Abstract
Intestinal mucositis is an important problem in the patients receiving cancer treatment. We aimed to investigate the effect of anakinra, which is a well known anti-oxidant and anti-inflammatory agent, on methotrexate-induced small intestine mucositis in rats. Forty rats were divided into 4 groups with 10 in each group. The healthy group (HG) and the methotrexate group (MTXG) were given distilled water, while the methotrexate + anakinra 50 (MTX+ANA50) and the methotrexate + anakinra 100 (MTX+ANA100) groups were intraperitoneally administered 50 and 100 mg/kg of anakinra. After one hour, the MTXG, MTX+ANA50 and MTX+ANA100 groups were given oral methotrexate at a dose of 5 mg/kg. This procedure was repeated once a day for 7 days. After the rats had been sacrificed, the small intestine tissue of rats were removed for the assesment of biochemical markers, histopathological evaluation and gene expression analyze. Statistical analyses of the data were performed using one-way ANOVA. Malondialdehyde (MDA), myeloperoxidase (MPO) and interleukin-6 (IL-6) levels were significantly higher, whereas total glutathione (tGSH) levels were significantly lower in MTXG (P<0.001) compared to other groups. MTX also increased IL-1β and TNF-α gene expression levels in MTXG (P<0.001). Inflammatory cell infiltration and damage to the villus were observed histopathologically in the MTXG group, whereas only mild inflammation was seen in the MTX+ANA100 group. A dose of 100 mg/kg of anakinra prevented the increase of the biochemical markers and gene expression levels better than a dose of 50 mg/kg. Intestinal mucositis caused by MTX may be preventible by co-administered anakinra.
Keywords: anakinra, mucositis, oxidative stress, rat
Introduction
Methotrexate (MTX), a folic acid antimetabolite, is used as a chemotherapeutic agent in the treatment of various cancer types. It is also used to treat inflammatory diseases such as psoriasis, systemic lupus erythematosus and rheumatoid arthritis [1]. MTX is used at high doses for the treatment of malignancies [5]. One of the most common adverse effect of high dose treatment is small intestinal mucositis. It has been reported that intestinal mucositis is seen in 40% of patients receiving chemotherapy at standart doses, while this rate is as almost 100% at high doses [12, 14, 21]. Elevation in the oxidant parameters and reduction in the antioxidant parameters have been reported in patients receiving high doses of chemotherapy [18, 21].
Oxidative stress is one of the main mechanisms responsible for the intestinal mucositis due to MTX. Oxidants such as myeloperoxidase (MPO) which is the marker of inflammatory response and malondialdehyde (MDA) which is the product of lipid peroxidation are increased and antioxidants such as glutathione (tGSH) are decreased in MTX induced mucositis [2]. It is also shown that MTX increases the production of proinflammatory cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in the small intestine tissue of rats [19]. In the light of this knowledge, antioxidants and IL-1β antagonists may be beneficial in the prevention of MTX mucositis.
Anakinra, a recombinant human IL-1 receptor antagonist, is the first biological agent which has been demonstrated to block pro-inflammatory effects in patients with rheumatoid arthritis [6]. It has been reported that anakinra protects brain tissue from oxidative damage after trauma by decreasing the oxidative parameters such as IL-1β [10]. Anakinra also protects the ovarian tissue from ischemia-reperfusion injury by anti-inflammatory and antioxidant activity [15]. Therefore, we hypothesized that anakinra may be protective against MTX induced mucositis according to these results.
In conclusion, there has been no studies that evaluated the protective effect of anakinra against mucositis induced by MTX. We aimed to assess the impact of anakinra on duodenal, jejunal and ileal mucositis induced by MTX in rats by evaluating biochemical changes and analyzing gene expression.
Materials and Methods
Animals
Totally 40 male albino Wistar rats, each weighing between 235–245 g, supplied from the Ataturk University Medical Experiments Application and Research Center were used in the study. Four groups were created with ten in each group before the experiment and the rats were kept and fed in the laboratory at the 22°C. Animal experiments were performed in accordance with the National Guidelines for the Use and Care of Laboratory Animals and were approved by the local animal ethics committee of Ataturk University, Erzurum, Turkey (Ethics Committee No: 2015/184, dated November 27, 2015).
Chemical agents
The ketamine, anakinra (Kineret) and methotrexate used in the experiment were supplied from Pfizer (Istanbul, Turkey), Swedish Orphan Biovitrum AB (Stockholm, Sweden) and Med-Drug (Istanbul, Turkey), respectively.
Experimental procedure
Experimental animals were divided into four groups as healthy group (HG), methotrexate group (MTXG), methotrexate + anakinra 50 group (MTX+ANA50), and methotrexate + anakinra 100 group (MTX+ANA100). The MTX+ANA50 and MTX+ANA100 groups were intraperitoneally administered 50 and 100 mg/kg of anakinra, respectively. Distilled water was given to the MTXG and HG groups as a solvent in the same way. One hour after the administration of anakinra and distilled water, MTXG, MTX+ANA50 and MTX+ANA100 groups were given oral methotrexate at a dose of 5 mg/kg. These procedures were repeated once a day for 7 days. At the end of this period, all the rats were sacrificed with a high dose of ketamine hydrochloride anesthesia. Blood samples were taken for leukocyte and platelet count from the tails of all animals before sacrifice. Duodenal, jejunal and ileal tissues of the rats were removed to measure the MDA, MPO, tGSH, and interleukin-6 (IL-6) levels. Gene expression of IL-1β and TNF- α was also assessed.
Biochemical analyses
A sample of 25 mg of tissue was homogenized using a solution of 1.15% KCl (Merck, Darmstadt, Germany). The homogenates were centrifuged at 4,000 rpm for 30 min at 4°C and the supernatants were separated by decantation for the measurement of MDA.
Another 25 mg of tissue was washed with isotonic solution of sodium chloride (NaCl; IE Ulagay) for tGSH analysis. First, the NaCl solution was removed from the samples, then the final volume was brought up to 2 ml by adding a phosphate buffer solution [0.213 g of NaH2PO4∙2H2O, 1.563 g of Na2HPO4∙2H2O (Merck), 0.038 g of EDTA (Sigma-Aldrich) in 100 ml of distilled water at pH 7.4].
The tissues were homogenized in an icy environment and centrifuged at 1,000 rpm for 15 min at a temperature of 4°C. The supernatants were separated and used for the measurement of protein concentration according to the method described by Bradford [4].
MDA analysis: The MDA levels were determined spectrophotometrically at 532 nm according to the method described by Ohkawa et al. [17]. This method is based on spectrophotometric measurement of absorbance of the pink-colored complex which is formed by thiobarbituric acid and MDA at a high temperature (95°C). The 0.1 ml of supernatant separated for the measurement of GSH-Px and MDA was added to a solution containing 0.1 ml of 8.1% sodium dodecylsulfate, 1.5 ml of 20% acetic acid (Merck), 1.5 ml of 0.9% thiobarbituric acid (Sigma-Aldrich) and 0.3 ml of dH2O. The mixture was incubated at 95°C for 1 h. Upon cooling, 5 ml of n-butanol:pyridine (v/v, 15: 1; Merck) was added. The mixture was vortexed for 1 min and centrifuged for 30 min at 4,000 rpm. The absorbances of the 0.15 ml final solutions were measured by spectrophotometry at 532 nm. The standard curve was obtained by using 1,1,3,3-tetramethoxypropane (Sigma-Aldrich).
MPO analysis: For determination of MPO in the small intestine tissue homogenates, pH 6 potassium phosphate buffer containing 0.5% HDTMAB (0.5% hexadecyl-trimethyl ammonium bromide) was prepared. The mixture then was centrifuged at 10,000 rpm at 4°C for 15 min. Supernatant part was used for analyzing the sample. In determination of MPO enzyme activity, oxidation reaction with MPO mediated H2O2 which included 4-amino antipyrine phenol solution as substrate was used.
tGSH analysis: tGSH was measured according to the method described by Sedlak and Lindsay [20]. In this method, sulfhydryl groups of GSH form a yellow-color TNB (5-thio-2-nitrobenzoic acid) following a chemical reaction with DTNB (5,5’-dithiobis[2-nitrobenzoic acid]). The intensity of this color is measured spectrophotometrically at 412 nm. For measurement, a cocktail solution (5.85 ml 100 mM Na phosphate buffer, 2.8 ml 1 mM DTNB, 3.75 ml 1 mM NADPH and 80 µl 625 U/l glutathione reductase; Sigma-Aldrich) was prepared. Before measurement, 0.1 ml of meta-phosphoric acid (Sigma-Aldrich) was added to 0.1 ml of homogenate to precipitate the proteins, followed by centrifugation at 2,000 rpm for 2 min to remove the precipitated proteins. After this, the 0.15 ml cocktail solution was added to the 50 µl of supernatants described above. The results were compared with the standard curves obtained by using GSSG (Sigma-Aldrich).
IL-6 analysis: We measured weight of samples and cut all of them. After that, these samples were rapidly frozen with liquid nitrogen and homogenized by pestle and mortar. All of the samples were maintained at 2–8°C after melting. We added PBS (pH 7.4), 1/10 (w/v), after that vortex for 10 s, the mixture then was centrifuged 20 min at 10,000 rpm to collect the supernatants carefully. The levels of IL-6 (ng/L) were measured using according to a commercial kit supplied by Eastbiopharm Co., Ltd. ELISA kit, China.
Leukocyte and platelet count: Blood samples were preserved in ethylenediaminetetraacetic acid-containing tubes. Complete blood count analysis was made using Coulter Hematology Analyzer (Coulter Beckman HMX, Miami, FL, USA).
Gene expression of IL-1β and TNF-α
RNA isolation: The Roche Magna Pure Compact LC device (Roche, Mannheim, Germany) was used for isolation of RNA from the homogenized intestinal tissue samples. We assessed the quantity and quality of the isolated RNA by nucleic acid measurement device (Maestro, Nano). The 50-µl RNA samples were stored at −80°C.
cDNA synthesis: cDNA was synthesized from the isolated RNA samples using the Transcriptor First Strand cDNA Synthesis Kit (Roche). For each subject, 1 µl of ddH2O, 10 µl of RNA and 2 µl of random primer were combined and incubated in a thermal cycler for 10 min at 65°C. After incubation, 4 µl of reaction buffer, 0.5 µl of RNAase, 2 µl of deoxynucleotide mix and 0.5 µl of reverse transcriptase were added. The reactions were incubated for 10 min at 25°C, 30 min at 55°C and 5 min at 85°C, and then held at 4°C.
Quantitative gene expression evaluation by real-time polymerase chain reaction: For each cDNA sample, gene expression of IL-1β and TNF-α, and the reference gene (G6pd) was analyzed using the Roche LightCycler 480 II Real-Time PCR instrument. PCRs were recorded in a final volume of 20 µl: 5 µl of cDNA, 3 µl of distilled water, 10 µl of LightCycler 480 Probes Master (Roche) and 2 µl primer-probe set (Real-Time Ready single assay, Roche). The cycle conditions of the relative quantitative PCR were preincubation at 95°C for 10 min followed by 45 amplification cycles of 95°C for 10 s, 6°C for 30 s, 72°C for 1 s, followed by cooling at 40°C for 30 s. LightCycler 480 Software, version 1.5 (Roche) was used for quantitative PCR analysis and calculation of quantification cycle (Cq) values for relative quantification. Relative quantitative amounts were calculated as the ratio of the target genes to the expression level of the reference gene. We used the reference gene for normalization of target gene expression.
Histopathological examination
All of the tissue samples were first fixated in a 10% formaldehyde solution for light microscope assessment. Following the fixation process, tissue samples were washed under tap water in cassettes for 24 h. Samples were then treated with conventional grade of alcohol (70%, 80%, 90%, and 100%) to remove the water within tissues. Tissues were then passed through xylol and embedded in paraffin. Four-to-five micron sections were cut from the paraffin blocks and hematoxylin–eosin staining was administered. Their photos were taken following the Olympus DP2-SAL firmware program (Olympus Inc., Tokyo, Japan) assessment. Histopathological assessment was carried out by the pathologist blind for the study groups.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences for Windows version 19.0 (IBM Corp. Released 2010. IBM SPSS Statistics for Windows, Version 19.0. IBM Corp. Armonk, NY, USA). Descriptive statistics for each variable were determined. The results for continuous variables were recorded as the mean ± SD. The significance of differences between the groups was determined using the one-way analysis of variance (ANOVA) test followed by Tukey’s analysis. A P value <0.05 was considered significant.
Results
Biochemical results
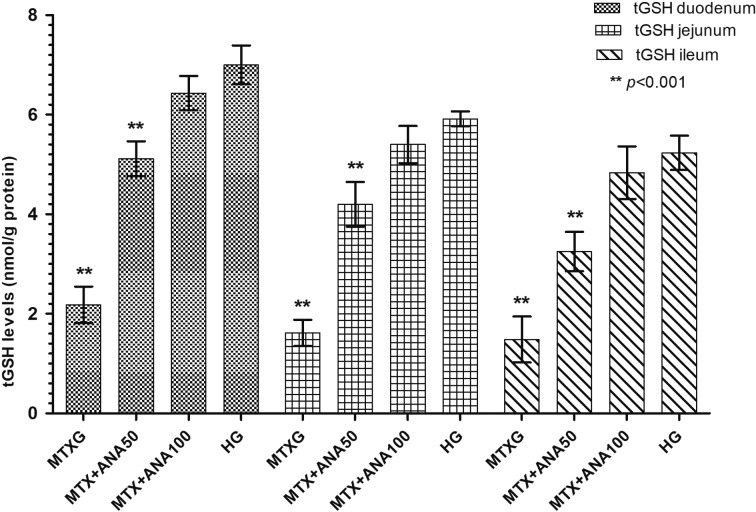
As shown in Fig. 1, MTX significantly increased the amount of MDA in the rat duodenum, jejunum and ileum tissue compared to the HG (P<0.001). Anakinra at doses of 50 and 100 mg/kg also significantly inhibited the increase of MDA by MTX in the small intestine tissue (P<0.001). There was a significant difference in the MDA levels between the MTX+ANA50 and HG, while the levels of MDA were almost the same between MTX+ANA100 and HG. MPO activity was also increased in the group administered MTX, anakinra at doses of 50 and 100 mg/kg significantly inhibited the increase of MPO by MTX in the small intestine tissue (P<0.001; Fig. 2). MTX significantly decreased the levels of tGSH in the duodenal, jejunal and ileal tissue compared to the MTX+ANA50, MTX+ANA100 and HG (P<0.001; Fig. 3). The levels of tGSH decreased in the MTX+ANA50 group. However, levels of tGSH were not affected by MTX in the intestinal tissue of the MTX+ANA100 group (Fig. 3). Anakinra significantly inhibited the decrease of tGSH levels by MTX in the duodenal, jejunal and ileal tissue (P<0.001).
Fig. 1.
The effects of anakinra on MDA levels in the duodenum, jejunum and ileum tissues of rats given methotrexate. Bars are mean ± SD. The healthy group is compared with the MTXG, MTX+ANA50 and MTX+ANA100 groups (The data derived from a single experiment). MDA: malondialdehyde; HG: healthy group; MTXG: methotrexate group; MTX+ANA50: methotrexate + anakinra 50 group; MTX+ANA100: methotrexate + anakinra 100 group.
Fig. 2.
The effects of anakinra on MPO levels in the duodenum, jejunum and ileum tissues of rats given methotrexate (Performed in the second experiment). Bars are mean ± SD. The healthy group is compared with the MTXG, MTX+ANA50 and MTX+ANA100 groups. MPO: myeloperoxidase; HG: healthy group; MTXG: methotrexate group; MTX+ANA50: methotrexate + anakinra 50 group; MTX+ANA100: methotrexate + anakinra 100 group.
Fig. 3.
The effects of anakinra on tGSH levels in the duodenum, jejunum and ileum tissues of rats given methotrexate. Bars are mean ± SD. The healthy group is compared with the MTXG, MTX+ANA50 and MTX+ANA100 groups (The data derived from a single experiment). tGSH: total glutathione; HG: healthy group; MTXG: methotrexate group; MTX+ANA50: methotrexate + anakinra 50 group; MTX+ANA100: methotrexate + anakinra 100 group.
IL-6 levels were found to be higher in the small intestine tissue of the rats that received MTX, compared to other groups (P<0.001; Fig. 4). IL-6 levels decreased significantly in the groups administered anakinra (P<0.001), and reached to the levels similar to those in the HG (P>0.05).
Fig. 4.
The effects of anakinra on IL-6 levels in the duodenum, jejunum and ileum tissues of rats given methotrexate (Performed in the second experiment). Bars are mean ± SD. The healthy group is compared with the MTXG, MTX+ANA50 and MTX+ANA100 groups. IL-6: interleukin-6; HG: healthy group; MTXG: methotrexate group; MTX+ANA50: methotrexate + anakinra 50 group; MTX+ANA100: methotrexate + anakinra 100 group.
Leukocyte counts were respectively found as 6.47 ± 1.35 × 103 /µL, 3.71 ± 0.6 × 103 /µL, 4.48 ± 0.96 × 103 /µL and 7.17 ± 0.24 × 103 /µL in the HG, MTXG, MTX+ANA50 and MTX+ANA100 groups. MTX significantly decreased leukocyte count compared to HG (P<0.05). However, there was no significant difference in terms of leukocyte count between HG and the MTX+ANA50 (P>0.05) and MTX+ANA100 groups (P>0.05).
Platelet counts were respectively found as 719 ± 69 × 103 /µL, 610 ± 183 × 103 /µL, 992 ± 81 × 103 /µL and 993 ± 142 × 103 /µL in the HG, MTXG, MTX+ANA50 and MTX+ANA100 groups. We did not find statistically significant difference between the HG and other groups in terms of platelet count (P>0.05)
IL-1β and TNF-α gene expression levels were found to be higher in the duodenal, jejunal and ileal tissue of the rats that received MTX, compared to the MTX+ANA50, MTX+ANA100 and HG (P<0.001; Figs. 5 and 6). The differences between the MTX+ANA100 and HG in terms of these parameters were not statistically significant (P>0.05), but the differences between the MTX+ANA50 and HG were found to be highly significant (P<0.001). MTX caused a significant increase in the levels of IL-1β and TNF-α. Anakinra was better at preventing an increase in the levels of IL-1β and TNF-α in rats at a 100 mg/kg dose compared to a 50 mg/kg dose.
Fig. 5.
The effects of anakinra on IL-1β gene expression levels in the duodenum, jejunum and ileum tissues of rats given methotrexate. Bars are mean ± SD. The healthy group is compared with the MTXG, MTX+ANA50 and MTX+ANA100 groups (The data derived from a single experiment). IL-1β: interleukin-1β; HG: healthy group; MTXG: methotrexate group; MTX+ANA50: methotrexate + anakinra 50 group; MTX+ANA100: methotrexate + anakinra 100 group.
Fig. 6.
The effects of anakinra on TNF-α gene expression levels in the duodenum, jejunum and ileum tissues of rats given methotrexate (Performed in the second experiment). Bars are mean ± SD. The healthy group is compared with the MTXG, MTX+ANA50 and MTX+ANA100 groups. TNF-α: tumor necrosis factor-α; HG: healthy group; MTXG: methotrexate group; MTX+ANA50: methotrexate + anakinra 50 group; MTX+ANA100: methotrexate + anakinra 100 group.
Histopathological findings: Histological examination of duedenal tissue in HG revealed the sharp villi, long and normal crypts, normal lining epithelium with mucus-secretion cells, normal architecture of mucosa with Brunner glands and normal vasculature (Fig. 7a). In MTXG, villi appearance which is characteristic structure of small intestine were totally lost and mucosa contained flattened, vacuolated cells. Lamina propria was infiltrated by mononuclear and polymorphonuclear cells. Both mucosal and muscularis layers were edematous and also various vacuolated bodies were detected in mucosa (Fig. 7b). Blood vessels were dilated and highly congested. Necrotic areas were noticeable in some areas of mucosa and inflammatory cell infiltration was observed as scattered throughout the full-thickness of the tissue (Fig. 7c). In MTX+ANA50 group, we observed blunted villi and degenerated epithelial cells, in some areas crypt architecture and Brunner glands were preserved. Inflammatory cell infiltration and muscularis layer edematous appearance were still intense and blood vessels were congested (Fig. 7d). In MTX+ANA100 group, the structure of villi and crypt formation were normal, epithelial cells and lamina propria were well organized. Brunner glands were normal in terms of location and density as in control samples. Occasional congested blood vessels attracted attention. In addition, edema decreased in muscularis layer (Fig. 7e).
Fig. 7.
Sections of the duodenum (a–e). (a) normal duodenal tissue of the control group, EP: epithelium, LP: lamina propria, GL: Brunner glands, ↔: villi, ▶: crypt, MS: muscularis layer, ★: blood vessel; (b) ➜: vacuolated bodies, ✸: inflammatory cell infiltration, MS: edematous muscularis layer, ★: dilated and congested blood vessel of the MTXG; (c) ➢: necrotic areas, ✸: inflammatory cell infiltration, MS: edematous muscularis layer, ★: dilated and congested blood vessel of the MTXG; (d) EP: degenerated epithelium, LP: lamina propria, GL: occasional Brunner glands, ↔: blunted villi, ▶: crypt, MS: edematous muscularis layer, ★: congested blood vessel of the MTX+ANA 50 group; (e) EP: epithelium, LP: lamina propria, GL: Brunner glands, ↔: villi, ▶: crypt, MS: mild edema in muscularis layer, ★: congested blood vessel of the MTX+ANA 100 group. HE. Original magnification. a,b,c,d,e ×200. (Performed in the second experiment).
When the samples of the HG jejunal tissue were examined, it was observed that the epithelial line, lamina propria and muscularis layers were in normal histological structure. Villus structures and crypt depths were also normal (Fig. 8a). In MTXG, we detected a widespread degeneration followed along the entire jejunal tissue wall. Epithelial tissue was replaced by necrotic tissue and inflammatory cell infiltration throughout the mucosal layer was intense. In some rare areas, crypts were still existed. Profound dilatation and congestion were observed in blood vessels. In other respects, muscularis layer were edematous and there were vacuolated bodies scattered the layer (Figs. 8b and c). In MTX+ANA50 group, the presence of short and irregular villi was observed, although epithelial borders were preserved. There was a less intense inflammatory cell infiltration compared to the metotrexate group. We detected mild edematous appearance in muscularis layer (Fig. 8d). In MTX+ANA100 group, a microscopic examination showed, still blunted but more organized villi, smooth crypts, an intact epithelial layer and a near-normal lamina propria. Muscularis layer was normal as well (Fig. 8e).
Fig. 8.
Sections of the jejunum (a–e). (a) normal jejunal tissue of the control group, EP: epithelium, LP: lamina propria, ↔: villi, ▶: crypt, MS: muscularis layer, ★: blood vessel; (b) ➢: necrotic areas, ➜: vacuolated bodies, ✸: inflammatory cell infiltration, MS: highly edematous muscularis layer, ★: dilated and congested blood vessel of the MTXG; (c) ➢: intense necrotic areas, ✸: inflammatory cell infiltration, ▶: crypt, MS: highly edematous muscularis layer of the MTXG; (d) EP: epithelium, LP: lamina propria, ↔: short and irregular villi, ▶: mostly blunted crypt, MS: mild edema in muscularis layer of the MTX+ANA 50 group; (e) EP: epithelium, LP: lamina propria, ↔: villi, ▶: smooth crypt, MS: muscularis layer of the MTX+ANA 100 group. HE. Original magnification. a,b,c,d,e ×200. (Performed in the second experiment).
In rats ileum tissues in HG showed, sharp villi, straight crypts, intact lining epithelium, normal vasculature and Peyer patches localized in mucosa (Fig. 9a). In MTXG, full-thickness degeneration attracted the attention. Mucosa exhibited villus blunting with flattened and vacuolated cells andcrypts were necrotic associated with intense inflammatory cell infiltration. There were sizeable vacuolated bodies in mucosa. Blood vessels were dilated and congested. Muscularis layer were highly edematous (Fig. 9b,c). In MTX+ANA50 group, we observed mostly protected crypt structures, mainly organized Peyer patches, a near-normal muscularis layer. But at the same time, occasional irregularities were observed in the epithelial layer and there was marked inflammatory cell infiltration in the lamina propria. Also blood vessels were dilated and congested (Fig. 9d). In MTX+ANA100 group, ileum tissue samples showed well preserved and more continuous epithelial layer, which was almost normal crypt structure with long villi. Inflammatory cell infiltration was mild in lamina propria when compared to the MTXG and MTX+ANA50 group. Peyer patches, muscularis layer and blood vessels were normal and well organized (Fig. 9e).
Fig. 9.
Sections of the ileum (a–e). (a) normal ileum tissue of the control group, EP: epithelium, LP: lamina propria, PP: Peyer patches, MS: muscularis layer, ★: blood vessel; (b) ➢: necrotic areas, ➜: sizeable vacuolated bodies, ✸: intense inflammatory cell infiltration, MS: highly edematous muscularis layer, ★: dilated and congested blood vessel of the MTXG; (c) ➢: necrotic areas, ➜: sizeable vacuolated bodies, ✸: intense inflammatory cell infiltration, MS: highly edematous muscularis layer, ★: dilated and congested blood vessel of the MTXG; (d) EP: irregular epithelium, LP: lamina propria, ✸: intense inflammatory cell infiltration, MS: muscularis layer, PP: Peyer patches, ★: dilated and congested blood vessel of the MTX+ANA 50 group; (e) EP: epithelium, LP: lamina propria, ✸: mild inflammatory cell infiltration, MS: muscularis layer, PP: Peyer patches of the MTX+ANA 100 group. HE. Original magnification. a,b,c ×200; d,e ×100. (Performed in the second experiment).
Discussion
This study investigated the protective effect of anakinra on small intestine mucositis induced by MTX in rats. Our biochemical results indicate that MTX increased the levels of MDA and MPO which are oxidant parameters and decreased the levels of tGSH which is an antioxidant parameter in the duodenal, jejunal and ileal tissues of rats. Serum levels of IL-6 were significantly lower in the groups administered anakinra, when compared to MTXG. We found also that MTX significantly increased the IL-1β and TNF-α gene expression levels in the small intestine tissues of rats. When we compared the rats treated with 100 mg/kg of anakinra with the rats given only MTX, decreased MDA, MPO and IL-6 levels and IL-1β and TNF-α gene expression levels but increased tGSH levels in the small intestine tissues of rats were shown in the anakinra group. MTX caused mixed inflammatory cell infiltration, damage to the villus and crypt structures and dilated congested blood vessels in the small intestine tissues of rats. Small intestinal damage due to MTX was improved in the histopathological examination of the anakinra given groups. Mild irregularities in the villus and crypt structures and a near-normal epithelial layer are seen in the small intestine tissues of the animals treated with anakinra. It was observed that histopathologically and biochemically, anakinra at a dose of 100 mg/kg better protects the small intestinal tissue against the oxidative damage of MTX, compared to a dose of 50 mg/kg. Reactive oxygen species are known to have a role in the oxidative stress caused by MTX [11]. Arslan et al. reported that MDA level which is the end product of lipid peroxidation was increased; tGSH, glutathione reductase, glutathione peroxidase, catalase and superoxide dismutase which are antioxidants were decreased in the small intestine mucositis caused by MTX [3]. MTX caused oxidative stress by increasing MDA levels and decreasing tGSH levels in the small intestine tissue and confirmed histopathological changes [2]. It has been also reported that MTX caused increased MDA and decreased tGSH levels in the other organs [13]. In the light of this knowledge, studies focus on antioxidants to reduce the side effects of MTX [8]. In our study, anakinra suppressed MTX-induced lipid peroxidation. It has been demonstrated that anakinra inhibits the increase of MDA and prevented the decrease of enzymatic antioxidants activities in experimentally induced spinal cord injury in animals [9].
In our study, we showed that proinflammatory IL-1β and TNF-α gene expression levels were also increased in the MTXG where the balance between oxidant antioxidant in the small intestine tissues changed in favor of oxidants. In a study by de Araújo et al., they reported that proinflammatory IL-1β was markedly increased in addition to oxidants in the damaged intestinal tissue [7].
Odabasoglu et al. reported that the amount of MDA increased and tGSH decreased in the inflamed tissue [16]. These studies showed that MTX causes both oxidative stress and inflammatory damage in the small intestine. It has been reported that anakinra inhibits the production of oxidant and proinflammatory IL-1β [13]. Nayki et al. reported that anakinra at a dose of 100 mg/kg inhibits the histopathological damage better than at a dose of 50 mg/kg and also makes better suppression of the oxidants production and IL-1β expression compared to at a dose of 50 mg/kg [15]. Our biochemical and histopathological results were consistent with the literature.
Our results showed that MTX changed the oxidant antioxidant balance in favor of oxidants and increased proinflammatory IL-1β and TNF-α gene expression levels in small intestine tissue. The gene expression and biochemical results of this study were fully consistent with the histopathological results. Mixed inflammatory cell infiltration, damaged villus and crypt structures and dilated congested blood vessels were observed in the small intestine tissue samples of MTXG, where we found high values of MDA, MPO and IL-6, but low values of tGSH. Anakinra inhibited the alteration of oxidant antioxidant balance against the oxidants and suppressed the increase of IL-1β and TNF-α gene expressions in the small intestine tissue due to MTX. Anakinra at a dose of 100 mg/kg better protects the small intestinal tissue against the oxidative damage of MTX, compared to a dose of 50 mg/kg. Anakinra prevents small intestine mucositis induced by MTX in a dose dependent manner.
In conclusion; anakinra can be used as a preventive measure in the MTX-induced intestinal injury by strong antioxidant and anti-inflammatory activity.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interests
The authors have no conflicts of interest.
References
- 1.Abolmaali S.S., Tamaddon A.M., Dinarvand R.2013. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother. Pharmacol. 71: 1115–1130. doi: 10.1007/s00280-012-2062-0 [DOI] [PubMed] [Google Scholar]
- 2.Arslan A., Ozcicek F., Keskin Cimen F., Altuner D., Yarali O., Kurt N., Tumkaya L., Ozturk C., Suleyman H.2015. Protective effect of resveratrol against methotrexate-induced oxidative stress in the small intestinal tissues of rats. Int. J. Clin. Exp. Med. 8: 10491–10500. [PMC free article] [PubMed] [Google Scholar]
- 3.Arslan A., Ozcicek A., Suleyman B., Coban T.A., Cimen F.K., Nalkiran H.S., Kuzucu M., Altuner D., Cetin N., Suleyman H.2016. Effects of nimesulide on the small intestine mucositis induced by methotrexate in rats. Exp. Anim. 65: 329–336. doi: 10.1538/expanim.15-0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M.M.1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. doi: 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- 5.Chabner B., Goodman K.B.2011. Gilman’s the pharmacological basis of therapeutics 12th ed., New York, McGraw-Hill. [Google Scholar]
- 6.Cvetkovic R.S., Keating G.2002. Anakinra. BioDrugs 16: 303–311, discussion 313–314. doi: 10.2165/00063030-200216040-00005 [DOI] [PubMed] [Google Scholar]
- 7.de Araújo A.A., Borba P.B., de Souza F.H., Nogueira A.C., Saldanha T.S., Araújo T.E., da Silva A.I., de Araújo Júnior R.F.2015. In a methotrexate-induced model of intestinal mucositis, olmesartan reduced inflammation and induced enteropathy characterized by severe diarrhea, weight loss, and reduced sucrose activity. Biol. Pharm. Bull. 38: 746–752. doi: 10.1248/bpb.b14-00847 [DOI] [PubMed] [Google Scholar]
- 8.Gulgun M., Erdem O., Oztas E., Kesik V., Balamtekin N., Vurucu S., Kul M., Kismet E., Koseoglu V.2010. Proanthocyanidin prevents methotrexate-induced intestinal damage and oxidative stress. Exp. Toxicol. Pathol. 62: 109–115. doi: 10.1016/j.etp.2009.02.120 [DOI] [PubMed] [Google Scholar]
- 9.Hasturk A.E., Yilmaz E.R., Turkoglu E., Arikan M., Togral G., Hayirli N., Erguder B.I., Evirgen O.2015. Potential neuroprotective effect of Anakinra in spinal cord injury in an in vivo experimental animal model. Neurosciences (Riyadh) 20: 124–130. doi: 10.17712/nsj.2015.2.20140483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasturk A.E., Yilmaz E.R., Turkoglu E., Kertmen H., Horasanli B., Hayirli N., Erguder I.B., Evirgen O.2015. Therapeutic evaluation of interleukin 1-beta antagonist Anakinra against traumatic brain injury in rats. Ulus. Travma Acil Cerrahi Derg. 21: 1–8. doi: 10.5505/tjtes.2015.57894 [DOI] [PubMed] [Google Scholar]
- 11.Huang C., Hsu P., Hung Y., Liao Y., Liu C., Hour C., Kao M., Tsay G.J., Hung H., Liu G.Y.2005. Ornithine decarboxylase prevents methotrexate-induced apoptosis by reducing intracellular reactive oxygen species production. Apoptosis 10: 895–907. doi: 10.1007/s10495-005-2947-z [DOI] [PubMed] [Google Scholar]
- 12.Keefe D.M., Cummins A.G., Dale B.M., Kotasek D., Robb T.A., Sage R.E.1997. Effect of high-dose chemotherapy on intestinal permeability in humans. Clin. Sci. (Lond.) 92: 385–389. doi: 10.1042/cs0920385 [DOI] [PubMed] [Google Scholar]
- 13.Kuyrukluyıldız U., Küpeli İ., Bedir Z., Özmen Ö., Onk D., Süleyman B., Mammadov R., Süleyman H.2016. The Effect of Anakinra on Paclitaxel-Induced Peripheral Neuropathic Pain in Rats. Turk. J. Anaesthesiol. Reanim. 44: 287–294. doi: 10.5152/TJAR.2016.02212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin J.Q.2002. Effect of nutrition intervention on antioxidant capacity and lipid peroxide in patients with bone marrow transplantation. J. First Mil. Med. Univ. 22: 530–532. [PubMed] [Google Scholar]
- 15.Nayki U.A., Nayki C., Cetin N., Cimen F.K., Coban A., Mammadov R., Tas I.H., Malkoc I.2016. Effect of Kineret® on ovarian ischemia reperfusion injury in a rat model. J. Obstet. Gynaecol. Res. 42: 1525–1533. doi: 10.1111/jog.13095 [DOI] [PubMed] [Google Scholar]
- 16.Odabasoglu F., Halici Z., Aygun H., Halici M., Atalay F., Cakir A., Cadirci E., Bayir Y., Suleyman H.2011. α-Lipoic acid has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced acute and cotton pellet-induced chronic inflammations. Br. J. Nutr. 105: 31–43. doi: 10.1017/S0007114510003107 [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H., Ohishi N., Yagi K.1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95: 351–358. doi: 10.1016/0003-2697(79)90738-3 [DOI] [PubMed] [Google Scholar]
- 18.Onk D., Mammadov R., Suleyman B., Cimen F.K., Cankaya M., Gul V., Altuner D., Senol O., Kadioglu Y., Malkoc I., Suleyman H.2018. The effect of thiamine and its metabolites on peripheral neuropathic pain induced by cisplatin in rats. Exp. Anim. 67: 259–269. doi: 10.1538/expanim.17-0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozcicek A., Cetin N., Keskin Cimen F., Tumkaya L., Malkoc I., Gulaboglu M., Yarali O., Suleyman B.2016. The Impact of Resveratrol on Oxidative Stress Induced by Methotrexate in Rat Ileum Tissue: Evaluation of Biochemical and Histopathological Features and Analysis of Gene Expression. Med. Princ. Pract. 25: 181–186. doi: 10.1159/000442020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedlak J., Lindsay R.H.1968. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 25: 192–205. doi: 10.1016/0003-2697(68)90092-4 [DOI] [PubMed] [Google Scholar]
- 21.Weijl N.I., Cleton F.J., Osanto S.1997. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat. Rev. 23: 209–240. doi: 10.1016/S0305-7372(97)90012-8 [DOI] [PubMed] [Google Scholar]