Abstract
Triclosan (TCS) is a broad-spectrum antibacterial and anti-fungal agent used in a broad variety of personal care products (PCPs) throughout the world. However, the molecular mechanism of TCS’s effects on the gill and ovary of fish is not clear. In this study, the effects of TCS exposure on expression of antioxidant- and apoptosis-related genes were investigated in the gill and ovary of zebrafish (Danio rerio). Zebrafish were exposed to 0, 17, 34, or 68 µg/l TCS for 42 days. Antioxidant-related genes (SOD, GPx1a, CAT, sMT-B, and MT-2) in the gill were significantly downregulated in the 34 (except GPx1a) and 68 µg/l TCS groups, and these genes (except MT-2) in the ovary were significantly downregulated in the 68 µg/l TCS group. Apoptosis-related gene (Bax and p53) expression level in the gill were significantly downregulated in the 68 µg/l TCS group, while the ratios of BCL-2 to Bax and MDM2 gene were significantly upregulated. The Bax gene in the ovary was significantly upregulated in the 34 and 68 µg/l TCS groups, while the ratio of BCL-2 to Bax was significantly downregulated. Moreover, the p53 gene in the ovary in the 34 µg/l TCS group was significantly upregulated. In addition, the MDA contents in the gill in the 34 and 68 μg/l TCS treated groups and in the ovary in 68 μg/l group were significantly increased. The results showed that the higher dose of TCS might cause oxidative damage in the gills and ovaries and accelerate ROS-dependent ovary apoptosis in zebrafish.
Keywords: antioxidation, apoptosis, gill, ovary, triclosan
Introduction
Triclosan (TCS) is an efficient, lipophilic synthetic broad-spectrum antibacterial and anti-fungal agent used in a broad variety of personal care products (PCPs), including toothpaste, soaps, shampoos, and other sanitation goods [9, 40]. Currently, widespread pollution with TCS has been detected in aquatic ecosystems of many countries [9]. TCS has been found in sewage treatment plant effluent in quantities up to micromolar (μg/l) concentrations in North America, Europe, and Asia [24, 25, 31, 32, 47]. Although most TCS is removed during sewage treatment, a survey of over 100 streams in the United States (USA) indicated that TCS was present in 57.6% of sites examined, with the maximum concentration being 2.3 μg/l [24]. Similar levels of TCS are also found in many rivers and lakes of Europe, Oceania, and Asia [9]. TCS or its derivative, methyl-triclosan, may bioaccumulate in animal tissues, and it has also been found in the urine and breast milk of people [16]. Therefore, TCS is considered to be the priority control pollutant by the European Union, USA, and China and to be a carcinogen by the International Agency for Research on Cancer (IARC) [41, 46].
TCS has effects on nontarget organisms. It is reported that exposure to TCS in the environment causes biological genotoxicity, developmental and reproductive toxicity, hepatotoxicity, endocrine toxicity, immunotoxicity, neurotoxicity, cardiotoxicity, and carcinogenesis effects [49]. Results obtained in the freshwater mussel Dreissena polymorpha by using in vitro and in vivo experiments offered evidence that TCS caused oxidative stress, which was possibly one of the main toxicity mechanisms of TCS [4, 5, 36].
Oxidative stress has become an important theme in aquatic toxicology in recent years, and more and more attention has been paid to the mechanism of oxidative damage and cellular response in biological systems [22]. Reactive oxygen species (ROS), such as superoxide anion radicals (O2•¯), hydrogen peroxide (H2O2), and hydroxyl radicals (·OH), are continuously produced in oxygen-consuming organisms. Exposure to toxic chemical pollutants may lead to an imbalance between these endogenous and exogenous ROS and can subsequently cause a decrease in antioxidant defenses or lead to oxidative damage in organisms [42].
Vertebrates try to decrease the damage from oxidative stress using an antioxidant defense system which includes antioxidant molecules (such as glutathione[GSH] and vitamins C and E), antioxidant enzymes (such as superoxide dismutase [SOD], catalase [CAT], and glutathione peroxidase [GPx]), and other antioxidant proteins (such as, metallothionein-like protein). Recently, measurements of antioxidative enzyme activities in fish have been used to assess the oxidative damage caused by toxic chemical pollutants in aquatic ecosystems [7, 17, 20]. Correspondingly, several studies have shown that TCS can cause oxidative stress by increasing the level of ROS and products of oxidative damage (such as lipid peroxides) and by affecting antioxidant enzyme activities in some organisms [11, 35]. It is reported that TCS exposure could cause an increase in ROS content (%) and glutathione transferase (GST) enzymatic activity in the monogonont rotifer (Brachionus koreanus) [15]. More recently, our previous study also confirmed that TCS could increase the antioxidant enzyme of CAT and the GSH contents and that it even induced excessive ROS production and DNA damage in the liver of goldfish after 14 d of exposure. These findings suggest that sub-chronic TCS concentrations can lead to oxidative damage in goldfish (Carassius auratus) [43].
Anti-oxidants such as N-acetylcysteine (NAC) can hinder apoptosis [29], suggesting that excessive oxidative stress may induce cell apoptosis. Apoptosis can be regulated by specific proteins which produce pro-apoptotic and anti-apoptotic signals. Bcl-2 family proteins are anti-apoptotic proteins [48]. Bax can promote apoptosis of cells. Bcl-2 can interact with bax protein to regulate the onset apoptosis [19]. P53 can upregulate the expression of Bax and downregulate the expression of Bcl-2 to promote apoptosis [37]. It can also induce apoptosis through the death signal receptor protein pathway. MDM2 is p53’s negative regulator [10]. These findings show that the molecular mechanism of apoptosis is extremely complex.
The zebrafish (Danio rerio) is an in vivo experimental animal model. The gill is usually one of the main target organs of fish for toxic effects of toxic chemicals [50]. There are reports of reproductive health effects related to TCS [21], and our previous experiments confirmed that TCS had an interference effect on the reproductive endocrine system in female Yellow River carp (Cyprinus carpio) [44]. Gene transcription level changes would cause earlier detection and measurement of toxicant effects [12]. Therefore, in the present study, the effects of environmental-related concentrations of TCS exposure on expression of antioxidant- and apoptosis-related genes were investigated in gill and ovary of zebrafish. In addition, we also examined malondialdehyde (MDA), a marker of oxidative stress, in the gill and ovary. This information is intended to provide new insights into the toxicological mechanism of TCS.
Materials and Methods
Test chemicals
TCS (CAS: 3380-34-5; 99.8%) was purchased from Jiangsu Equalchem Co., Ltd (Jiangsu, China), and stock solutions were prepared by dissolving the powder in dimethyl sulfoxide (DMSO; Tianjin Kemiou Chemical Reagent Co., Ltd., Tianjin, China) at a concentration of 2,000 mg/l. The stock solutions were used to prepare TCS treatment solutions via dilution to the required concentrations in the present study. DMSO in the test solution was kept in 0.07%.
Experimental fish
All animal use was approved by School of Life Sciences, Luoyang Normal University Institutional Animal Care Committee and complied with the Institutional Guidelines for the Care and Use of Laboratory Animals. Wild-type (AB strain) zebrafish (Danio rerio) were obtained from China Zebrafish Resource Center (CZRC) at 7 weeks old. The mean body weights and lengths were 0.06 ± 0.01 g and 1.62± 0.31 cm. Zebrafish were maintained in 60-l glass tanks containing 30 l aerated tap water at 26 ± 2°C under a photoperiod 14:10 h light/dark in the Ecotoxicology Laboratory at Luoyang Normal University (Luoyang, China). The fish were fed twice a day with Artemia nauplii ad libitum. After feeding, the remaining food and feces were removed within 30 min. At the end of 2 weeks of acclimation, the zebrafish TCS exposure experiment began.
TCS exposure
Zebrafish were continuously exposed to 0 (control), 17, 34, or 68 µg/l TCS for 42 d. Experimental concentrations were chosen based on the LC50 (340 µg/l) at 96 h and the environmental concentration of TCS for zebrafish [34]. Two hundred and forty fish (twenty fish/tank, three replicates) were used for each control or TCS treatment group. The TCS test solution in each tank was replaced with half-fresh TCS test solution every day. The other conditions were in accordance with those during the acclimation period.
TCS concentrations in tested solutions were measured by high-performance liquid chromatography with ultraviolet detection. Detailed procedures for analysis of tank TCS concentrations were conducted as described in our previously established protocols [44]. Based on analysis of the test solutions, the TCS concentrations in the TCS-treated tanks during the 42-day exposure experiment were as follows: 13.5 ± 3.4, 28.3 ± 6.7, and 56.4 ± 9.2 µg/l, respectively. The TCS concentration in the control group was lower than the lower limit of detection.
The gills and ovaries excised from zebrafish in each exposure tank were divided into one sample of each was collected from three zebrafish in each experimental group, resulting in six samples for each experiment group. These samples were immediately snap-frozen in liquid nitrogen and then stored in an ultralow temperature refrigerator (−80°C) until use. No mortality was observed in any of the experimental groups during the whole exposure period.
Gene expression analysis
Total RNA was isolated from the pooled gills and ovaries of the zebrafish using RNAfast200 reagent (Fastagen Biotech, Shanghai, China) according to the manufacturer’s instructions. The ratio of A260 to A280 was checked by ultramicro spectrophotometer and 1% agarose gel electrophoresis and used to verify the quality of the total RNA.
Subsequently, the RNA was denatured at 65°C for 5 min. cDNA was synthesized using First Strand cDNA Synthesis Kit (Toyobo Co., Ltd., Osaka, Japan) according to the manufacturer’s protocol. Denatured RNA was used as the template and was reverse- transcribed to cDNA in a final reaction volume of 10.0 μl. Tt was then incubate at 37°C for 15 min and then 98°C for 5 min to inactivate the enzyme. The reversed cDNA was adjusted to the appropriate concentration and stored in a refrigerator.
Real-time quantitative PCR (RT-qPCR) was performed using a SYBR Green PCR kit (DBI® Bioscience, Ludwigshafen, Germany) and analyzed on an Bio-Rad CFX96 Touch Sequence Detector System. Specific primers were used to detect the gene expression of β-actin, SOD, GPx1a, CAT, sMT-B, MT-2, Bcl-2, Bax, p53, and MDM2, and the detailed information is shown for them in Table 1. RT-qPCR amplification was carried out in 25-µl reaction mixtures which included 10 µl Bestar SybrGreen qPCR mastermix, 1 µl first-strand cDNA (template), 0.5 µl PCR Forward Primer, and 0.5 µl PCR Reverse Primer. The reaction was performed at 95°C for 2 min followed by 40 cycles of 95°C for 10 s, 55°C for 30 s, and 72°C for 30 s. As an internal reference gene, β-actin transcript was used to standardize the results, and each target gene mRNA level was expressed as its ratio to β-actin mRNA. The relative quantification of each target gene expression among the experiment groups was analyzed by the 2-ΔΔct method.
Table 1. Sequences of primer pairs used in the real-time quantitative PCR reactions.
| Primer Name | Sequence (5’ to 3’) | Accession number | Tm (°C) |
|---|---|---|---|
| β-actin | CGGAATCCACCAAACCACCTAATCTCCTTCTGCATCCTGTGA | NM_181601.5 | 56 |
| SOD | GGCCAACCGATAGTGTTAGACCAGCGTTGCCAGTTTTTAG | NM_131294.1 | 57 |
| GPx1a | ACCTGTCCGCGAAACTATTGTGACTGTTGTGCCTCAAAGC | NM_001007281.2 | 58 |
| CAT | AGGGCAACTGGGATCTTACATTTATGGGACCAGACCTTGG | XM_021470442.1 | 58 |
| sMT-B | TGCTCCAAATCTGGATCTTGGCAGTCCTTCTTGCCCTTAC | NM_001201469.1 | 58 |
| MT-2 | AGACTGGAACTTGCAACTGTGGTCAGCTGGAGCCACAGGAATT | NM_001131053.3 | 62 |
| Bcl-2 | F:ATGTGCGTGGAAAGCGTCAACR:GAAGGCATCCCAACCTCCATT | NM_001030253.2 | 56 |
| p53 | F:GGGCAGGGAGCGTTATGAR:AGAGTCGCTTCTTCCTTCGTC | NM_001271820.1 | 56 |
| MDM2 | F:CCGACGCCTCCACTTCTCR:ATAAGGTGCCCAGTCCTTCC | AF010255.1 | 56 |
| Bax | F:CGATACGGGCAGTGGCAR:TCGGCTGAAGATTAGAGTTGTT | AF231015.1 | 56 |
Assay of MDA in the gill and ovary
The whole process of preparation of gill and ovary homogenates of zebrafish was performed on ice. First of all, the gills and ovaries were thawed, then were washed with a 0.86% normal saline at 4°C, dried and weighed, and put into a tissue grinder immediately with 0.01 mol/l Tris–HCl buffer solution at pH 8.0 using a mass ratio of 1:9. The homogenates were then collected in centrifuge tubes and centrifuged at 1,006 g for 20 min. The supernatants were collected to determine the levels of MDA and protein.
MDA and protein were measured by using MDA and protein determination kits according to the manufacturer’s protocol (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). MDA in gill and ovary homogenates was determined in terms of thiobarbituric acid reactive substances (TBARSs) formation with maximal absorbance at 532 nm by following the protocol. The concentration of MDA in the gill and ovary of the zebrafish was calculated by comparing the absorbance to that produced by the control standard 1, 1, 3,3-tetraethoxypropane and expressed as nmol/mg prot.
Statistics analysis
Data analyses were conducted using the SPSS software (Ver.17.0,SPSS Inc., Chicago, IL, USA). Data were expressed as means ± SD. One-way ANOVA (Duncan’s multiple comparison test) was applied for significance tests to verify differences between the control and the different TCS treatment groups.
Results
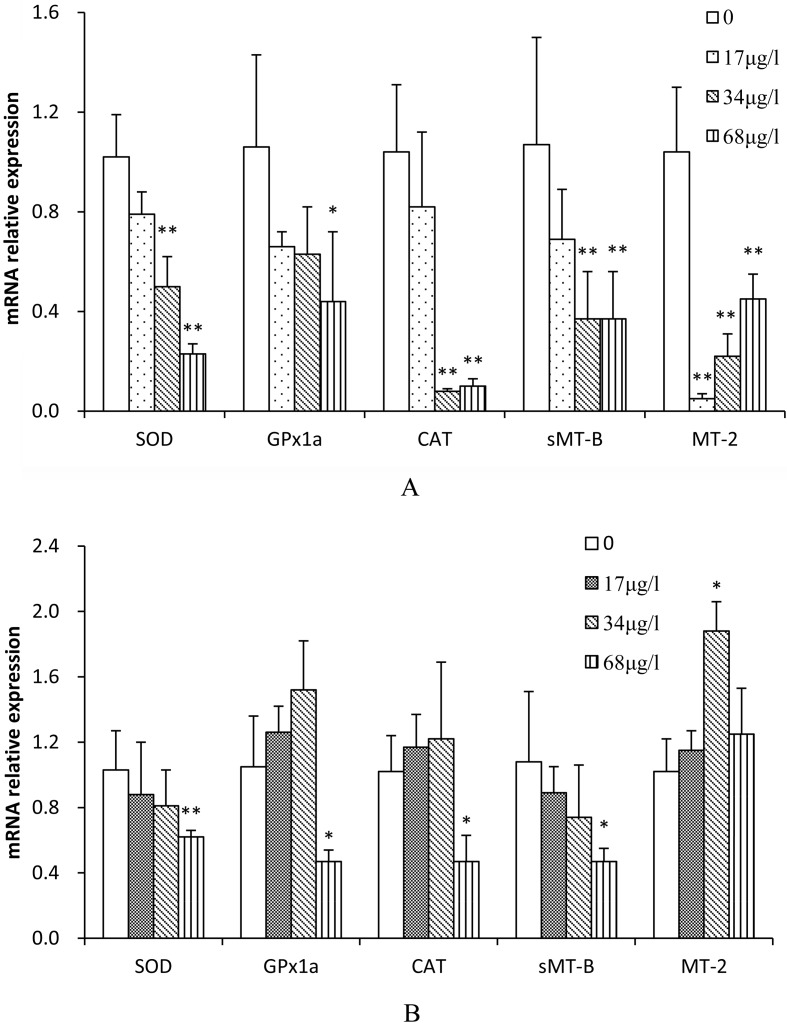
Effects of TCS on mRNA expression of oxidative stress-related genes in the gill and ovary of zebrafish
Treatment with TCS resulted in decreased oxidative stress-related mRNA levels in the gill of zebrafish (Fig. 2A). The inhibitory effects on the SOD, CAT, and sMT-B genes were all highly significant for 34 and 68 μg/l TCS (50.6% and 77.7%, 94.1% and 90.2% and 65.4% and 65.4%, respectively, P<0.01). The GPx1a inhibitory effect was significant for 68 μg/l TCS (58.4%, P<0.05). The MT-2 inhibitory effects were highly significant for 17, 34, and 68 μg/l TCS (95.1%, 78.5%, and 56.7%, respectively, P<0.01).
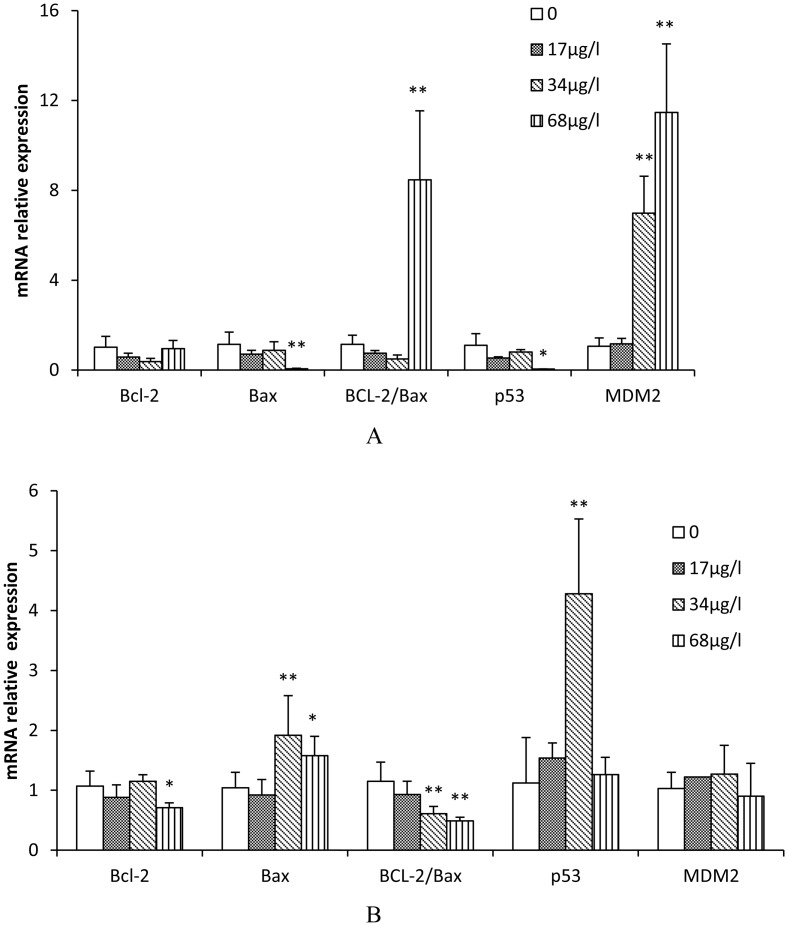
Fig. 2.
Expression of apoptosis-related genes in zebrafish (A, gill; B, ovary) exposed to various concentrations of TCS for 42 d. Values were normalized against β-actin (used as a house-keeping gene) and presented as the mean mRNA expression value ± SD (n=6) relative to those of the controls. Asterisks indicate statistically significant differences when compared with the controls. * P<0.05; ** P<0.01.
The effects of TCS on the expression of oxidative stress-related genes in the ovary of zebrafish were shown in Fig. 1B. The inhibitory effects in SOD, CAT, GPx1a, and sMT-B genes were also highly significant or significant for 68 μg/l TCS (39.8%, 55.2%, 53.9%, and 56.5%, respectively), while the induction effects of MT-2 were significant for 34 μg/l TCS (1.84 fold, P<0.05).
Fig. 1.
Expression of antioxidant-related genes in zebrafish (A, gill; B, ovary) exposed to various concentrations of TCS for 42 d. Values were normalized against β-actin (used as a house-keeping gene) and presented as the mean mRNA expression value ± SD (n=6) relative to those of the controls. Asterisks indicate statistically significant differences when compared with the controls. * P<0.05; **P<0.01.
Effects of TCS on mRNA expression of apoptosis-related genes in the gill and ovary of zebrafish
The effects of TCS on the expression of apoptosis-related genes in the gill and ovary of zebrafish are shown in Figs. 2A and B, respectively. The Bax and p53 mRNA relative expression levels in the gill in the 68 μg/l TCS group were significantly decreased compared with the control (P<0.05 for Bax; P<0.01 for p53). The inhibitory rates were 94.9% and 95.3%, respectively. However, the Bax gene in the ovary in the 34 and 68 μg/L TCS treated groups and p53 in the 34 μg/l TCS treated group were significantly increased compared with the control (1.52 fold, 1.84 fold, and 3.82 fold, respectively; P<0.05 for Bax ; P<0.01 for p53). The ratio of BCL-2 to Bax in the gill in the 68 μg/l TCS treated group was significantly increased compared with the control (P<0.01), while the ratio of BCL-2 to Bax in the ovary in the 34 and 68μg/l TCS treated groups was significantly decreased (P<0.01). In addition, the MDM2 mRNA relative expression levels in the gill in the 34 and 68 μg/l TCS groups were significantly increased compared with the control (6.6 fold and 10.8 fold, respectively, P<0.01). The results showed that the effects of TCS on the regulation of apoptosis-related genes expression in the ovaries and gills of zebrafish are different.
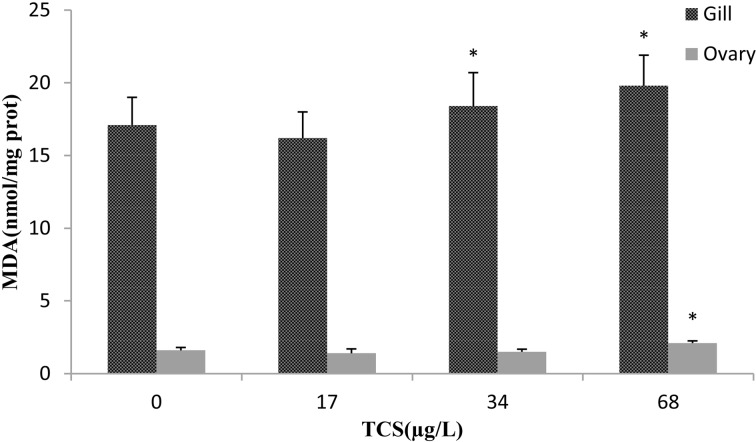
Effects of TCS on MDA in the gill and ovary of zebrafish
MDA is widely used as a biomarker for assaying oxidative stress in the field of toxicology and pharmacology. In the present study, the MDA contents in the gill in the 34 and 68 μg/l TCS groups were significantly increased compared with the control, and in the ovary in the 68 μg/l TCS group, it was also significantly increased (Fig. 3). The results showed that a higher concentration of TCS caused oxidative damage to the gills and ovaries of zebrafish.
Fig. 3.
. Effects of TCS on MDA in the gill and ovary of zebrafish exposed to various concentrations of TCS for 42 d. Asterisks indicate statistically significant differences from the control group (*P<0.05). Data are presented as the mean ± SD (n=6).
Discussion
When an organism is exposed to environmental pollutants, oxidative stress, defined as an imbalance between the production and consumption of ROS [23, 42],often occurs Recently, differential transcription levels of genes encoding stress-related proteins and antioxidant enzymes have been used to detect biological toxicity and/or to discuss the effects of chemical pollutants [1, 20, 27]. For this reason, we examined the transcription levels of representative genes, which encode proteins and antioxidant enzymes that are used to resist oxidative stress. In order to confirm whether TCS causes oxidative stress in zebrafish, we also examined MDA, a marker of oxidative stress.
Antioxidant enzymes include important ROS scavenging enzymes, such as SOD, CAT, and GPx, which are the first line of defense against ROS [18]. In the present study, SOD, CAT, and GPx gene expression levels in the gill were significantly downregulated in the 34 (except GPx1a) and 68 µg/l TCS groups, and the levels of these genes in the ovary were also significantly downregulated in the 68 µg/l TCS group. These results showed that a high dose of TCS significantly affected the transcription levels of antioxidant enzymes-related genes, which may influence the expression of antioxidant enzymes. Decreased gene expression of SOD, CAT, and GPx1a observed in the gill and ovary at the higher doses of TCS may be a consequence of an inhibition caused directly by the xenobiotic action via ROS accumulation or via key damages in the structure of the enzymes induced by ROS [39]. The mechanism of direct inhibition of antioxidant enzymes by xenobiotics is unclear and mainly depends on the type of compound. In addition, the action time and concentration of a xenobiotic determine the amount of ROS accumulated, which could result in inhibition of SOD, CAT, and GPx, respectively [28]. Therefore, a high dose of TCS could cause accumulation of excessive ROS, resulting in decreased SOD, CAT, and GPx gene expression.
Metallothioneins (MTs) are thiol-rich metal-binding proteins with a wide range of functions, one of which is defense against oxidative damage [13]. Previous studies have shown that MTs have antioxidant activity and together with GSH, play an important role in regulating redox balance and scavenging superoxide and hydroxyl radicals [33]. There are many types of MTs in zebrafish. In the present study, the MT2 gene expression levels in the gill in the TCS treated groups and the sMT-B gene expression levels in the 34 and 68 µg/l TCS groups were significantly decreased compared with the control, while the MDA contents were significantly increased. These results further confirm that a high dose TCS may cause oxidative damage to the gills of zebrafish. In addition, the sMT-B gene expression in the ovary in the 68 µg/l TCS group was significantly decreased compared with the control, while the MT-2 gene expression in the 34 µg/l TCS group was significantly increased. The results showed that MT-2 in the ovary in the 34µgl TCS group can be used as a sensor and amplification system for oxidative stress, in which the oxidation of MT-2 releases zinc through thioprotein and GSH. Findings of several previous reports are in agreement with the findings in the present study [6, 45]. Therefore, it is possible that increased MT-2 enhanced the antioxidative capacity in the ovary in the 34µg/l TCS group of zebrafish. However, the results showed that the MDA content in the ovary in the 68µg/l TCS group was significantly increased, while the decrease in sMT-B gene expression in the ovary in the 68µgl TCS group showed that TCS caused oxidative damage to the ovary under this condition.
Mitochondrial dysfunction following oxidative damage is one of the early events in apoptotic cell death, as the pro-apoptotic factor cytochrome C (Cyt C) is released into the cytoplasm [38]. Release of Cyt C from the mitochondria can be triggered by the pro-apoptogenic Bax [3], while the anti-apoptotic Bcl-2 can prevent the release of Cyt C [2], and Bcl-2 can interact with bax protein to regulate the onset of apoptosis [19]. Moreover, the increased ROS production decreases the Bcl-2/Bax ratio [8]. In the present study, Bax gene expression in the ovary in the 68 µg/l TCS group was significantly increased, while the Bcl-2/Bax ratio and Bcl-2 gene expression were significantly decreased in the 34 and 68 µg/l TCS groups. These results showed that a high dose of TCS could cause mitochondrial dysfunction to accelerate ovary apoptosis. However, in the gill, Bcl-2 gene expression in the TCS groups was not significantly changed, whereas the Bax mRNA expression and Bcl-2/Bax ratio in the 68μg/l TCS group were significantly decreased and increased, respectively, suggesting that ROS generation was not related to apoptosis in the gill of zebrafish exposed to TCS and that a high dose of TCS inhibited gill apoptosis in our study.
ROS are involved in the signaling pathways that cause the mitochondrial migration of p53, which is involved in triggering ROS-dependent cell apoptosis [14, 26]. Elevated levels of p53 upregulate the expression of the proto-oncogene MDM2 [10, 30]. Moreover, p53 can promote apoptosis by upregulating the expression of Bax and downregulating the expression of Bcl-2 [37]. In the ovary in the present study, p53 gene expression was significantly increased in the 34µg/l TCS group, and MDM2 gene expression was unchanged in all TCS groups; p53 gene expression was significantly decreased in the 68μg/lgroup, and NDM2 gene expression was significantly increased in the 34 and 68 μg/l TCS groups. These results further confirmed that a higher dose of TCS might accelerate ovary ROS-dependent apoptosis but inhibit gill apoptosis.
In conclusion, a high dose of TCS exposure down-regulates significantly the expression of antioxidant-related genes, which may lead to oxidative damage to the gill and ovary of zebrafish. In addition, a higher dose of TCS might accelerate ovary ROS-dependent apoptosis. To our knowledge, this is the first report to study the oxidative stress and apoptosis-related transcription level effects of TCS on zebrafish. Thus, the information presented in the present study is helpful for understanding the mechanism of TCS-induced oxidative stress and apoptosis in fish.
Acknowledgments
This study was supported by the National Nature Science Foundation of China (grants 31971524) and the Joint Fund for Fostering Talents of the National Natural Science Foundation of China and Henan Province (grants U1504303). All authors declare that there are no conflicts of interest.
References
- 1.Abdel-Gawad F.2014. Effect of Polycyclic Aromatic Hydrocarbons (PAHs) on Modulate Genes Encoding Stress Related Proteins and Antioxidant Enzymes in Different Marine Fish Species of Red Sea Water. World Appl. Sci. J. 32: 2337–2347. [Google Scholar]
- 2.Adams J.M., Cory S.1998. The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326. doi: 10.1126/science.281.5381.1322 [DOI] [PubMed] [Google Scholar]
- 3.Antonsson B., Montessuit S., Lauper S., Eskes R., Martinou J.C.2000. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 345: 271–278. doi: 10.1042/bj3450271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binelli A., Cogni D., Parolini M., Riva C., Provini A.2009. In vivo experiments for the evaluation of genotoxic and cytotoxic effects of Triclosan in Zebra mussel hemocytes. Aquat. Toxicol. 91: 238–244. doi: 10.1016/j.aquatox.2008.11.008 [DOI] [PubMed] [Google Scholar]
- 5.Binelli A., Cogni D., Parolini M., Riva C., Provini A.2009. Cytotoxic and genotoxic effects of in vitro exposure to triclosan and trimethoprim on zebra mussel (Dreissena polymorpha) hemocytes. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 150: 50–56. doi: 10.1016/j.cbpc.2009.02.005 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y., Maret W.2001. Catalytic selenols couple the redox cycles of metallothionein and glutathione. Eur. J. Biochem. 268: 3346–3353. doi: 10.1046/j.1432-1327.2001.02250.x [DOI] [PubMed] [Google Scholar]
- 7.Chen Y., Zeng S.F., Cao Y.F.2012. Oxidative stress response in zebrafish (Danio rerio) gill experimentally exposed to subchronic microcystin-LR. Environ. Monit. Assess. 184: 6775–6787. doi: 10.1007/s10661-011-2457-0 [DOI] [PubMed] [Google Scholar]
- 8.Chien C.T., Lee P.H., Chen C.F., Ma M.C., Lai M.K., Hsu S.M.2001. De novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J. Am. Soc. Nephrol. 12: 973–982. [DOI] [PubMed] [Google Scholar]
- 9.Dann A.B., Hontela A.2011. Triclosan: environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 31: 285–311. doi: 10.1002/jat.1660 [DOI] [PubMed] [Google Scholar]
- 10.de Rozieres S., Maya R., Oren M., Lozano G.2000. The loss of mdm2 induces p53-mediated apoptosis. Oncogene 19: 1691–1697. doi: 10.1038/sj.onc.1203468 [DOI] [PubMed] [Google Scholar]
- 11.Falisse E., Voisin A.S., Silvestre F.2017. Impacts of triclosan exposure on zebrafish early-life stage: Toxicity and acclimation mechanisms. Aquat. Toxicol. 189: 97–107. doi: 10.1016/j.aquatox.2017.06.003 [DOI] [PubMed] [Google Scholar]
- 12.Ghelichpour M., Taheri Mirghaed A., Hoseinifar S.H., Khalili M., Yousefi M., Van Doan H., Perez-Jimenez A.2019. Expression of immune, antioxidant and stress related genes in different organs of common carp exposed to indoxacarb. Aquat. Toxicol. 208: 208–216. doi: 10.1016/j.aquatox.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Iglesias H., Alvarez L., García M., Petrash C., Sanz-Medel A., Coca-Prados M.2014. Metallothioneins (MTs) in the human eye: a perspective article on the zinc-MT redox cycle. Metallomics 6: 201–208. doi: 10.1039/c3mt00298e [DOI] [PubMed] [Google Scholar]
- 14.Gu Z.T., Wang H., Li L., Liu Y.S., Deng X.B., Huo S.F., Yuan F.F., Liu Z.F., Tong H.S., Su L.2014. Heat stress induces apoptosis through transcription-independent p53-mediated mitochondrial pathways in human umbilical vein endothelial cell. Sci. Rep. 4: 4469. doi: 10.1038/srep04469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J., Won E.J., Hwang U.K., Kim I.C., Yim J.H., Lee J.S.2016. Triclosan (TCS) and Triclocarban (TCC) cause lifespan reduction and reproductive impairment through oxidative stress-mediated expression of the defensome in the monogonont rotifer (Brachionus koreanus). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 185-186: 131–137. doi: 10.1016/j.cbpc.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 16.Hinther A., Bromba C.M., Wulff J.E., Helbing C.C.2011. Effects of triclocarban, triclosan, and methyl triclosan on thyroid hormone action and stress in frog and mammalian culture systems. Environ. Sci. Technol. 45: 5395–5402. doi: 10.1021/es1041942 [DOI] [PubMed] [Google Scholar]
- 17.Hou J., Li L., Xue T., Long M., Su Y., Wu N.2014. Damage and recovery of the ovary in female zebrafish i.p.-injected with MC-LR. Aquat. Toxicol. 155: 110–118. doi: 10.1016/j.aquatox.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 18.Ighodaro O.M., Akinloye O.A.2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defense grid. AJM 54: 287–293. [Google Scholar]
- 19.Jarskog L.F., Selinger E.S., Lieberman J.A., Gilmore J.H.2004. Apoptotic proteins in the temporal cortex in schizophrenia: high Bax/Bcl-2 ratio without caspase-3 activation. Am. J. Psychiatry 161: 109–115. doi: 10.1176/appi.ajp.161.1.109 [DOI] [PubMed] [Google Scholar]
- 20.Jin Y., Zhang X., Shu L., Chen L., Sun L., Qian H., Liu W., Fu Z.2010. Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78: 846–852. doi: 10.1016/j.chemosphere.2009.11.044 [DOI] [PubMed] [Google Scholar]
- 21.Johnson P.I., Koustas E., Vesterinen H.M., Sutton P., Atchley D.S., Kim A.N., Campbell M., Donald J.M., Sen S., Bero L., Zeise L., Woodruff T.J.2016. Application of the Navigation Guide systematic review methodology to the evidence for developmental and reproductive toxicity of triclosan. Environ. Int. 92-93: 716–728. doi: 10.1016/j.envint.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly K.A., Havrilla C.M., Brady T.C., Abramo K.H., Levin E.D.1998. Oxidative stress in toxicology: established mammalian and emerging piscine model systems. Environ. Health Perspect. 106: 375–384. doi: 10.1289/ehp.98106375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klotz L.O., Steinbrenner H.2017. Cellular adaptation to xenobiotics: Interplay between xenosensors, reactive oxygen species and FOXO transcription factors. Redox Biol. 13: 646–654. doi: 10.1016/j.redox.2017.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koplin D.W., Furlong E.T., Meyer M.T., Meyer M.T., Thurman E.M., Zaugg S.D., Barber L.B., Buxton H.T.2002. Response to comment on “Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance”. Environ. Sci. Technol. 36: 4004. [DOI] [PubMed] [Google Scholar]
- 25.Kosma C.I., Lambropoulou D.A., Albanis T.A.2014. Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci. Total Environ. 466-467: 421–438. doi: 10.1016/j.scitotenv.2013.07.044 [DOI] [PubMed] [Google Scholar]
- 26.Lee S.J., Yang E.S., Kim S.Y., Kim S.Y., Shin S.W., Park J.W.2008. Regulation of heat shock-induced apoptosis by sensitive to apoptosis gene protein. Free Radic. Biol. Med. 45: 167–176. doi: 10.1016/j.freeradbiomed.2008.03.026 [DOI] [PubMed] [Google Scholar]
- 27.Liang X., Nie X., Ying G., An T., Li K.2013. Assessment of toxic effects of triclosan on the swordtail fish (Xiphophorus helleri) by a multi-biomarker approach. Chemosphere 90: 1281–1288. doi: 10.1016/j.chemosphere.2012.09.087 [DOI] [PubMed] [Google Scholar]
- 28.Ma X., Deng D., Che W.2017. Inhibitors and activators of SOD, GSH-Px, and CAT. In: Şentürk, M. (Ed.), Enzyme Inhibitors and Activators. IntechOpen. [Google Scholar]
- 29.McGowan A.J., Fernandes R.S., Samali A., Cotter T.G.1996. Anti-oxidants and apoptosis. Biochem. Soc. Trans. 24: 229–233. doi: 10.1042/bst0240229 [DOI] [PubMed] [Google Scholar]
- 30.Michael D., Oren M.2002. The p53 and Mdm2 families in cancer. Curr. Opin. Genet. Dev. 12: 53–59. doi: 10.1016/S0959-437X(01)00264-7 [DOI] [PubMed] [Google Scholar]
- 31.Mohan S., Balakrishnan P.2019. Triclosan in Treated Wastewater from a City Wastewater Treatment Plant and its Environmental Risk Assessment. Water Air Soil Pollut. 230: 69. doi: 10.1007/s11270-019-4098-9 [DOI] [Google Scholar]
- 32.Montaseri H., Forbes P.B.C.2016. A review of monitoring methods for triclosan and its occurrence in aquatic environments. Trends Analyt. Chem. 85: 221–231. doi: 10.1016/j.trac.2016.09.010 [DOI] [Google Scholar]
- 33.Nzengue Y., Steiman R., Rachidi W., Favier A., Guiraud P.2012. Oxidative stress induced by cadmium in the C6 cell line: role of copper and zinc. Biol. Trace Elem. Res. 146: 410–419. doi: 10.1007/s12011-011-9265-9 [DOI] [PubMed] [Google Scholar]
- 34.Oliveira R., Domingues I., Koppe Grisolia C., Soares A.M.2009. Effects of triclosan on zebrafish early-life stages and adults. Environ. Sci. Pollut. Res. Int. 16: 679–688. doi: 10.1007/s11356-009-0119-3 [DOI] [PubMed] [Google Scholar]
- 35.Park J.C., Han J., Lee M.C., Seo J.S., Lee J.S.2017. Effects of triclosan (TCS) on fecundity, the antioxidant system, and oxidative stress-mediated gene expression in the copepod Tigriopus japonicus. Aquat. Toxicol. 189: 16–24. doi: 10.1016/j.aquatox.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 36.Riva C., Cristoni S., Binelli A.2012. Effects of triclosan in the freshwater mussel Dreissena polymorpha: a proteomic investigation. Aquat. Toxicol. 118-119: 62–71. doi: 10.1016/j.aquatox.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 37.Selvakumaran M., Lin H.K., Miyashita T., Wang H.G., Krajewski S., Reed J.C., Hoffman B., Liebermann D.1994. Immediate early up-regulation of bax expression by p53 but not TGF beta 1: a paradigm for distinct apoptotic pathways. Oncogene 9: 1791–1798. [PubMed] [Google Scholar]
- 38.Salahudeen A.K., Huang H., Joshi M., Moore N.A., Jenkins J.K.2003. Involvement of the mitochondrial pathway in cold storage and rewarming-associated apoptosis of human renal proximal tubular cells. Am. J. Transplant. 3: 273–280. doi: 10.1034/j.1600-6143.2003.00042.x [DOI] [PubMed] [Google Scholar]
- 39.Slaninova A., Smutna M., Modra H., Svobodova Z.2009. A review: oxidative stress in fish induced by pesticides. Neuroendocrinol. Lett. 30:(Suppl 1): 2–12. [PubMed] [Google Scholar]
- 40.Stoker T.E., Gibson E.K., Zorrilla L.M.2010. Triclosan exposure modulates estrogen-dependent responses in the female wistar rat. Toxicol. Sci. 117: 45–53. doi: 10.1093/toxsci/kfq180 [DOI] [PubMed] [Google Scholar]
- 41.USEPA. 1991. Water Quality Criteria Summary.
- 42.Valavanidis A., Vlahogianni T., Dassenakis M., Scoullos M.2006. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 64: 178–189. doi: 10.1016/j.ecoenv.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 43.Wang F., Xu R., Zheng F., Liu H.2018. Effects of triclosan on acute toxicity, genetic toxicity and oxidative stress in goldfish (Carassius auratus). Exp. Anim. 67: 219–227. doi: 10.1538/expanim.17-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang F., Guo X., Chen W., Sun Y., Fan C.2017. Effects of triclosan on hormones and reproductive axis in female Yellow River carp (Cyprinus carpio): Potential mechanisms underlying estrogen effect. Toxicol. Appl. Pharmacol. 336: 49–54. doi: 10.1016/j.taap.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 45.Wu S.M., Liu J.H., Shu L.H., Chen C.H.2015. Anti-oxidative responses of zebrafish (Danio rerio) gill, liver and brain tissues upon acute cold shock. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 187: 202–213. doi: 10.1016/j.cbpa.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 46.Xing L., Sun J., Liu H., Yu H.2012. Combined toxicity of three chlorophenols 2,4-dichlorophenol, 2,4,6-trichlorophenol and pentachlorophenol to Daphnia magna. J. Environ. Monit. 14: 1677–1683. doi: 10.1039/c2em30185g [DOI] [PubMed] [Google Scholar]
- 47.Xu J., Xu Y., Wang H., Guo C., Qiu H., He Y., Zhang Y., Li X., Meng W.2015. Occurrence of antibiotics and antibiotic resistance genes in a sewage treatment plant and its effluent-receiving river. Chemosphere 119: 1379–1385. doi: 10.1016/j.chemosphere.2014.02.040 [DOI] [PubMed] [Google Scholar]
- 48.Youle R.J., Strasser A.2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9: 47–59. doi: 10.1038/nrm2308 [DOI] [PubMed] [Google Scholar]
- 49.Yueh M.F., Tukey R.H.2016. Triclosan: a widespread environmental toxicant with many biological effects. Annu. Rev. Pharmacol. Toxicol. 56: 251–272. doi: 10.1146/annurev-pharmtox-010715-103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou B., Liu C., Wang J., Lam P.K., Wu R.S.2006. Primary cultured cells as sensitive in vitro model for assessment of toxicants-comparison to hepatocytes and gill epithelia. Aquat. Toxicol. 80: 109–118. doi: 10.1016/j.aquatox.2006.07.021 [DOI] [PubMed] [Google Scholar]