Abstract
Hyperglycemia in pregnancy (HIP) is related to adverse pregnancy outcomes. However, women with hyperglycemia in the second and third trimester of pregnancy (HISTTP) were not been observed. We aim to reveal associations between HISTTP and prematurity. To confirm which risk factor is better in predicting preterm delivery.
This retrospective study included 660 patients, of which 132 have HISTTP and 528 have euglycemia. Univariate analysis was used to extract risk factors and multivariates logistic regression analysis to obtain odds ratio (OR) for prematurity. Mean decrease gini (MDG) in random forest algorithm was used to rank the risk factors.
HISTTP women have higher prepregnancy BMI and a higher percentage of family history of hypertension, maternal adiposity, maternal anemia, gestational diabetes mellitus (GDM), prematurity, neonatal asphyxia in 1-minute (P < .05). Univariate analysis of prematurity showed that preterm women had higher rate of HISTTP (P < .01), second births, elderly pregnancy, hypertention, family history of hypertention and multiple perinatal infant (P < .05). Multivariate logistic regression analysis indicates that HISTTP (OR = 2.984, P = .0017), maternal hypertension (OR = 5.208, P = .001) and multiple perinatal infants (OR = 59.815, P < .0001) are independent risk factors for prematurity. After ranked the MDG, the top 3 risk factors were multiple perinatal infants, maternal hypertension, HISTTP. MDG of HISTTP is higher than that of GDM.
Women with HISTTP deserve to be concerned, whose prematurity rate are increased. HISTTP is an independent risk factor and a better predictor of prematurity.
Keywords: gestational diabetes mellitus, hyperglycemia and pregnancy outcome, pregnancy complications, prematurity
1. Introduction
Hyperglycemia in pregnancy (HIP) was divided into pre-existing diabetes complicating pregnancy, gestational diabetes mellitus (GDM) and gestational overt diabetes mellitus.[1] According to the study by International Diabetes Federation (IDF), approximately 16% of the births are complicated by HIP, with more than 86% of cases being due to GDM annually. But there are many forms of gestational hyperglycemia, such as insulin-treated type 1 or type 2 diabetes complicating pregnancy, overt diabetes during pregnancy, and gestational diabetes with or without insulin therapy. Different hyperglycemia states during pregnancy have various effects on parturient and their offspring, and the ways of health care are different. In various diabetes phenotypes, as we found in clinical observation, some pregnant women did not have hyperglycemia before pregnancy and in the early stages of pregnancy, but over time, hyperglycemia occurred in the second and third trimester of pregnancy.
Previous observations have shown that any form of blood glucose problems during pregnancy can have adverse effects on pregnant women and offspring. Among the reported adverse outcomes,[2,3] premature birth,[4] and neonatal asphyxia were serious consequences. The famous hyperglycemia adverse pregnancy outcome (HAPO) study demonstrated increasing risks of preterm delivery with increasing maternal glucose levels in women with no diabetes.[5] Studies also demonstrated associations of maternal prepregnancy Type 1 diabetes,[6] gestational diabetes, maternal obesity, insulin-treated pregestational diabetes and type 2 diabetes not treated with insulin with prematurity.[7] The report on the association between maternal diabetes and preterm delivery, the risk in insulin-treated diabetes group was significantly increased, mainly for moderate prematurity.[7] Mechanisms that may contribute to prematurity for mothers with obesity and diabetes include hyperglycemia, lipotoxicity, insulin resistance, and oxidative stress leading to endothelial dysfunction.[8,9]
Meanwhile, preterm birth also has other reported risk factors.[10–12] A national population study in Taiwan showed that women with type1diabetes had an increased risk of having a premature offspring.[13] A cohort study of 46,230 pregnancies found that gestational diabetes and lower degrees of maternal hyperglycemia (than gestational diabetes) during pregnancy mildly increased the risk of spontaneous preterm birth.[14] A study based on a large population cohort of 1.6 million births showed that maternal prepregnancy underweight, overweight, and obesity are associated with increased risks of preterm delivery, especially extremely preterm.[15] Increased risk of prematurity for mothers with obesity is reported to be associated with medical complications, including diabetes, anemia, and hypertension.[16] A Chinese prospective cohort analysis show that maternal obesity in early pregnancy in associated with preterm birth.[17] Furthermore, diabetes increases the risk of preeclampsia, which is associated with higher risk for preterm delivery.[18] In the study of maternal body mass index (BMI) and adverse outcomes, a systematic review and meta-analysis showed that underweight women had a higher risk of an offspring with low birth weight and prematurity compared with mothers with normal weight.[19]
Although previous studies have demonstrated associations of various forms HIP with prematurity, the associations between women with hyperglycemia occurred in the second and third trimester of pregnancy and GDM have not been studied. The potential implications of HISTTP with prematurity have not been well reported. Women who were observed in this study with hyperglycemia in the second and third trimester of pregnancy (HISTTP) met the criteria that the FBG in the early trimester (1–13 gestational weeks) was lower than 5.1 mmol/L, while the FBG were higher than 5.1 mmol/l during the second trimester (13–27 weeks) and the third trimester (28 weeks to delivery). They were not diagnosed with any type of diabetes before pregnancy and were not treated with oral drugs or insulin during pregnancy. This study aims to examine the associations of HISTTP and adverse pregnancy outcomes, especially preterm delivery, and to clarify the importance of HISTTP to prematurity. If we just focus on GDM patients, women in the HISTTP group who were at high risk for preterm delivery would have been filtered out.
2. Methods
2.1. Study population
About 9000 pregnant women who gave birth in the maternity ward of Beijing Luhe hospital and maternity clinic from 2016 to 2018. These women consistently completed maternity check and underwent fasting blood glucose (FBG) testing during the three periods of pregnancy. On the basis of meeting the above preconditions, all the subjects also met the requirement that the FBG in the early trimester (1–13 gestational weeks) was lower than 5.1 mmol/L, and they had not been diagnosed with any type of diabetes before pregnancy. In addition, the FBG of the case group was higher than 5.1 mmol/l during the second trimester (13–27 weeks) and the third trimester (28 weeks to delivery), while the FBG of the control group was lower than 5.1 mmol/l during the second and third trimester. They were not diagnosed with any type of diabetes before pregnancy and were not treated with oral drugs or insulin during pregnancy. We also extracted relevant data of patients from the database of perinatal examination and inpatient medical records registered in the hospital. The exclusion criteria were incomplete case data, patients with severe systemic diseases such as tumors, and patients with definite Type 1 or Type 2 diabetes. This study protocol was approved by the medical ethics committee of Luhe hospital affiliated to capital medical university. All patients gave informed consent to the study and all the methodological issues related to medical ethics met the requirements of the Helsinki declaration.
2.2. Data sources and assessment
The information of subjects such as maternal demographic, clinical care, and anthropometric characteristics, pregnancy outcomes were obtained from the Pregnancy examination database, which restricted the inclusion of data to 2016 as the earliest. The maternal prepregnancy height and weight was self-reported at first prenatal visit on the 10th week of pregnancy. Data on maternal prepregnancy BMI , from the first prenatal visit, was calculated as weight in kilograms divided by height in meters squared. Other information were obtained from the perinatal examination database and electronic medical record database registered in the hospital. The information include the date of hospitalization, date of birth, height, weight of prepregnancy, pregnancy age, delivery date, the last menstrual date, early-middle and late pregnancy test date, postpartum blood loss, delivery times, and the pregnancy risk factors such as maternal hypertension, maternal anemia, history of spontaneous abortion, history of macrosomia, the delivery mode (cesarean delivery or natural birth), delivery gestational age (weeks), number of perinatal infant, birth outcomes, such as number of live births, stillbirth. The definitive diagnosis of gestational diabetes mellitus was obtained from the medical record, which followed the Guidelines for diagnosis and treatment of gestational diabetes mellitus of China (2014), which based on the International Association of Diabetes and Pregnancy study Groups (IADPSG) 2010 criteria.
Fetal information includes sex of infant, birth length, birth weight, 1-minute Apgar scores, 5-minute Apgar scores, 10-minute Apgar scores. According to gestational age and birth weight, newborns were classified into premature infants, postterm infants, low birth weight infants, small-for-gestational-age infants (SGA) and macrosomia. Premature delivery was defined as gestational age less than 37 weeks, and postterm infant was defined as gestational age greater than 42 weeks. Newborns with birth weight below 2500 g are considered as low birth weight infants. Newborns with a gestational age between 37 and 42 weeks and a birth weight of less than 2500 g are considered small-for-gestational-age infants (SGA). Newborns with birth weight more than 4000 g were defined as macrosomia.
Apgar score is a method used to evaluate the presence and severity of asphyxia at birth. Breathing, heart rate, muscle tone, skin color and response to stimulation were scored 1, 5 and 10 minutes after birth; 0–2 points for each item, the full score is 10 points. For example, the total score of the 5 items is 0–3 for severe neonatal asphyxia, 4–7 for mild neonatal asphyxia, and 8–10 for no neonatal asphyxia. In this study, neonatal asphyxia score ≤7 points is asphyxia, and more than 8 points (including 8 points) is normal.
2.3. Statistical analysis
Continuous variables were presented with mean ± SD and categorical variables were presented with the number (percentages). Statistical significance of differences was analyzed using independent t test or Mann–Whitney U test for continuous variables and the χ2 test or Fisher exact test for categorical variables. Multivariate logistic regression analysis was applied to extract risk factors and calculate odds ratio (OR) with 95% confidence intervals (CI) of hyperglycemia on prematurity events during pregnancy. Mean decrease gini (MDG) involved in random forest algorithm was used to rank the associated factors with premature delivery. MDG provides the ways to quantify which factors contribute most to classification accuracy. Greater MDG will indicate that the degree of impurity arising from category could be reduced farthest by one variable, and thus suggests an important associated factor. Statistical analysis was computed using JMP 13.0 Pro by SAS and random Forest package of R software (http://www.r-project.org). All of the statistical tests were 2-sided and considered statistically significant if P < .05.
3. Results
3.1. Demographic data for pregnancy women
The study population consisted of 660 subjects, 132 (20%) patients were included in the HISTTP group, also known as the case group. A total of 528 patients in euglycemia group were included as control group. Of the euglycemia group, patients were adjusted for pregnancy age and fasting glucose in first trimaster of pregnancy. As shown in Table 1, the pregnancy age of the women in different groups were similar, which was 30.9 ± 4.7 years in the case group and 30.8 ± 4.6 years in the control group (P > .05). After adjusted for fasting glucose in first trimaster of pregnancy, the fasting glucose levels in both groups were 4.9 ± 0.2 mmol/L. Mean fasting blood glucose levels in the middle and later stages of pregnancy in the case group were higher than those in the control group (case group vs control group, 5.5 ± 0.5 vs 4.6 ± 0.3, 5.6 ± 0.9 vs 4.5 ± 0.3 mmol/L, P < .001).
Table 1.
Maternal characteristics and pregnancy outcomes of HISTTP and euglycemia group.
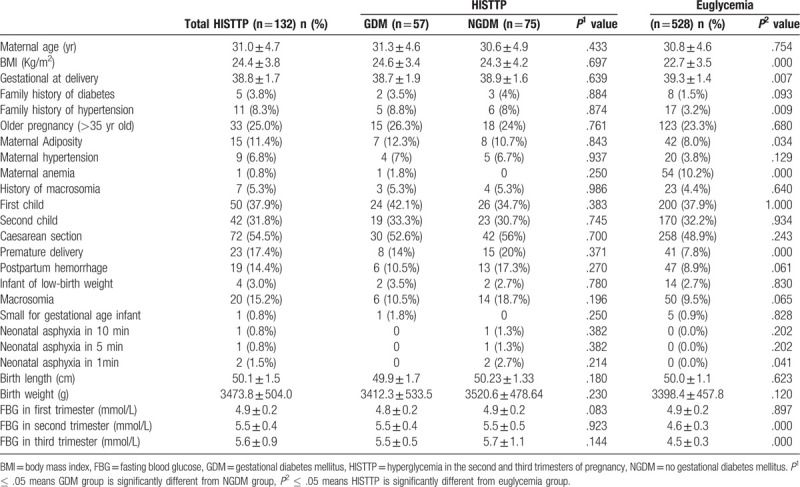
Compared with the women with euglycemia, HISTTP group tended to have higher mean prepregnancy BMI and less weeks of gestational length. In addition, the case group had a higher percentage of family history of hypertension, maternal adiposity, maternal anemia, prevalence of GDM, premature delivery, neonatal asphyxia in one minute after giving birth (P < .05). However, there was no significant difference in the incidence of maternal risk factors, such as the percentage of family history of diabetes, older pregnancy (>35 years old), maternal hypertention, multiple spontaneous abortions, history of macrosomia, etc. Other adverse pregnancy outcomes of maternal and infant were similar, such as the percentage of caesarean section, post maturity, postpartum hemorrhage, infant of low-birth weight, macrosomia, small for gestational age infant, neonatal asphyxia in 5 or 10 minutes after giving birth. Additionally, the length and birth weight of infant were no difference between groups. We regrouped HISTTP according to whether the subject had GDM. There was no significant difference in preterm delivery between GDM and NGDM group (P1 = .371). However, in the HISTTP group we observed, the preterm delivery rate was higher than that of the euglycemia group (P2 = .000). This means that women with HISTTP deserve to be concerned because of the higher rate of preterm delivery rate than euglycemia group. If we just focus on GDM patients, women in the HISTTP group who were at high risk for preterm delivery would have been filtered out.
3.2. Risk factors of prematurity
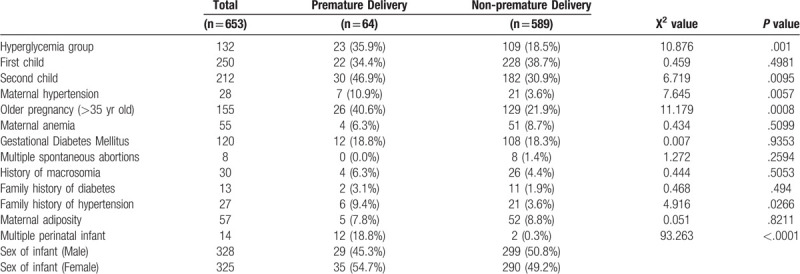
Among 660 subjects, the gestational age of delivery was available in 653 patients, so we classified the preterm and non-preterm groups according to whether the gestational age of delivery was less than 37 weeks. There were 64 (9.8%) patients in the preterm birth group and 589 (90.2%) in the non-preterm birth group. As shown in Table 2, compared to the women without premature delivery, the preterm pregnant women had a higher percentage of hyperglycemia as we defined (P < .01). Additionally, the proportion of second births, elderly parturient women (>35 years old), maternal hypertention, family history of hypertention and multiple perinatal infant in preterm delivery group were higher than those in non-premature delivery women (P < .05). There was no difference in other risk factors, such as the percentage of maternal anemia, gestational diabetes mellitus, multiple spontaneous abortions, history of macrosomia, maternal adiposity, family history of diabetes, between the 2 groups. This study found that the proportion of patients with elevated fasting blood glucose during the second and third trimaster of pregnancy in the preterm delivery group was higher than that in the non-preterm delivery group.
Table 2.
Comparisons risk factors of premature delivery between groups.
3.3. Logistic regression analysis for risk factors of prematurity
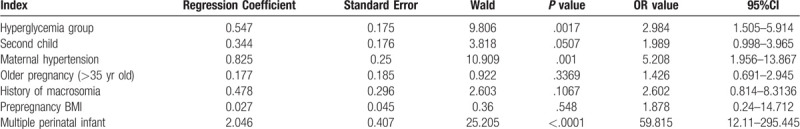
Multiple factors logistic regression was performed to evaluate independent risk factors for prematurity. The risk factors assessed in the logistic model included those found by univariate analysis in Table 2, such as hyperglycemia group, second child, maternal hypertension, elderly pregnant women (>35 years old), history of macrosomia, prepregnancy BMI, multiple perinatal infant. As shown in Table 3, the hyperglycemia, maternal hypertension and the multiple perinatal infant are independent risk factors for preterm delivery (P < .01).
Table 3.
Multivariate logistic regression analysis of independent risk factors for premature delivery patients.
3.4. Random forest algorithm to rank the risk factors with prematurity
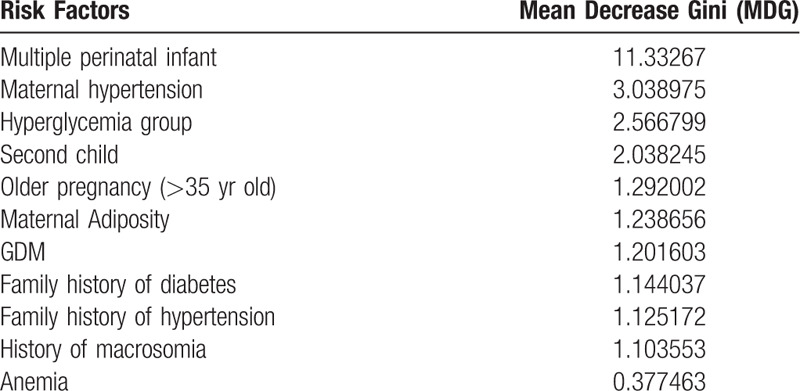
We assessed 11 potential factors associated with prematurity based on univariate analysis. The 11 risk factors included multiple perinatal infant, maternal hypertension, hyperglycemia group, second child, elder pregnancy (>35 years old), maternal adiposity, gestational diabetes mellitus, family history of diabetes, family history of hypertension, history of macrosomia, maternal anemia. With preterm delivery as the dependent variable, we randomly selected 70% of the 653 patients as the prediction set of the random forest model, and the other 30% of the data as the validation set. The accuracy of this random forest algorithm model is 88.57%. The MDG represents the weight of each risk factor in this model. With MDG sequencing, we can observe the importance of each risk factor associated to preterm delivery. As shown in Table 4, the top 5 ranked factors were multiple perinatal infant, maternal hypertension, hyperglycemia group, second child, elder pregnancy (>35 years old). Obviously, we found that the MDG of hyperglycemia group, which defined as higher fasting blood glucose in the second and third trimester during the pregnancy, is higher than that of gestational diabetes mellitus. Among the various risk factors, hyperglycemia ranks in the top three, while GDM appears to be less important in comparison.
Table 4.
The rank of factors associated with premature delivery.
4. Discussion
In this retrospective study, women with normal blood glucose levels in prepregnancy and early pregnancy but abnormal fasting blood glucose in the second and third trimester of pregnancy are defined as HISTTP. We found the HISTTP women deserve to be attentioned because of a higher percentage of premature delivery and neonatal asphyxia in one minute after giving birth than those women without hyperglycemia (P < .05). The hyperglycemia adverse pregnancy outcome (HAPO) study demonstrated increasing risks of preterm delivery with increasing maternal glucose levels in women without diabetes.[7] Furthermore, the rate of preterm birth has been reported increased because of multiple pregnancies and maternal hypertension. Diabetes increases the risk of preeclampsia, which is associated with higher risk for preterm births.[18] The risk factors for preterm delivery found in our study included HISTTP, maternal hypertension and multiple pregnancies, which were consistent with those reported in the literature. These risk factors are almost consistent with the results of previous studies. Previous studies have shown that the impacts of high BMI on preeclampsia, gestational diabetes and preterm delivery in Chinese women might be stronger than that in Caucasian,[20] and there are several conclusions about the relationship between maternal diabetes and premature birth of offspring. Mothers with Type 1 diabetes have an increased risk of preterm birth (data came from Taiwan OR, 4.21 [95%CI, 3.78–4.71], and the United States OR, 1.42[95%CI, 1.15–1.77]).[13,14] Mothers who are underweight, obese or severely obese have a slightly higher risk of preterm birth.[15] Despite that, our study shows that prepregnancy BMI does not seem to have a significant effect on the risk of preterm delivery. There are several possible reasons about this. First, the small sample size limits the conclusion. Second, in a post study, it is mentioned that prematurity was increased for mothers with Type 2 diabetes, independent of prepregnancy BMI.[7] According to their conclusion, in utero exposure to maternal diabetes treated with insulin appeared to be associated with prematurity regardless of the maternal prepregnancy BMI.
In the random forest algorithm section, we included previously reported risk factors for preterm delivery, such as multiple pregnancy, maternal hypertension, elderly pregnancy, prepregnancy obesity, gestational diabetes mellitus, family history of hypertension and diabetes, history of macrosomia, maternal anemia. We randomly selected 70% of the preterm women as a training dataset, and the remaining 30% as a prediction dataset. To avoid the problem of overfitting, we adopt 5-fold cross-validation to implement the analysis.[21] The classification accuracy rate was 88.57% and the specificity of the model is 96.84%. According to our study, the top 3 ranked risk factors associated with prematurity is multiple pregnancy, HISTTP, maternal hypertension. These results provide further support to our findings in multivariants logistic regression analysis section. As to the association of gestational diabetes mellitus with preterm delivery, we found the MDG of GDM is lower than that of HISTTP, which means their effects on prematurity is different.
This is consistent with a conclusion came from a study, which has reported a markedly high aOR of diabetes with insulin treatment, and a smaller, but clearly statistically significant aOR of type 2 diabetes, but no association with gestational diabetes.[7] Mechanisms behind this phenomenon has been suggested by some studies, which demonstrated that mothers with obesity and diabetes have a higher rate of preterm delivery due to hyperglycemia, lipotoxicity, insulin resistance, and oxidative stress leading to endothelial dysfunction.[8,9]
Several limitations of this study should be taken into account. First of all, the overall number of patients included in the study and the number of adverse outcomes (e.g., neonatal asphyxia) need to be observed are small, which is not conducive to effective conclusions. Second, it was a retrospective study and data on risk factors of pregnancy, maternal complications and grade of diabetes control during pregnancy were not available. The diagnosis of GDM comes from the discharge diagnosis certificate of the patient's electronic medical record. Although this diagnosis was judged by a professional obstetrician based on the values of 75 g OGTT test results at 24 to 28 weeks of gestation and the guidelines for gestational diabetes mellitus, we were unable to obtain blood glucose value at each time point. Therefore, for the diagnosis of GDM, we can only indirectly control the quality. Thirdly, the prepregnancy weight was self-reported in the first antenatal examination during the early stages of pregnancy. Our follow-up work will focus on a series of prospective studies to make up for the shortcomings of retrospective studies. Expanding the sample size and verifying the conclusions in a larger population is also one of our future work goals. In addition, we will follow up the offspring of HISTTP women to determine the blood glucose metabolism of them.
5. Conclusion
Women with HISTTP are different from GDM, who deserve to be concerned. The risk of preterm delivery and neonatal asphyxia are increased in people with HISTTP. With regard to the predictor of prematurity, HISTTP may be a more significant factor than GDM. These findings may have implications for antenatal counseling and managing pregnancies to prevent adverse birth outcomes.
Acknowledgment
The authors thank all participants involved in this study.
Author contributions
DZ, JKY conceived and designed the experiments. DZ, SSY, YM, YXA, YXY performed the experiments. DZ analyzed the data and contributed reagents/materials/analysis tools, DZ, JKY wrote the paper.
Writing – original draft: Dong Zhao.
Jin-Kui Yang orcid: 0000-0002-5430-2149.
Footnotes
Abbreviations: GDM = gestational diabetes mellitus, HIP = Hyperglycemia in pregnancy, HISTTP = hyperglycemia in the second and third trimester of pregnancy, MDG = Mean Decrease Gini, OR = odds ratio.
How to cite this article: Zhao D, Yuan SS, Ma Y, An YX, Yang YX, Yang JK. Associations of maternal hyperglycemia in the second and third trimesters of pregnancy with prematurity. Medicine 2020;99:17(e19663)
The authors have no conflicts of interest to disclose.
References
- [1].Hod M, Kapur A, Sacks DA, et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynaecol Obstet 2015;131: Suppl 3: S173–211. [DOI] [PubMed] [Google Scholar]
- [2].Lowe WL, Jr, Lowe LP, Kuang A, et al. Maternal glucose levels during pregnancy and childhood adiposity in the hyperglycemia and adverse pregnancy outcome follow-up study. Diabetologia 2019;62:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Black MH, Sacks DA, Xiang AH, et al. Clinical outcomes of pregnancies complicated by mild gestational diabetes mellitus differ by combinations of abnormal oral glucose tolerance test values. Diabetes Care 2010;33:2524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen KH, Chen IC, Yang YC, et al. The trends and associated factors of preterm deliveries from 2001 to 2011 in Taiwan. Medicine (Baltimore) 2019;98:e15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- [6].Klemetti M, Nuutila M, Tikkanen M, et al. Trends in maternal BMI, glycaemic control and perinatal outcome among type 1 diabetic pregnant women in 1989–2008. Diabetologia 2012;55:2327–34. [DOI] [PubMed] [Google Scholar]
- [7].Kong L, Nilsson IAK, Gissler M, et al. Associations of maternal diabetes and body mass index with offspring birth weight and prematurity. JAMA pediatrics 2019;173:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ramsay JE, Ferrell WR, Crawford L, et al. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 2002;87:4231–7. [DOI] [PubMed] [Google Scholar]
- [9].Jarvie E, Hauguel-de-Mouzon S, Nelson SM, et al. Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 2010;119:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schempf AH, Branum AM, Lukacs SL, et al. Maternal age and parity-associated risks of preterm birth: differences by race/ethnicity. Paediatr Perinat Epidemiol 2007;21:34–43. [DOI] [PubMed] [Google Scholar]
- [11].Schmidt L, Sobotka T, Bentzen JG, et al. Demographic and medical consequences of the postponement of parenthood. Hum Reprod Update 2012;18:29–43. [DOI] [PubMed] [Google Scholar]
- [12].Waldenstrom U, Cnattingius S, Vixner L, et al. Advanced maternal age increases the risk of very preterm birth, irrespective of parity: a population-based register study. BJOG 2017;124:1235–44. [DOI] [PubMed] [Google Scholar]
- [13].Lin SF, Kuo CF, Chiou MJ, et al. Maternal and fetal outcomes of pregnant women with type 1 diabetes, a national population study. Oncotarget 2017;8:80679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hedderson MM, Ferrara A, Sacks DA. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol 2003;102:850–6. [DOI] [PubMed] [Google Scholar]
- [15].Cnattingius S, Villamor E, Johansson S, et al. Maternal obesity and risk of preterm delivery. JAMA 2013;309:2362–70. [DOI] [PubMed] [Google Scholar]
- [16].Aly H, Hammad T, Nada A, et al. Maternal obesity, associated complications and risk of prematurity. J Perinatol 2010;30:447–51. [DOI] [PubMed] [Google Scholar]
- [17].Zhou Y, Li H, Zhang Y, et al. Association of maternal obesity in early pregnancy with adverse pregnancy outcomes: a Chinese prospective cohort analysis. Obesity (Silver Spring) 2019;27:1030–6. [DOI] [PubMed] [Google Scholar]
- [18].Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Han Z, Mulla S, Beyene J, et al. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol 2011;40:65–101. [DOI] [PubMed] [Google Scholar]
- [20].Leung TY, Leung TN, Sahota DS, et al. Trends in maternal obesity and associated risks of adverse pregnancy outcomes in a population of Chinese women. BJOG 2008;115:1529–37. [DOI] [PubMed] [Google Scholar]
- [21].Chi TY, Zhu HM, Zhang M. Risk factors associated with nonsteroidal anti-inflammatory drugs (NSAIDs)-induced gastrointestinal bleeding resulting on people over 60 years old in Beijing. Medicine (Baltimore) 2018;97:e0665. [DOI] [PMC free article] [PubMed] [Google Scholar]


