaccine 2018;36:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wunderink RG, Waterer GW. Community-acquired pneumonia. N Engl J Med 2014;370:543–51. [DOI] [PubMed] [Google Scholar]
- [3].Schuetz P, Amin DN, Greenwald JL. Role of procalcitonin in managing adult patients with respiratory tract infections. Chest 2012;141:1063–73. [DOI] [PubMed] [Google Scholar]
- [4].Saleh MAA, van de Garde EMW, van Hasselt JGC. Host-response biomarkers for the diagnosis of bacterial respiratory tract infections. Clin Chem Lab Med 2019;57:442–51. [DOI] [PubMed] [Google Scholar]
- [5].Luo Q, He X, Ning P, et al. Admission pentraxin-3 level predicts severity of community-acquired pneumonia independently of etiology. Proteomics Clin Appl 2019;13:e1800117. [DOI] [PubMed] [Google Scholar]
- [6].Caironi P, Masson S, Mauri T, et al. ALBIOS Biomarkers Study Investigators. Pentraxin 3 in patients with severe sepsis or shock: the ALBIOS trial. Eur J Clin Invest 2017;47:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jie H, Li Y, Pu X, et al. Pentraxin 3, a predicator for 28-day mortality in patients with septic shock. Am J Med Sci 2017;353:242–6. [DOI] [PubMed] [Google Scholar]
- [8].Kim HS, Won S, Lee EK, et al. Pentraxin 3 as a clinical marker in children with lower respiratory tract infection. Pediatr Pulmonol 2016;51:42–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bilgin H, Haliloglu M, Yaman A, et al. Sequential measurements of pentraxin 3 serum levels in patients with ventilator-associated pneumonia: a nested case-control study. Can J Infect Dis Med Microbiol 2018;2018:4074169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. J BMC Med Res Methodol 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lin Q, Fu F, Shen L, et al. Pentraxin 3 in the assessment of ventilator-associated pneumonia: an early marker of severity. Heart Lung 2013;42:139–45. [DOI] [PubMed] [Google Scholar]
- [12].Mauri T, Coppadoro A, Bombino M, et al. Alveolar pentraxin 3 as an early marker of microbiologically confirmed pneumonia: a threshold-finding prospective observational study. Crit Care 2014;18:562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Thulborn SJ, Dilpazir M, Haldar K, et al. Investigating the role of pentraxin 3 as a biomarker for bacterial infection in subjects with COPD. Int J Chron Obstruct Pulmon Dis 2017;12:1199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elmahalawy II, Ammar AS, Fathy WM, et al. Pentraxin 3 as an early marker in diagnosis of ventilator associated pneumonia. Egypt J Chest Dis Tuberc 2017;66:709–12. [Google Scholar]
- [15].Kabbani D, Bhaskaran A, Singer LG, et al. Pentraxin 3 levels in bronchoalveolar lavage fluid of lung transplant recipients with invasive aspergillosis. J Heart Lung Transplant 2017;36:973–9. [DOI] [PubMed] [Google Scholar]
- [16].Tekerek NU, Akyildiz BN, Ercal BD, et al. New biomarkers to diagnose ventilator associated pneumonia: pentraxin 3 and surfactant protein D. Indian J Pediatr 2018;85:426–32. [DOI] [PubMed] [Google Scholar]
- [17].Li H, Liu L, Zhou W, et al. Pentraxin 3 in bronchoalveolar lavage fluid and plasma in non-neutropenic patients with pulmonary aspergillosis. Clin Microbiol Infect 2019;25:504–10. [DOI] [PubMed] [Google Scholar]
- [18].Meili M, Kutz A, Briel M, et al. Infection biomarkers in primary care patients with acute respiratory tract infections-comparison of Procalcitonin and C-reactive protein. BMC Pulm Med 2016;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Le Bel J, Hausfater P, Chenevier-Gobeaux C, et al. ESCAPED study group. Diagnostic accuracy of C-reactive protein and procalcitonin in suspected community-acquired pneumonia adults visiting emergency department and having a systematic thoracic CT scan. Crit Care 2015;19:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fahmey SS, Mostafa N. Pentraxin 3 as a novel diagnostic marker in neonatal sepsis. J Neonatal Perinatal Med 2019;12:437–42. [DOI] [PubMed] [Google Scholar]
- [21].de Oliveira THC, Souza DG, Teixeira MM, et al. Tissue dependent role of PTX3 during ischemia-reperfusion injury. Front Immunol 2019;10:1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guo H, Qiu X, Deis J, et al. Pentraxin 3 deficiency exacerbates lipopolysaccharide-induced inflammation in adipose tissue. Int J Obes (Lond) 2020;44:525–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Brusselaers N, Labeau S, Vogelaers D, et al. Value of lower respiratory tract surveillance cultures to predict bacterial pathogens in ventilator-associated pneumonia: systematic review and diagnostic test accuracy meta-analysis. Intensive Care Med 2013;39:365–75. [DOI] [PubMed] [Google Scholar]
- [24].Xie C, Huang T, Teng Z, et al. A meta-analysis of the diagnostic value of microRNA-1246 for malignant tumors. Medicine (Baltimore) 2019;98:e15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].García-Laorden MI, Lorente JA, Flores C, et al. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med 2017;5:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tschiedel E, Goralski A, Steinmann J, et al. Multiplex PCR of bronchoalveolar lavage fluid in children enhances the rate of pathogen detection. BMC Pulm Med 2019;19:132. [DOI] [PMC free article] [PubMed] [Google Scholar]


