Abstract
Background:
Oral mucositis (OM) is a common, disabling, and severe early effect of chemotherapy and radiotherapy that limits the effectiveness of anticancer therapy. The prevention and treatment of OM in patients with malignant tumors is an urgent problem in the field of anticancer therapy.
Methods:
Databases including PubMed, Embase, Scopus, The Cochrane Library, and Google Scholar were searched to collect published randomized control trials (RCTs) about the effects of different oral care solutions on the prevention of OM from inception to January 2019. We used the Cochrane Handbook to assess the methodological quality of the RCTs. Two of the authors independently extracted the articles and predefined data. Network meta-analysis was then performed using Stata 15.0 software.
Results:
A total of 28 RCTs involving 1861 patients were included. The results of network meta-analysis showed that chlorhexidine, benzydamine, honey, and curcumin were more effective than placebo (P < .05) and that honey and curcumin were more effective than povidone-iodine (P < .05). Probability ranking according to the Surface Under the Cumulative Ranking curve showed the following treatments: curcumin, honey, benzydamine, chlorhexidine, allopurinol, sucralfate, granulocyte-macrophage colony-stimulating factor, povidone-iodine, and aloe.
Conclusion:
Our preliminary results indicate that curcumin and honey may serve as the preferred options for patients to prevent OM. The findings may offer an important theoretical basis for clinical prevention and treatment. However, this conclusion still requires an RCT with a larger sample size for further verification.
Keywords: cancer, mucositis, network meta-analysis, nursing, oral
1. Introduction
Oral mucositis (OM) is described as a common and painful debilitating inflammation of the oral mucosa in patients with cancer that varies from mild mucosal erythema to severe ulcerations.[1] OM is one of the main side effects of anticancer treatment with an incidence rate of 40% to 100%, related to tumor type, oral hygiene, treatment method, age, and nutritional status.[2–4] Depending on its severity, OM may trigger an inability to tolerate food or fluids, which leads to malnutrition, dehydration, and weight loss.[5] Furthermore, it limits the effectiveness of anticancer therapy, increases hospitalization costs, and may even lead to interruption in chemoradiotherapy protocols, which reduces the chances of healing and patients’ survival.[6,7] Therefore, there is a need to develop therapeutic strategies to prevent and treat OM.
Oral care plays a critical role in the prevention and treatment of OM. Oral care, including regular oral care before and during cancer treatment and gentle flushing with saline or sodium bicarbonate, has long been considered the basis of oral hygiene for patients receiving cancer treatment.[8] It is considered important to maintain oral cleansing, reduce the risk of oral infections, and promote oral comfort; the evidence for a role in preventing or treating OM has been both scarce and inconsistent for basic oral care.[9] However, the mechanisms by which various basic oral care strategies may directly affect the pathogenesis of OM are unclear, although most have few complex interactions that may affect the molecular factors that cause mucosal tissue damage.[10] Mixed medication mouthwashes, usually consisting of topical coatings, anesthetics, and possibly other agents, have little or no direct effect on the pathogenesis of OM. Finally, some oral rinses are known to have specific biological activity, such as antibacterial drugs chlorhexidine and povidone-iodine, which do not affect the primary pathways involved in mucositis pathogenesis. Despite this, basic oral care is considered the backbone of supportive care for patients receiving cancer treatment.[9,11]
Different oral care solutions have been investigated for the prevention and treatment of OM, such as chlorhexidine, benzydamine, sucralfate, povidone-iodine, and honey, which have been found to prevent mucositis or reduce the severity of mucositis; however, no approach has been completely successful.[12–14] Although several systematic reviews and meta-analyses have been conducted to examine the effects of the different oral care solutions, evidence was limited due to the lack of multiple comparisons of classical meta-analysis. Bayesian network meta-analysis is a method combining all available direct and indirect evidences on the relative treatment effects, enabling a unified, coherent analysis of all RCTs.[15–17]The objective of this study was to evaluate the effect of different oral care solutions. These treatments were compared from 28 randomized controlled trials (RCTs) by network meta-analysis, which calculates the relative effects for all treatments.[18] The aim was to provide hierarchies of the prevention of OM for 9 treatments. This may provide valuable information for OM treatment research in the future.
2. Materials and methods
2.1. Ethical statement
Ethical approval and informed consent are not required, as the study will be a literature review and will not involve direct contact with patients or alterations to patient care.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: RCTs; studies that assessed the effects of different oral care solutions on the prevention of OM in patients with cancer who underwent chemotherapy, radiotherapy, or both; OM outcomes reported by trial authors (incidence of mucositis); articles written in English; and the subjects rinsed with different oral care solutions.
The exclusion criteria were as follows: duplicate publications; studies with insufficient data; subjects underwent intravenous, oral, subcutaneous, or inhalation treatment methods; and nonrandomized studies, retrospective studies, review articles, conference abstracts, letters, or case reports.
2.3. Literature search
We searched the PubMed, Embase, Scopus, Cochrane Library, and Google Scholar databases for studies related to the prevention of OM that were conducted before January 2019. We also manually searched the bibliographies of relevant literature to further identify any other research related to our analysis. Articles with the following key words and Medical Subject Headings were also searched: “Mucositis,” “Mouthwashes,” “Stomatitis,” “Mouth,” “Nursing care,” “Oral,” “Ulcer,” “Cancer,” “Chemotherapy,” “Radiotherapy,” and “RCT.”
2.4. Data extraction
Two researchers independently extracted information such as patient characteristics, first author, publication year, country of origin, treatment, and incidence of mucositis. A third researcher resolved any disagreement between the reviewers.
2.5. Risk of bias assessment
The Cochrane Risk of Bias Tool was used to assess the quality of the included studies by 2 reviewers. The tool is based on assessing random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other biases. The judgment classification in each domain is low risk of bias, unclear risk of bias, or high risk of bias.[19]
2.6. Statistical analysis
We estimated the relative risk (RR) with 95% confidence intervals (CIs) for dichotomous variables. Statistical analysis was performed using Stata software, version 15.0 (Stata Corporation, College Station, TX). P < .05 was considered statistically significant. Network meta-analysis compares multiple treatments simultaneously by combining direct and indirect evidence of the relative treatment effects.[20] We used inconsistency factors (IFs) to estimate heterogeneity in each closed loop, and a 95% CI (IF) value of zero indicated the absence of statistical significance. Funnel plot analysis was used to estimate small-study effects. We ranked the 9 interventions for treating OM according to the Surface Under the Cumulative Ranking curve (SUCRA), which represents the percentage of the area under the curve.[21]
3. Results
3.1. Study characteristics
We identified 2561 articles. We excluded 909 duplicate articles and a further 1518 articles after reviewing titles and abstracts. After screening the full text of the remaining 134 articles, we included 28 articles in our network meta-analysis.[22–49]Figure 1 shows a flow chart of the entire sample selection process. Table 1 provides a summary of the studies included in the present meta-analysis. A total of 28 studies were RCTs directly comparing alternative treatments, with a total of 1861 patients.
Figure 1.
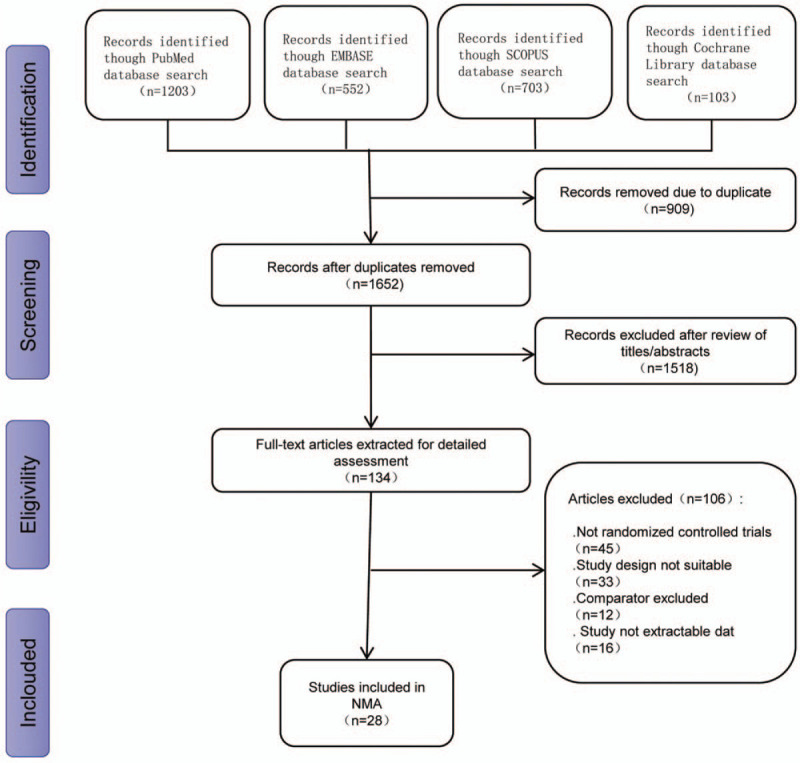
Flow diagram of study selection.
Table 1.
Characteristics of the included studies.
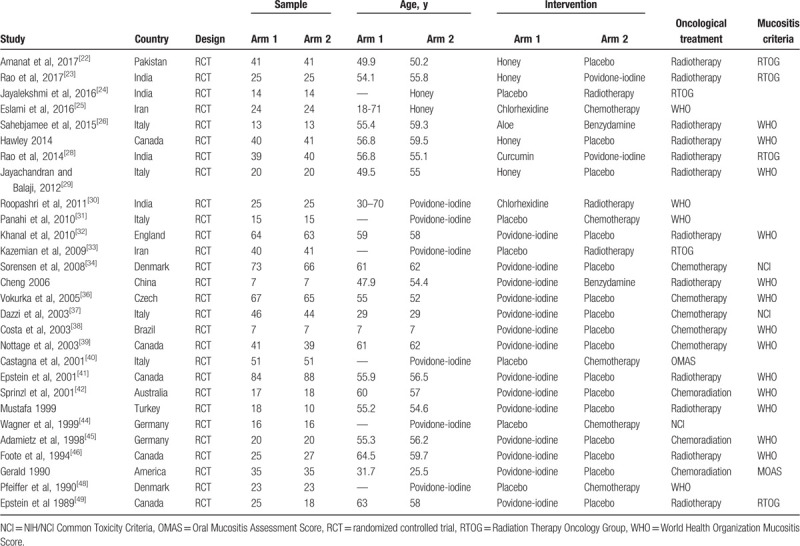
3.2. Quality assessment
Although all the studies involved randomization, 21 trials incorporated an adequate randomization technique. Only 7 articles reported information regarding allocation concealment. Regarding contamination between treatment groups, 18 trials were at a low risk of bias, whereas 18 trials were at a low risk of bias due to selective outcome reporting. Figure 2 shows the Cochrane risk of bias assessment of the included studies.
Figure 2.
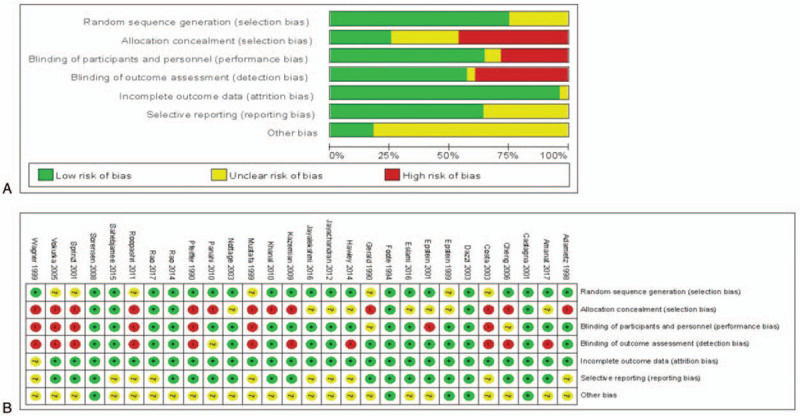
Risk-of-bias analysis. (A) Review authors’ judgments on each risk of bias items presented as percentages across all included studies. (B) Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
3.3. Evidence network
As shown in Figure 3, the lines in the evidence network represent a direct comparison between the 2 directly related interventions. Interventions without connections are compared indirectly through the network meta-analysis. The width of the lines represents the number of trials, and the size of the node represents the total sample size of multiple treatments.
Figure 3.
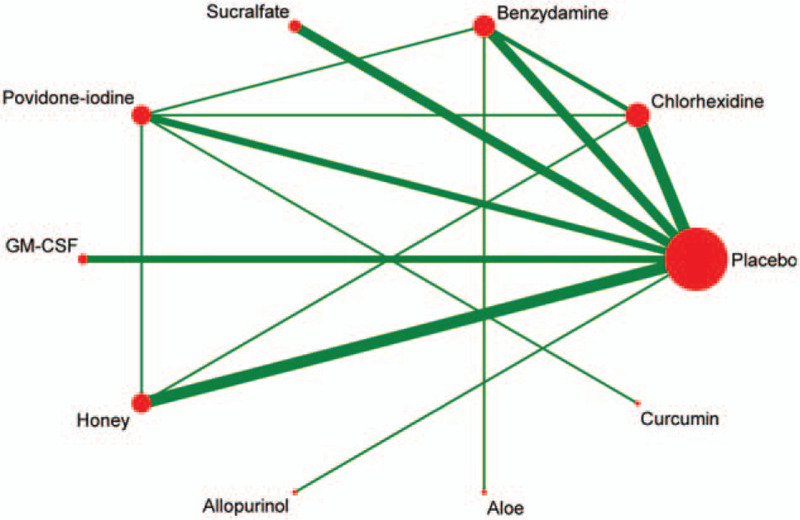
Evidence network of the RCT in the network meta-analysis.
3.4. Inconsistency test
Figure 4 shows an inconsistency plot for assessing the heterogeneity among studies in the closed loop of the network meta-analysis. It was composed of 7 loops with a 95% CI (IF) value of zero, which indicates that our network analysis data were consistent. In addition, all P values were >0.05, indicating that the indirect and direct comparisons of the various treatments were consistent.
Figure 4.
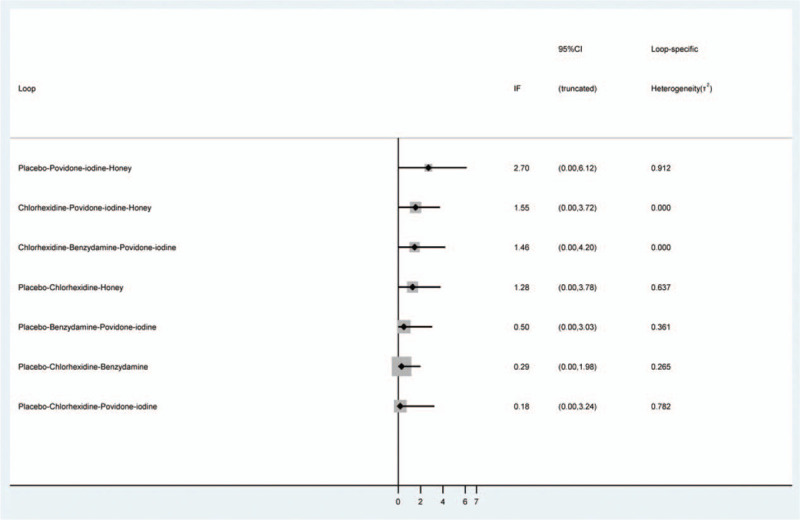
Inconsistency test for direct and indirect comparisons of the enrolled studies in the network meta-analysis.
3.5. Network meta-analysis
The results of network meta-analysis showed that chlorhexidine, benzydamine, honey, and curcumin were more effective than placebo (chlorhexidine: RR = 0.39; 95% CI, 0.18–0.82; benzydamine: RR = 0.30; 95% CI, 0.13–0.68; honey: RR = 0.25; 95% CI, 0.11–0.56; curcumin: RR = 0.08; 95% CI, 0.01–0.60) and that honey and curcumin were more effective than povidone-iodine (honey: RR = 0.32; 95% CI, 0.11–0.97; curcumin: RR = 0.10; 95% CI, 0.02–0.60). Other comparisons were not statistically significant (Fig. 5).
Figure 5.
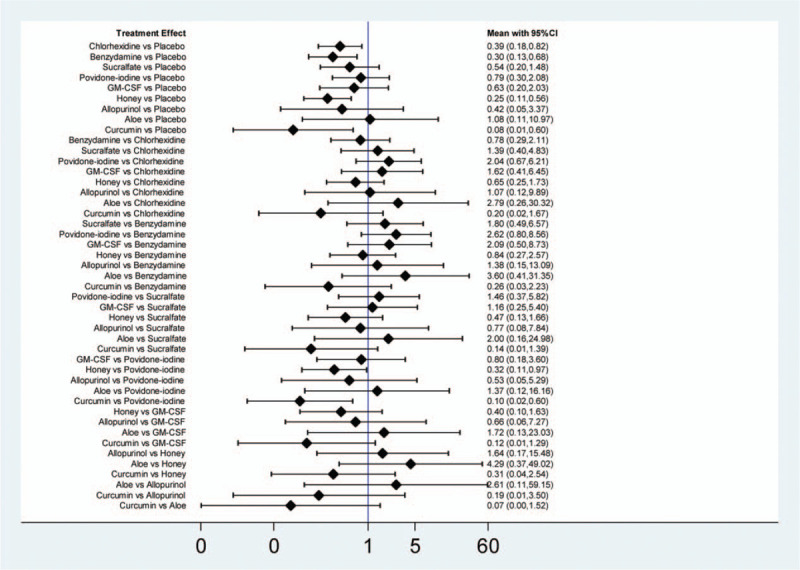
Results of the network meta- analysis.
3.6. Ranking probability
A ranking graph of the distribution of probabilities on remission is presented in Figure 6. Based on SUCRA, curcumin had the highest SUCRA rank, which was the first efficacy possibility. The SUCRA result showed the following efficacy ranking: curcumin > honey > benzydamine > chlorhexidine > allopurinol > sucralfate > granulocyte-macrophage colony-stimulating factor (GM-CSF) > povidone-iodine > aloe > placebo (Fig. 6).
Figure 6.
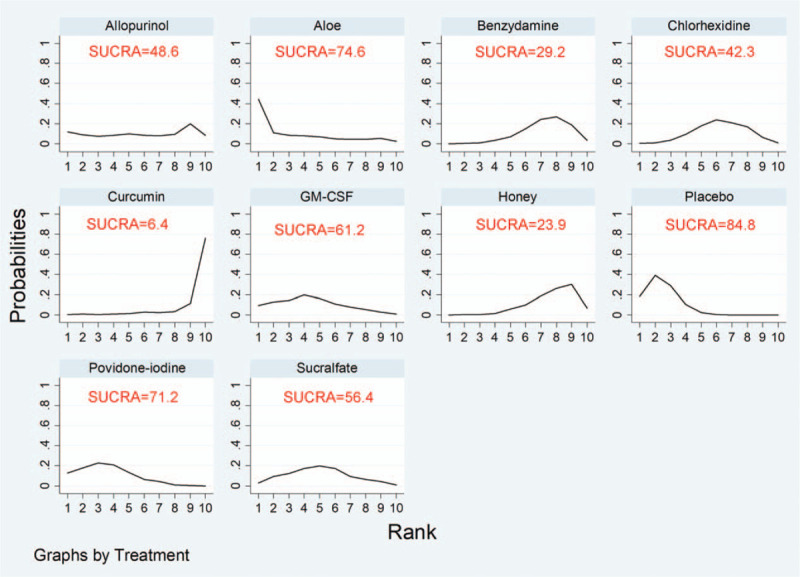
SUCRA probabilities of the different oral care solutions on prevention of oral mucositis. SUCRA = surface under the cumulative ranking.
3.7. Publication biases
The funnel plot suggested that the results for chlorhexidine might be affected by publication bias and small-study effects, which might have a significant impact on the estimated treatment effect (Fig. 7).
Figure 7.
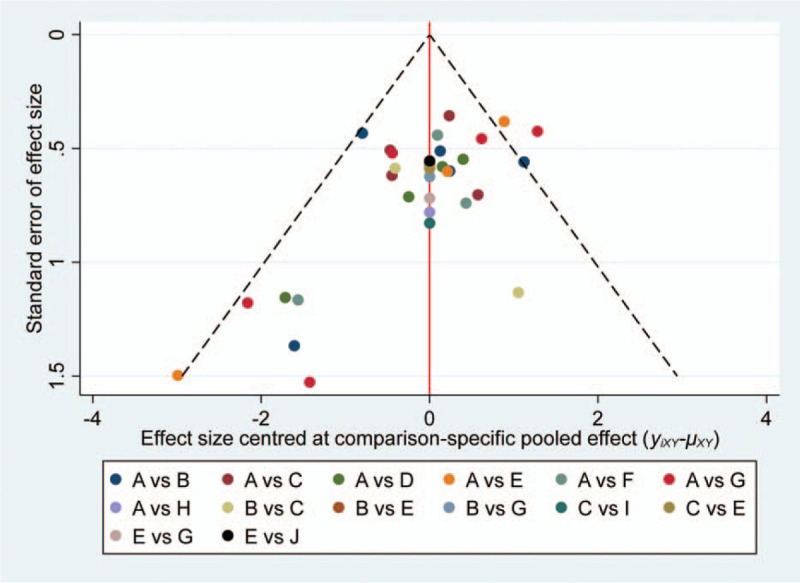
The comparison-adjusted funnel plot of multiple treatments for oral mucositis. A = Placebo, B = Chlorhexidine, C = Benzydamine, D = Sucralfate, E = Povidone-iodine, F = GM-CSF, G = Honey, H = Allopurinol, I = Aloe, J = Curcumin.
4. Discussion
OM is a common, disabling, and severe early effect of chemotherapy and radiotherapy. The prevention and treatment of OM in patients with malignant tumors is an urgent problem in the field of anticancer therapy. Recently, oral care was suggested to play a role in OM progression. Many RCTs and meta-analyses have been conducted for the prevention and treatment of OM with different oral care solutions. However, traditional meta-analysis is not conclusive in assessing >2 oral care solutions. Our study is the first to assess different oral care solutions for OM in patients receiving anti-cancer treatment based on network meta-analysis. Network meta-analysis is used to compare multiple interventions through direct and indirect comparisons.
A total of 28 RCTs involving 1861 patients and 9 oral care solutions were included. This article may present current evidence for OM treatment research in the future.
The results of network meta-analysis showed that chlorhexidine, benzydamine, honey, and curcumin were more effective than placebo (P < .05) and that honey and curcumin were more effective than povidone-iodine (P < .05). SUCRA showed the following result: curcumin, honey, benzydamine, chlorhexidine, allopurinol, sucralfate, GM-CSF, povidone-iodine, and aloe.
Members of the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) recently completed the process of updating the MASCC/ISOO Clinical Practice Guidelines for the prevention and treatment of mucositis.[50] These guidelines were originally published in 2004,[51] and then updated in 2007[9] and 2014.[52] In agreement with our data, the current guidelines recommend that benzydamine mouthwash be used to prevent OM in patients with cancer (level I). Furthermore, the panel suggests that chlorhexidine and GM-CSF mouthwash not be used to prevent OM in patients receiving radiation therapy for head and neck cancer (level II).[52] This guide roughly aligns with our findings. In this context, due to inadequate evidence, no guideline was possible in relation to other agents of natural origin reviewed, including honey, aloe vera, and Chinese herbs.[52] However, there were 8 articles included in our study that reported curcumin (Chinese herbs) and honey as oral care solutions. Only 2 articles were published before 2014, and 6 were published after 2014. Increasingly more studies confirm the role of natural medicines in the prevention and treatment of OM. Our research may provide a basis for updating the guidelines.
Active components are extracted from the rhizomes of the turmeric plant. In recent years, numerous studies have demonstrated the potentially important effects of curcumin as a potential therapeutic agent, such as antioxidant, anticancer, and antiulcer activities.[53,54] Of the total of 28 articles included, only 1 reported treatment with curcumin. However, numerous studies have shown that curcumin plays an important role in OM. Patil et al[55] also confirmed that the effect of curcumin may be better in the management of radiochemotherapy-induced OM compared with chlorhexidine. Meanwhile, this effect may be due to the different study populations used. A non-RCT found that curcumin combined with honey also has significant advantages for OM.[56] Elad et al[57] assessed the tolerability of a curcumin mouthwash for the prevention of OM in pediatric patients, and no adverse events were documented. Large-scale, long-term studies are needed to evaluate the role of curcumin in the treatment of OM.
Previous reviews and meta-analyses have reported that honey is beneficial to prevent the development of severe mucositis, compared with controls.[58,59] This study updated previous data as it added 3 recent clinical trials to the previous systematic reviews and meta-analyses. The additional studies used chlorhexidine and povidone-iodine as the controls, and these studies have shown that honey plays an important role in OM. However, the apprehension that honey would enhance the radiation-related caries in cancer patients topically applying them during the course of the radiation is a major concern as this would enhance dental caries and compromise the quality of life of the cancer survivors.[60,61] In contrast, when compared with honey, turmeric may not have long-term adjunct effects as studies have shown it to be also beneficial in the treatment of various periodontal diseases.[62,63] Clinically, although clinically chlorhexidine is a therapy that may be routinely used or recommended in cancer patients with OM, the current evidence does not support the routine prescription and cost of chlorhexidine for the prevention or treatment of OM until further studies are performed. Well, in this study, probability ranking according to SUCRA indicates that curcumin and honey have great potential in preventing OM until further studies are performed.
The outcome measurement of mucositis is fairly uniform with the Radiation Therapy Oncology Group, World Health Organization, National Cancer Institute, and OM Assessment Scale. These evaluation standards were the scales used in most of the studies included. These scales are fairly similar, all with grades 0 and 1 indicating tolerable or less severe mucositis and grades 2 to 4 indicating intolerable or severe mucositis. In consequence, to standardize the outcome indicators, the occurrence of OM was defined as a grade of >1. The benefit of using a standardized reporting system will facilitate better pooling of results from different studies.[64]
This network meta-analysis has several limitations. First, we focused on the occurrence of OM in patients with cancer and did not consider other outcomes, such as the severity of mucositis and side effects of different mouthwashes because these data were not available. Second, due to the lack of data to yield outcomes (such as incidence rate) in most trials included in our article, we could only extract the change according to various international groups (RTOG, WHO, NCI, and OMAS) to evaluate the effectiveness of various treatments. Third, some of the treatments, including curcumin, allopurinol, and aloe, were respectively covered by just 1 study, and the number of patients involved in some treatments was relatively small. In addition to the variety of agents, chemotherapy regimens may also affect the prognosis of patients while included in this network meta-analysis.
5. Conclusion
In summary, our preliminary results indicate that curcumin and honey may serve as the preferred options for patients to prevent OM. The findings may offer an important theoretical basis for clinical prevention and treatment. Hence, in the future, well-designed, high-quality, large-scale RCTs are necessary.
Author contributions
Conceptualization: Ya-Ying Yu, Xin Zhou.
Data curation: Ya-Ying Yu, Xian-Rong Jin.
Formal analysis: Ya-Ying Yu.
Funding acquisition: Ya-Ying Yu, Xin Zhou.
Investigation: Xin Zhou, Xiao-Hua Zhang.
Project administration: Zu Zhong Zhang.
Resources: Zu Zhong Zhang.
Software: Xin Zhou, Jia-Lin Deng.
Supervision: Zu Zhong Zhang.
Validation: Zu Zhong Zhang.
Visualization: Zu Zhong Zhang.
Writing – original draft: Ya-Ying Yu.
Writing – review & editing: Xin Zhou.
Footnotes
Abbreviations: CI = confidence interval, GM-CSF = granulocyte-macrophage colony-stimulating factor, IF = inconsistency factor, MASCC/ISOO = Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology, NCI = National Cancer Institute, OM = oral mucositis, OMAS = Oral Mucositis Assessment Scale, RCT = randomized controlled trial, RR = relative risk, RTOG = Radiation Therapy Oncology Group, SUCRA = surface under the cumulative ranking, WHO = World Health Organization.
How to cite this article: Yu YY, Deng JL, Jin XR, Zhang ZZ, Zhang XH, Zhou X. Effects of nine oral care solutions on the prevention of oral mucositis: A network meta-analysis of randomized controlled trials. Medicine. 2020;99:16(e19661).
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 81502329); Program of Science and Technology of Chongqing Commission (Grant No. KJ1600228); and Programs of Yongchuan Hospital of Chongqing Medical University (Grant Nos. YJYB20120012; YJZQN 201514; YCZQN201511).
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Sonis ST. The pathobiology of mucositis. Nat Rev Cancer 2004;4:277–84. [DOI] [PubMed] [Google Scholar]
- [2].Raber-Durlacher JE, Weijl NI, Abu SM, et al. Oral mucositis in patients treated with chemotherapy for solid tumors: a retrospective analysis of 150 cases. Support Care Cancer 2000;8:366–71. [DOI] [PubMed] [Google Scholar]
- [3].Maria OM, Eliopoulos N, Muanza T. Radiation-induced oral mucositis. Front Oncol 2017;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oronsky B, Goyal S, Kim MM, et al. A review of clinical radioprotection and chemoprotection for oral mucositis. Transl Oncol 2018;11:771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Georgiou M, Patapatiou G, Domoxoudis S, et al. Oral mucositis: understanding the pathology and management. Hippokratia 2012;16:215–6. [PMC free article] [PubMed] [Google Scholar]
- [6].Villa A, Sonis ST. Mucositis: pathobiology and management. Curr Opin Oncol 2015;27:159–64. [DOI] [PubMed] [Google Scholar]
- [7].Bressan V, Bagnasco A, Aleo G, et al. The life experience of nutrition impact symptoms during treatment for head and neck cancer patients: a systematic review and meta-synthesis. Support Care Cancer 2017;25:1699–712. [DOI] [PubMed] [Google Scholar]
- [8].McGuire DB, Fulton JS, Park J, et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients. Support Care Cancer 2013;21:3165–77. [DOI] [PubMed] [Google Scholar]
- [9].Keefe DM, Schubert MM, Elting LS, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 2007;109:820–31. [DOI] [PubMed] [Google Scholar]
- [10].Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004;100:1995–2025. [DOI] [PubMed] [Google Scholar]
- [11].McGuire DB, Correa ME, Johnson J, et al. The role of basic oral care and good clinical practice principles in the management of oral mucositis. Support Care Cancer 2006;14:541–7. [DOI] [PubMed] [Google Scholar]
- [12].Niikura N, Ota Y, Hayashi N, et al. Evaluation of oral care to prevent oral mucositis in estrogen receptor-positive metastatic breast cancer patients treated with everolimus (Oral Care-BC): randomized controlled phase III trial, Jpn. J Clin Oncol 2016;46:879–82. [DOI] [PubMed] [Google Scholar]
- [13].Wasko-Grabowska A, Rzepecki P, Oborska S, et al. A supersaturated calcium phosphate solution seems to effectively prevent and treat oral mucositis in haematopoietic stem cell transplanted cancer patients—single centre experience. J BUON 2012;17:363–8. [PubMed] [Google Scholar]
- [14].Garavito AA, Cardona AF, Reveiz L, et al. Colchicine mouth washings to improve oral mucositis in patients with hematological malignancies: a clinical trial. Palliat Support Care 2008;6:371–6. [DOI] [PubMed] [Google Scholar]
- [15].Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24. [DOI] [PubMed] [Google Scholar]
- [17].Sun XL, Wang S, Xia YN. Predictive-trend-aware composition of web services with time-varying quality-of-service. IEEE Access 2019. [Google Scholar]
- [18].Garcia-Perdomo HA. Network meta-analysis, a new statistical technique at urologists’ disposal to improve decision making. Int Braz J Urol 2018;44:422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].De Zordo T, Ahmad N, Ødegaard F, et al. US-guided therapy of calcific tendinopathy: clinical and radiological outcome assessment in shoulder and non-shoulder tendons. Ultraschall Med 2011;32: suppl 1: S117–23. [DOI] [PubMed] [Google Scholar]
- [20].Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [22].Amanat A, Ahmed A, Kazmi A, et al. The effect of honey on radiation-induced oral mucositis in head and neck cancer patients. Indian J Palliat Care 2017;23:317–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rao S, Hegde SK, Rao P, et al. Honey mitigates radiation-induced oral mucositis in head and neck cancer patients without affecting the tumor response. Foods 2017;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jayalekshmi JL, Lakshmi R, Mukerji A. Honey on oral mucositis: a randomized controlled trial. Gulf J Oncolog 2016;1:30–7. [PubMed] [Google Scholar]
- [25].Eslami H, Pouralibaba F, Falsafi P, et al. Efficacy of Hypozalix spray and propolis mouthwash for prevention of chemotherapy-induced oral mucositis in leukemic patients: a double-blind randomized clinical trial. J Dent Res Dent Clin Dent Prospects 2016;10:226–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sahebjamee M, Mansourian A, Hajimirzamohammad M, et al. Comparative efficacy of aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: a triple-blind, randomised, controlled clinical trial. Oral Health Prev Dent 2015;13:309–15. [DOI] [PubMed] [Google Scholar]
- [27].Hawley P, Hovan A, McGahan CE, et al. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Support Care Cancer 2014;22:751–61. [DOI] [PubMed] [Google Scholar]
- [28].Rao S, Dinkar C, Vaishnav LK, et al. The Indian spice turmeric delays and mitigates radiation-induced oral mucositis in patients undergoing treatment for head and neck cancer: an investigational study. Integr Cancer Ther 2014;13:201–10. [DOI] [PubMed] [Google Scholar]
- [29].Jayachandran S, Balaji N. Evaluating the effectiveness of topical application of natural honey and benzydamine hydrochloride in the management of radiation mucositis. Indian J Palliat Care 2012;18:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Roopashri G, Jayanthi K, Guruprasad R. Efficacy of benzydamine hydrochloride, chlorhexidine, and povidone iodine in the treatment of oral mucositis among patients undergoing radiotherapy in head and neck malignancies: a drug trail. Contemp Clin Dent 2011;2:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Panahi Y, Ala S, Saeedi M, et al. Allopurinol mouth rinse for prophylaxis of fluorouracil-induced mucositis. Eur J Cancer Care (Engl) 2010;19:308–12. [DOI] [PubMed] [Google Scholar]
- [32].Khanal B, Baliga M, Uppal N. Effect of topical honey on limitation of radiation-induced oral mucositis: an intervention study. Int J Oral Maxillofac Surg 2010;39:1181–5. [DOI] [PubMed] [Google Scholar]
- [33].Kazemian A, Kamian S, Aghili M, et al. Benzydamine for prophylaxis of radiation-induced oral mucositis in head and neck cancers: a double-blind placebo-controlled randomized clinical trial. Eur J Cancer Care (Engl) 2009;18:174–8. [DOI] [PubMed] [Google Scholar]
- [34].Sorensen JB, Skovsgaard T, Bork E, et al. Double-blind, placebo-controlled, randomized study of chlorhexidine prophylaxis for 5-fluorouracil-based chemotherapy-induced oral mucositis with nonblinded randomized comparison to oral cooling (cryotherapy) in gastrointestinal malignancies. Cancer 2008;112:1600–6. [DOI] [PubMed] [Google Scholar]
- [35].Kin-Fong CK, Ka TYJ. A pilot study of chlorhexidine and benzydamine oral rinses for the prevention and treatment of irradiation mucositis in patients with head and neck cancer. Cancer Nurs 2006;29:423–30. [DOI] [PubMed] [Google Scholar]
- [36].Vokurka S, Bystrická E, Koza V, et al. The comparative effects of povidone-iodine and normal saline mouthwashes on oral mucositis in patients after high-dose chemotherapy and APBSCT--results of a randomized multicentre study. Support Care Cancer 2005;13:554–8. [DOI] [PubMed] [Google Scholar]
- [37].Dazzi C, Cariello A, Giovanis P, et al. Prophylaxis with GM-CSF mouthwashes does not reduce frequency and duration of severe oral mucositis in patients with solid tumors undergoing high-dose chemotherapy with autologous peripheral blood stem cell transplantation rescue: a double blind, randomized, placebo-controlled study. Ann Oncol 2003;14:559–63. [DOI] [PubMed] [Google Scholar]
- [38].Costa EM, Fernandes MZ, Quinder LB, et al. Evaluation of an oral preventive protocol in children with acute lymphoblastic leukemia. Pesqui Odontol Bras 2003;17:147–50. [DOI] [PubMed] [Google Scholar]
- [39].Nottage M, McLachlan SA, Brittain MA, et al. Sucralfate mouthwash for prevention and treatment of 5-fluorouracil-induced mucositis: a randomized, placebo-controlled trial. Support Care Cancer 2003;11:41–7. [DOI] [PubMed] [Google Scholar]
- [40].Castagna L, Benhamou E, Pedraza E, et al. Prevention of mucositis in bone marrow transplantation: a double blind randomised controlled trial of sucralfate. Ann Oncol 2001;12:953–5. [DOI] [PubMed] [Google Scholar]
- [41].Epstein JB, Silverman S, Paggiarino DA, et al. Benzydamine HCl for prophylaxis of radiation-induced oral mucositis: results from a multicenter, randomized, double-blind, placebo-controlled clinical trial. Cancer 2001;92:875–85. [DOI] [PubMed] [Google Scholar]
- [42].Sprinzl GM, Galvan O, de Vries A, et al. Local application of granulocyte-macrophage colony stimulating factor (GM-CSF) for the treatment of oral mucositis. Eur J Cancer 2001;37:2003–9. [DOI] [PubMed] [Google Scholar]
- [43].Cengiz M, Ozyar E, Oztürk D, et al. Sucralfate in the prevention of radiation-induced oral mucositis. J Clin Gastroenterol 1999;28:40–3. [DOI] [PubMed] [Google Scholar]
- [44].Wagner W, Alfrink M, Haus U, et al. Treatment of irradiation-induced mucositis with growth factors (rhGM-CSF) in patients with head and neck cancer. Anticancer Res 1999;19:799–803. [PubMed] [Google Scholar]
- [45].Adamietz IA, Rahn R, Böttcher HD, et al. Prophylaxis with povidone-iodine against induction of oral mucositis by radiochemotherapy. Support Care Cancer 1998;6:373–7. [DOI] [PubMed] [Google Scholar]
- [46].Foote RL, Loprinzi CL, Frank AR, et al. Randomized trial of a chlorhexidine mouthwash for alleviation of radiation-induced mucositis. J Clin Oncol 1994;12:2630–3. [DOI] [PubMed] [Google Scholar]
- [47].Ferretti GA, Raybould TP, Brown AT, et al. Chlorhexidine prophylaxis for chemotherapy- and radiotherapy-induced stomatitis: a randomized double-blind trial. Oral Surg Oral Med Oral Pathol 1990;69:331–8. [DOI] [PubMed] [Google Scholar]
- [48].Pfeiffer P, Madsen EL, Hansen O, et al. Effect of prophylactic sucralfate suspension on stomatitis induced by cancer chemotherapy. A randomized, double-blind cross-over study. Acta Oncol 1990;29:171–3. [DOI] [PubMed] [Google Scholar]
- [49].Epstein JB, Stevenson-Moore P, Jackson S, et al. Prevention of oral mucositis in radiation therapy: a controlled study with benzydamine hydrochloride rinse. Int J Radiat Oncol Biol Phys 1989;16:1571–5. [DOI] [PubMed] [Google Scholar]
- [50].Bowen JM, Elad S, Hutchins RD, et al. Methodology for the MASCC/ISOO Mucositis Clinical Practice Guidelines Update. Support Care Cancer 2013;21:303–8. [DOI] [PubMed] [Google Scholar]
- [51].Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 2004;100:2026–46. [DOI] [PubMed] [Google Scholar]
- [52].Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014;120:1453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol 2007;595:453–70. [DOI] [PubMed] [Google Scholar]
- [54].Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett 2007;255:170–81. [DOI] [PubMed] [Google Scholar]
- [55].Patil K, Guledgud MV, Kulkarni PK, et al. Use of curcumin mouthrinse in radio-chemotherapy induced oral mucositis patients: a pilot study. J Clin Diagn Res 2015;9:ZC59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Francis M, Williams S. Effectiveness of Indian turmeric powder with honey as complementary therapy on oral mucositis: a nursing perspective among cancer patients in Mysore. Nurs J India 2014;105:258–60. [PubMed] [Google Scholar]
- [57].Elad S, Meidan I, Sellam G, et al. Topical curcumin for the prevention of oral mucositis in pediatric patients: case series. Altern Ther Health Med 2013;19:21–4. [PubMed] [Google Scholar]
- [58].Co JL, Mejia MB, Que JC, et al. Effectiveness of honey on radiation-induced oral mucositis, time to mucositis, weight loss, and treatment interruptions among patients with head and neck malignancies: a meta-analysis and systematic review of literature. Head Neck 2016;38:1119–28. [DOI] [PubMed] [Google Scholar]
- [59].Xu JL, Xia R, Sun ZH, et al. Effects of honey use on the management of radio/chemotherapy-induced mucositis: a meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg 2016;45:1618–25. [DOI] [PubMed] [Google Scholar]
- [60].Santos-Silva AR, Rosa GB, Eduardo CP, et al. Increased risk for radiation-related caries in cancer patients using topical honey for the prevention of oral mucositis. Int J Oral Maxillofac Surg 2011;40:1335–6. author reply 1235. [DOI] [PubMed] [Google Scholar]
- [61].Van den Wyngaert T. Topical honey application to reduce radiation-induced oral mucositis: a therapy too sweet to ignore. J Evid Based Dent Pract 2012;12:203–5. [DOI] [PubMed] [Google Scholar]
- [62].Waghmare PF, Chaudhari AU, Karhadkar VM, et al. Comparative evaluation of turmeric and chlorhexidine gluconate mouthwash in prevention of plaque formation and gingivitis: a clinical and microbiological study. J Contemp Dent Pract 2011;12:221–4. [DOI] [PubMed] [Google Scholar]
- [63].Nagpal M, Sood S. Role of curcumin in systemic and oral health: an overview. J Nat Sci Biol Med 2013;4:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Logan RM, Gibson RJ, Bowen JM, et al. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol 2008;62:33–41. [DOI] [PubMed] [Google Scholar]


