Abstract
Lung adenocarcinoma (LUAD), a form of lung cancer, is reported to cause first and second-order cancer morbidity to men and women in China, respectively. We assessed the mRNA expression of GJB2 in LUAD patients in our study, based on data acquired from the cancer genome atlas (TCGA) and so as to increase further knowledge into the biological pathways involved in LUAD pathogenesis related to GJB2.
Information on gene expression and comparing clinical data were recognized and downloaded from TCGA. Gene set enrichment analysis (GSEA) created an arranged list of all genes is indicated by their connection with GJB2 expression.
Our study cohort included 265 (54.5%) female and 221 (36.0%) male patients. The scatter plot and paired plot showed the difference of GJB2 expression between normal and tumor samples (P < .01). Overall survival (OS) analysis demonstrated that LUAD with GJB2 -high had a more terrible prognosis than that with GJB2 -low (P < .01). Multivariate analysis with the cox proportional hazards model indicated that the expression of Cx26 (HR: 1.00; 95%CI: 1.00–1.01; P = .041) and stage (HR: 1.95; 95%CI: 1.23–3.09; P = .003) were independent prognostic factors for patients with LUAD. The GSEA results showed that cytosolic DNA sensing pathway, apoptosis, cytokine-cytokine receptor interaction, natural killer cell mediated cytotoxicity, regulation of actin cytoskeleton, toll-like receptor signaling pathway, small cell lung cancer and pathways in cancer are differentially enriched in GJB2 high expression phenotype.
Our study confirmed the significantly high levels of Cx26 expression in LUAD patients with several observed clinical features. GJB2 may be a potentially useful prognostic molecular biomarker of bad survival in LUAD, while further experimental ought to be performed to demonstrate the biologic effect of GJB2.
Keywords: GJB2, lung adenocarcinoma, prognosis, the cancer genome atlas
1. Introduction
Lung adenocarcinoma (LUAD), a form of lung cancer, is reported to cause first and second-order cancer morbidity to men and women in China, respectively.[1,2] Recently, the population of LUAD patients is on the rise due to elevated levels of atmospheric pollution, tobacco intake, and rapid aging that causes 1.8 million deaths annually.[3–5] Typically, the late diagnosis of LUAD lowers the 5-year overall survival rate of patients to 15%; however, more than 60% of LUAD patients experience targetable gene alterations, which could improve their survival rate.[6–7] Nevertheless, a general lack of specific biomarkers for early detection of LUAD leads to a high rate of metastasis and drug resistance in LUAD patients with high mortality rates, worldwide.[8] Consequently, the development of a novel therapeutic target molecule for effective diagnosis of LUAD is necessary.
Post-transcriptional modifications are vital for the initiation and progression of tumors. Gap junction protein beta 2 (GJB2), also known as connexin 26 (Cx26), belongs to a member of the gap junction protein family that causes mutations in the gene, which leads to 50% of pre-lingual, recessive deafness.[9] Studies reported that Cx26 plays a vital role in chemoresistance and metastasis during tumor prognosis of patients. Loewenstein et al first proposed that cell communication activities mediated by gap junctions were closely related to cellular growth control and tumorigenesis. More precisely, the PI3K/Akt signaling pathway connected to Cx26 is reported to cause acquired gefitinib resistance in NSCLC cells through the activation of epithelial-mesenchymal transition (EMT).[10,11] A recent study found that the internalization of Cx26 contributed to proliferation, EMT and migration, which caused non-small cell lung cancer (NSCLC) by aberrant activation of the P53/MDM2 signaling pathway under hypoxic conditions.[12] Studies reported that patients with high levels of Cx26 in the primary tumor had a high probability of developing lung metastasis.[1,13] Moreover, high expression of Cx26 in the colorectal carcinomas caused poor clinical outcomes.[14,15] Interestingly, Cx26 also promotes self-renewal of cancer stem cells (CSCs) that shape the signaling complex with the aid of pluripotency transcription factor NANOG and FAK, which lead to NANOG FAK activation in triple-negative breast cancer (TNBC).[16] Furthermore, the cytoplasmic Cx26 levels are reported to be related to lymphatic vessel invasion and poor relapse-free survival in breast tumor tissues.[17] However, the role of Cx26 in LUAD prognostic remains to be elucidated. We assessed the mRNA expression of Cx26 in LUAD patients in our study, based on data acquired from the cancer genome atlas (TCGA). Besides the clinical features of LUAD, we also conducted a Gene set enrichment analysis (GSEA) analysis to understand the biological pathways involved in Cx26-associated LUAD pathogenesis.
2. Materials and methods
2.1. Gene information and bioinformatics analysis
Information on gene expression and comparing clinical data (486 cases, Data Format: BCR XML) were recognized and downloaded from level 3 gene-expression information (FPKM normalized) of TCGA LUAD cohort. Boxplots were utilized to envision expression differences for discrete variables. The clinicopathological data collected included gender, age, stage, grade, T-classification, M-classification, N-classification, survival status and survival duration in days. As this study is a bioinformatics study, there is no need of ethics committee or institutional review board to approve the study.
2.2. GSEA enrichment
GSEA created an arranged list of all genes s indicated by their connection with Cx26 expression. Then, samples were divided into high- and low-Cx26 groups as training set to distinguish the potential function and elucidate the significant survival difference utilizing GSEA. Annotated gene sets c2.cp.kegg.v6.0.symbols.gmt was selected as the reference gene sets, which includes terms with FDR < 0.05. Gene set permutations were executed multiple times for every examination. The expression degree of UBE2T was applied as a phenotype label. The normalized enrichment score (NES) and nominal P value had been used to kind the pathways enriched in every phenotype.
2.3. Statistical analysis
The listwise deletion technique was utilized to deal with missing data, which excluded the entire sample from the investigation if any single value was absent. The connection between clinical factors and Cx26 were examined with the logistic regression, Wilcoxon signed-rank test and Kruskal test. Clinical factors related to overall survival utilizing Cox regression and the Kaplan–Meier approach. Multivariate Cox analysis was utilized to evaluate the impact of Cx26 expression on survival alongside other clinical attributes (such as age, gender, stage, distant metastasis). Benjamini–Hochberg way to transform the P values to FDRs. Data were examined with the R (version 3.5.3) and R Bioconductor packages. We use Perl language for data matrix and data processing according to P less than .5.
3. Results
3.1. Patients’ characteristics
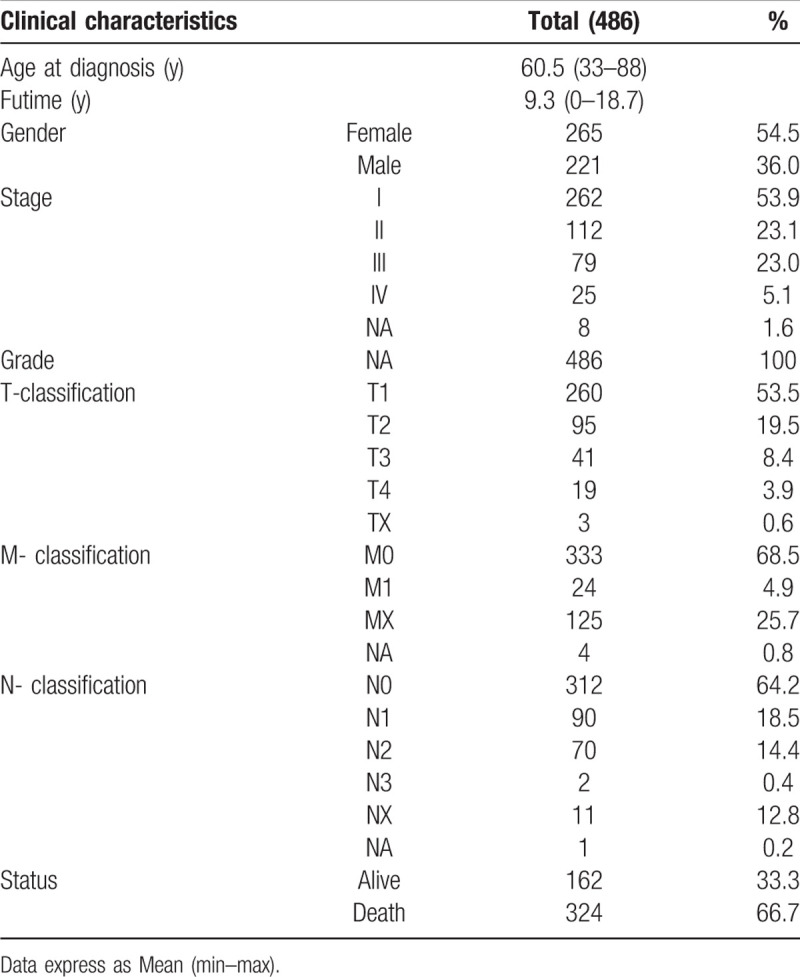
TCGA database contained 486 patients and the clinicopathological attributes of these samples are appear in Table 1. The middle age at diagnosis in TCGA was 60.5 years old (range 33–88 years) and median finally contact for subjects was 9.3 years (range 0–18.7 years). Meanwhile, follow-up for subjects conformed 162 (33.3%) alive and 324 (66.7%) death patients. Our study cohort included 265 (54.5%) female and 221 (36.0%) male patients. Stage I disease was located in 262 patients (53.9%), stage II in 112 (23.1%), stage III in 79 (23.0%) and stage IV in 25 (5.1%). Tumor stage was found T1 in 260 patients (53.5%), T2 in 95 (19.5%), T3 in 41 (8.4%) and T4 in 19 (3.9%). Node stage contained N0 in 321 (64.2%), N1 in 90 (18.5%), N2 in 70 (14.4%), N3 in 2 (0.4%). A total of 24 of 486 (4.9%) cases had distant metastases. All the subjects were adenomas or adenocarcinomas.
Table 1.
TCGA lung adenocarcinoma patient characteristics.
3.2. Association with Cx26 expression and clinicopathologic factors
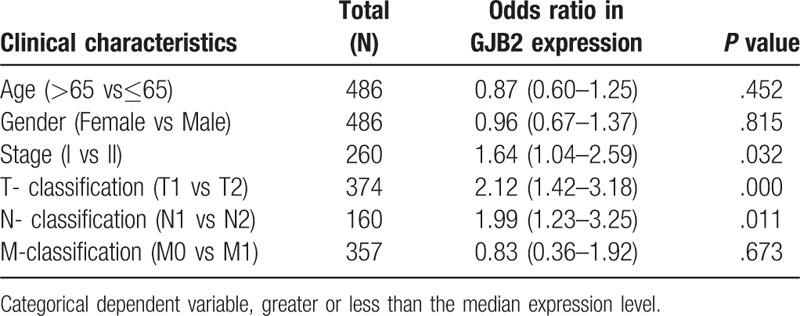
The scatter plot showed the difference of Cx26 expression between normal and tumor samples (P < .01) (Fig. 1A). We then use paired plot to demonstrated the Cx26 expression between normal and tumor from the same patients and the results was significant difference (P < .01, Fig. 1B). The outcomes suggested that the expression of Cx26 was significant difference and may play a vital role in regulating cancer development. The expression of Cx26 correlated significantly with the patient clinical stage, T-classification and N-classification (P < .05) (Fig. 1D–F). Univariate analysis utilizing logistic regression uncovered that Cx26 expression as a clear-cut ward variable was related to poor prognostic clinicopathologic factors (Table 2). Cx26 expression in LAUD as appreciably connected with stage (OR = 1.64; 95%CI = 1.02–2.59, I vs II), T-classification (OR = 2.12; 95%CI = 1.42–3.18, T1 vs T2) and N-classification (OR = 1.99; 95%CI = 1.23–3.25, N1 vs N2) indicated that patients with high Cx26 mRNA expression are inclined to advance to a further advanced stage than those with low Cx26 mRNA expression.
Figure 1.
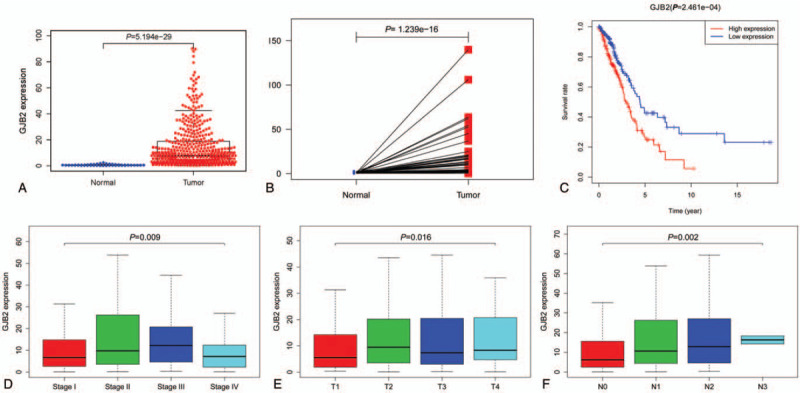
(A) The scatter plot showed the difference of Cx26 expression between normal and tumor samples (P < .01); (B) paired plot to demonstrated the Cx26 expression between normal and tumor from the same patients and the results was significant difference (P < .01); (C) Overall survival (OS) analysis demonstrated that LUAD with Cx26 -high had a more terrible prognosis than that with Cx26 -low (P < .01); (D-F) The expression of Cx26 correlated significantly with the patient clinical stage, T-classification and N-classification (P < .05).
Table 2.
GJB2 expression associated with clinical pathological characteristics (logistic regression).
3.3. Survival results and multivariate examination
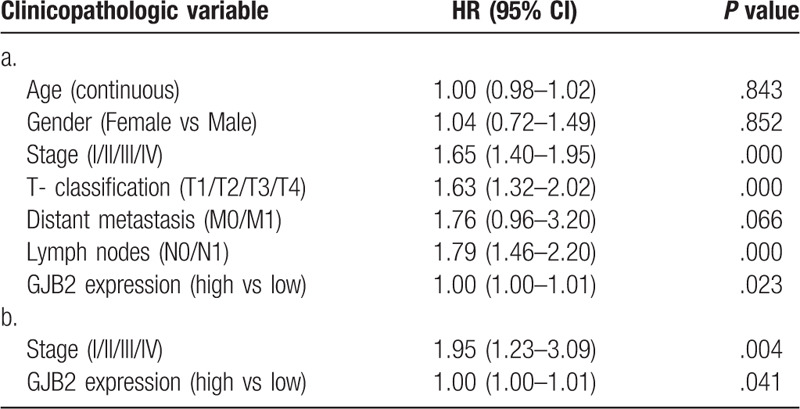
Overall survival (OS) analysis demonstrated that LUAD with Cx26 -high had a more terrible prognosis than that with Cx26 -low (P < .01) (Fig. 1C). The univariate analysis suggested that Cx26 associated essentially with stage (HR: 1.65; 95%CI: 1.40–1.95; P < .01), T-classification (HR: 1.63; 95%CI: 1.32–2.02; P < .01) and N-classification (HR: 1.79; 95%CI: 1.46–2.20; P < .01). Multivariate analysis with the cox proportional hazards model indicated that the expression of Cx26 (HR: 1.00; 95%CI: 1.00–1.01; P = .041) and stage (HR: 1.95; 95%CI: 1.23–3.09; P = .003) were independent prognostic factors for patients with LUAD. (Table 3)
Table 3.
a. Associations with overall survival and clinicopathologic characteristics in TCGA patients using Cox regression. b. Multivariate survival model after variable selection.
3.4. GSEA recognizes GJB2 related signaling pathway
In order to recognize signaling pathways which might be differentially initiated in LUAD, we led GSEA analysis among high and low Cx26 expression data sets (FDR P < .05, NOM P < .05). We chose the most significantly enriched signaling pathways dependent on normalized enrichment score (NES) Table 4. The results showed that cytosolic DNA sensing pathway, apoptosis, cytokine-cytokine receptor interaction, natural killer cell mediated cytotoxicity, regulation of actin cytoskeleton, toll-like receptor signaling pathway, small cell lung cancer and pathways in cancer are differentially enriched in Cx26 high expression phenotype (Fig. 2). The KEGG pathway of pathways in cancer and small cell lung cancer was shown in Figures 3 and 4.
Table 4.
Gene sets enriched in phenotype high.
Figure 2.
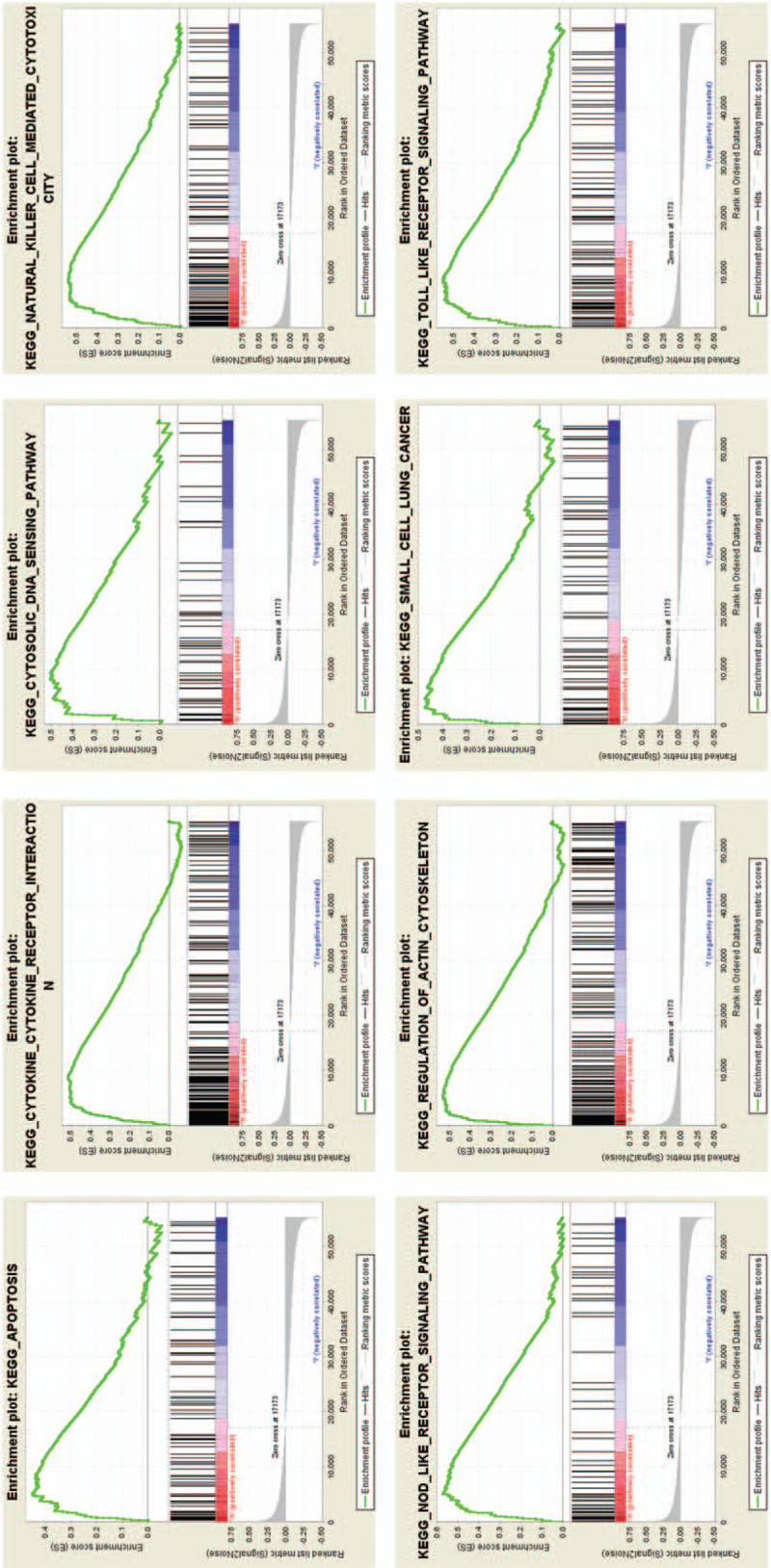
Enrichment plots from gene set enrichment analysis (GSEA).
Figure 3.
The KEGG pathway of pathways in cancer and small cell lung cancer.
Figure 4.
The KEGG pathway of pathways in small cell lung cancer.
4. Discussion
In this manuscript, we conducted a comprehensive and detailed assessment of Cx26 expression in LUAD patients to explore its association with clinicopathologic characteristics, survival rates, and functionality. A detailed understanding of a highly-expressed biomarker in LUAD-afflicted tumor cells allowed us to interpret its clinical survival patterns. Our results indicated that Cx26 expressions were prominent in healthy and tumor samples, respectively, and also regulated cancer progression. Therefore, Cx26 could be a potential target molecule for the development of diagnostic strategies for LUAD patients.
Although advance surgery is recommended for patients diagnosed with an early stage of cancer to facilitate their long-term survival rates, the low survival rates of such patients with metastasis account for 90% of cancer-related deaths.[18,19] Cytoplasmic connexins-mediated signaling is reported to modulate the chemoresistance of tumor cells and considered to be potential biomarkers for cancer prognosis. As an essential member of the gap junction protein family, Cx26 plays a critical role in tumor growth, migration, and invasion. Meanwhile, high cytoplasmic Cx26 expression is reported to be associated with lymph node metastasis and a high occurrence of intra-glandular dissemination in follicular thyroid cancer.[20] Similarly, high Cx26 levels in esophageal squamous cell carcinoma (ESCC) tissues are associated with high lymph node metastasis and reduced chances of survival of patients with ESCC. This pheromone was also found in melanoma and breast cancer cells.[21–22] Cx26 could significantly enhance both cell proliferation and tumorigenicity in head and neck squamous cell carcinoma (HNSCC);[23] whereas, a moderate increase of cytoplasmic Cx26 levels is also observed during tumor progression in malignant melanoma. Besides, the loss of correlation between STAT3 and Cx26 proteins in tumors of endometrial carcinogenesis shows more aggressive behavior.[24] To date, the expression of Cx26 and its potential prognostic effect on LUAD has not been investigated. Our results demonstrated that the expression of Cx26 in LUAD is related to advanced clinical-pathologic factors (clinical-stage, T-classification and N-classification), which provided strong evidence that Cx26 could participate in tumor migration and invasion. Survival analysis indicated that LUAD patients with high-Cx26 expression showed poorer prognosis than low-Cx26 expression (P < .01). Univariate analysis utilizing logistic regression uncovered that Cx26 expression, a clear-cut ward variable, is linked to poor prognostic clinicopathologic factors. Next, we used univariate and multivariate analysis to evaluate the impact of Cx26 expression in LUAD patients. The results suggested that Cx26 remained freely connected with OS, which proved that Cx26 could act as a potential prognostic biomarker and therapeutic target in LUAD. However, further research is necessary to establish a conclusive observation.
We also compared GSEA results between low and high Cx26 expression data sets to recognize differentially expressed signaling pathways in LUAD. The results showed that cytosolic DNA sensing pathways, apoptosis, cytokine-cytokine receptor interaction, natural killer cell-mediated cytotoxicity, regulation of actin cytoskeleton, toll-like receptor signaling pathway, small cell lung cancer were differentially enriched in Cx26 high expression phenotype. Furthermore, Cx26 could also be related to cell function, such as apoptosis, cytokine; as well as immune function and cancer development.[25] Protective immune sentries’ toll-like receptors (TLRs) are reported to induce the secretion of inflammatory cytokines, which allowed lymphocytes to mount an adaptive, activated antigen-specific immune response that eliminated the invading microbes.[26] Additionally, signals produced by TLRs are reported to transduce through MAP kinases and NF-κB signaling that recruited co-stimulatory molecules and pro-inflammatory cytokines, which eventually lead to inflammatory responses and severe systemic disorders such as autoimmune disorders and tumor growth.[27] Natural killer (NK) cells are cytokine-secreting and cytotoxic cells that can mediate potent anti-tumor activity.[28] Therefore, Cx26 modifications can also engage with the center hub of post-transcriptional regulation and immune infiltrates, which are firmly related to protein translation. Some studies attempted to investigate the role of Cx26 in lung cancer.[11,29] Shimizu et al first reported the abnormal methylation of the Cx26 gene in rats, which contributed to the development of LUAD-induced by N-nitrosobis (2-hydroxypropyl) amine (BHP).[30] Likewise, Chen et al found that the phenotypic transitions were consistent with the upregulation of Cx26 in small cell lung cancer (SCLC) in rats.[31] However, the role of Cx26 in human LUAD prognostic remains to be elucidated. So, we assessed the mRNA expression of Cx26 in LUAD and found that Cx26 played a critical role in LUAD pathogenesis. Our study confirmed the significantly high levels of Cx26 expression in LUAD patients with several observed clinical features. However, our research had limitations: Firstly, the clinical samples were not extensively verified. Secondly, precise clinical data were not available due to relatively few turnovers of LUAD patients because of which, we have to conduct follow-up research.
Author contributions
WZH and TY designed and analyzed the research study; WZH ZYJ and TY wrote and revised the manuscript, WZH collected the data and all authors contributed to and approved the final version of manuscript.
Investigation: Youjing Zhang.
Methodology: Youjing Zhang.
Resources: Zenghong Wu.
Supervision: Zenghong Wu.
Validation: Zenghong Wu.
Footnotes
Abbreviations: GJB2 = gap junction protein beta 2, GSEA = gene set enrichment analysis, LUAD = lung adenocarcinoma, NES = normalized enrichment score.
How to cite this article: Tang Y, Zhang Yj, Wu Zh. High GJB2 mRNA expression and its prognostic significance in lung adenocarcinoma: a study based on the TCGA database. Medicine. 2020;99:14(e19054).
YT and YJZ contributed equally to this study.
The authors report no conflicts of interest.
References
- [1].Youlden DR, Cramb SM, Baade PD. The International Epidemiology of Lung Cancer: geographical distribution and secular trends. J Thorac Oncol 2008;3:819–31. [DOI] [PubMed] [Google Scholar]
- [2].Tan WL, Jain A, Takano A, et al. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol 2016;17:e347–62. [DOI] [PubMed] [Google Scholar]
- [3].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [4].She J, Yang P, Hong Q, et al. Lung cancer in China: challenges and interventions. Chest 2013;143:1117–26. [DOI] [PubMed] [Google Scholar]
- [5].Zheng M. Classification and pathology of lung cancer. Surg Oncol Clin N Am 2016;25:447–68. [DOI] [PubMed] [Google Scholar]
- [6].Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [7].Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aris VM, Cody MJ, Cheng J, et al. Noise filtering and nonparametric analysis of microarray data underscores discriminating markers of oral, prostate, lung, ovarian and breast cancer. Bmc Bioinformatics 2004;5:185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kelsell DP, Dunlop J, Stevens HP, et al. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 1997;387:80–3. [DOI] [PubMed] [Google Scholar]
- [10].Loewenstein WR, Kanno Y. Intercellular communication and the control of tissuegrowth: lack of communication between cancer cells. Nature 1966;9:1248–9. [DOI] [PubMed] [Google Scholar]
- [11].Yang J, Qin G, Luo M, et al. Reciprocal positive regulation between Cx26 and PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC cells via GJIC-independent induction of EMT. Cell Death Dis 2015;6:e1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zeng SG, Lin X, Liu JC, et al. Hypoxia-induced internalization of connexin 26 and connexin 43 in pulmonary epithelial cells is involved in the occurrence of non-small cell lung cancer via the P53/MDM2 signaling pathway. Int J Oncol 2019;56:642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ezumi K, Yamamoto H, Murata K, et al. Aberrant expression of connexin 26 is associated with lung metastasis of colorectal cancer. Clin Cancer Res 2008;14:677–84. [DOI] [PubMed] [Google Scholar]
- [14].Nomura S, Maeda K, Noda E, et al. Clinical significance of the expression of connexin26 in colorectal cancer. J Exp Clin Cancer Res 2010;29:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Knosel T, Emde A, Schluns K, et al. Immunoprofiles of 11 biomarkers using tissue microarrays identify prognostic subgroups in colorectal cancer. Neoplasia 2005;7:741–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thiagarajan PS, Sinyuk M, Turaga SM, et al. Cx26 drives self-renewal in triple-negative breast cancer via interaction with NANOG and focal adhesion kinase. Nature Commun 2018;9:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang Y, Liu J, Ying X, et al. Twist-mediated epithelialmesenchymal transition promotes breast tumor cell invasion via inhibition of hippo pathway. Sci Rep The Author(s) 2016;6:24606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Duggan MA, Anderson WF, Altekruse S, et al. The surveillance, epidemiology, and end results (SEER) program and pathology: toward strengthening the critical relationship. Am J Surg Pathol 2016;40:e94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Riggi N, Aguet M, Stamenkovic I. Cancer metastasis: a reappraisal of its underlying mechanisms and their relevance to treatment. Annu Rev Pathol 2018;13:117–40. [DOI] [PubMed] [Google Scholar]
- [20].Naoi Y, Miyoshi Y, Taguchi T, et al. Connexin26 expression is associated with aggressive phenotype in human papillary and follicular thyroid cancers. Cancer Lett 2008;262:248–56. [DOI] [PubMed] [Google Scholar]
- [21].Inose T, Kato H, Kimura H, et al. Correlation between connexin 26 expression and poor prognosis of esophageal squamous cell carcinoma. Ann Surg Oncol 2009;16:1704–10. [DOI] [PubMed] [Google Scholar]
- [22].Stoletov K, Strnadel J, Zardouzian E, et al. Role of connexins in metastatic breast cancer and melanoma brain colonization. J Cell Sci 2013;126:904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nobuko I, Yohei Y, Yohei K, et al. Intrinsic oncogenic function of intracellular connexin26 protein in head and neck squamous cell carcinoma cells. Int J Mol Sci 2018;19:2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kiszner G, Balla P, Wichmann B, et al. Exploring differential connexin expression across melanocytic tumor progression involving the tumor microenvironment. Cancers (Basel) 2019;11:pii: E165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sulkowska U, Febp A, Sulkowski S. Association of STAT3 with Cx26 and Cx43 in human uterine endometrioid adenocarcinoma. Oncol Lett 2016;11:4134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 2010;11:373–84. [DOI] [PubMed] [Google Scholar]
- [27].Vidya MK, Kumar VG, Sejian V, et al. Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals. Int Rev Immunol 2017;1–7. [DOI] [PubMed] [Google Scholar]
- [28].Barrow AD, Colonna M. Tailoring Natural Killer cell immunotherapy to the tumour microenvironment. Semin Immunol 2017;30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aasen T, Sansano I, Montero MÁ, et al. Insight into the role and regulation of gap junction genes in lung cancer and identification of nuclear Cx43 as a putative biomarker of poor prognosis. Cancers (Basel) 2019;11:pii: E320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shimizu K, Shimoichi Y, Hinotsume D, et al. Reduced expression of the Connexin26 gene and its aberrant DNA methylation in rat lung adenocarcinomas induced by N-nitrosobis (2-Hydroxypropyl) amine. Mol Carcinog 2006;45:710–4. [DOI] [PubMed] [Google Scholar]
- [31].Chen Y, Pacyna-Gengelbach M, Deutschmann N, et al. 5-Bromodeoxyuridine induced differentiation of a human small cell lung cancer cell line is associated with alteration of gene expression. Biochem Biophys Res Commun 2007;353:559–64. [DOI] [PubMed] [Google Scholar]


