Abstract
The aim of this study is to evaluate the predictive value of carbohydrate antigen125 (CA125) and carcino embryonic antigen (CEA) expression and its guiding role of choosing chemotherapy regimen in post-operation patients with colorectal carcinoma.
The clinical data of all patients, including laboratory data and pathological data, were collected from the electronic medical records. Kaplan-Meier Log rank test, COX regression model and subgroup analyses were employed to assess the correlation between the expression of CA125 and CEA in patients with colorectal carcinoma and the survival, and the effect on chemotherapy efficacy.
Kaplan-Meier showed that CA125 expression is negatively related to the progression-free survival (PFS) of the post-operative patients, Median PFS was 1140 days in the patients with high expression, and Median PFS was 1387 days in the patients with low expression (χ2 = 4.715, P = .030); CEA expression is also negatively associated with the PFS of the post-operative patients, Median PFS was 1197 days in the patients with high expression, and Median PFS was 1424 days in the patients with low expression (χ2 = 4.992, P = .025). Subgroup analysis also showed that the patients with normal CA125 and CEA had better prognosis, median PFS was 1505 days, and the patients with CA125 and (or) CEA high expression had poor prognosis and median PFS was 1162 days (χ2 = 13.346, P = .001), and found that there was no statistical difference in patients with oxaliplatin plus capecitabine (XELOX) and oxaliplatin, 5-fluorouracil and Calcium folinate (FOLFOX) chemotherapy in patients with CA125 and CEA low expression. However, in these patients with CA125 or (and) CEA high expression, the median PFS of patients treated with XELOX was 1082 days, and the median PFS of patients treated with FOLFOX chemotherapy was 1335 (χ2 = 4.547, P = .033).
Expression of CA125 and CEA associated with the survival of patients, and have some guiding significance for chemotherapy in patients with colorectal cancer after operation; Compared with XELOX, FOLFOX chemotherapy is more effective for CA125 or (and) CEA high expression patients with colorectal carcinoma.
Keywords: carbohydrate antigen125, carcino embryonic antigen, colorectal carcinoma, progression-free survival
1. Introduction
Tumor markers refer to a class of substances produced by tumor cells and tissues in the process of tumorigenesis and progression or produced by normal tissue after stimulation of tumor. It plays an important role in tumor early diagnosis, tumor progression and monitoring therapeutic effect.[1] Carbohydrate antigen125 (CA125) and carcino embryonic antigen (CEA) are common clinical monitoring indexes about tumor, especially gastrointestinal tumors.[2–4] However, there is still a lack of more precise markers that can provide guidance for clinical chemotherapy.
Colorectal carcinoma is a health problem worldwide, and the morality and mortality are increased gradually in recent years,[5–8] which was also becoming the leading cause of death in the population.[9] For decades, translational medicine plays an important role in the treatment of malignant tumor and clinical research.[10] Therefore, some tumor related substances as biomarkers are used to diagnose early stage of cancers, assess tumor progression and efficacy of anti-carcinoma therapies.[9] Some drugs specifically targeting tumor markers have been used to treat the patients with colon carcinoma.[7,11,12] Some serum biomarkers, such as carbohydrate antigen 199 (CA199), CA125, and CEA, are associated with the prognosis of patients with carcinoma, and it is easy to detect in the clinical work. CA199 is a kind of tumor marker of digestive system cancer. The increase of CA199 is mainly seen in pancreatic cancer. Second, in cholangiocarcinoma and colorectal cancer, the level of CA199 will also increase.[13–15] CEA is mainly produced by gastrointestinal tumors. Its chemical component is glycoprotein. As an adhesion molecule, CEA promotes the aggregation and invasion of tumor cell. Elevated CEA levels often indicate tumor progression and recurrence. At present, it is mainly used as a marker of gastrointestinal tumors.[15] CA125 interacts with some receptors to inhibit the apoptosis of cancer cells and promote the implantation and metastasis of tumor cells.[16]
Research on biomarkers of tumors has a great progress in the last 2 decades. The value of potential diagnostic and therapeutic of biomarkers is also becoming more and more evident. In this study, the clinical data of 206 patients, including laboratory data and pathological data, were collected from the electronic medical records of the Second Hospital of Lanzhou University, and investigated the correlation between the survival and clinicopathological parameters in patients with colorectal carcinoma, and to explore serum tumor markers that can be used to guide clinical treatment.
2. Materials and methods
2.1. Patients
We enrolled 206 patients with colorectal carcinoma who underwent tumor resection at Lanzhou University Second Hospital (Gansu Province, China) between 2010 and 2015. The study was approved by the Ethics Committee of at the Lanzhou University Second Hospital, and the data were anonymously obtained and analyzed. Informed consents from participants were also waived due to the complete anonymity of the patients. The data of all patients, including laboratory data and pathological data, were collected from the electronic medical records of the Lanzhou University Second Hospital. This study was performed in accordance with the relevant guidelines and regulations, and conformed to the Declaration of Helsinki.[17] The inclusion criteria of patients included complete pathological and follow-up data, without long distance metastasis, no other chronic diseases, and without any treatments before the surgery. The exclusion criteria: patients who suffered from other tumor or other chronic diseases, or died from accidental death or other diseases, lack of pathological and follow-up data, long distance metastasis before the surgery. Ultimately, a total of 206 cases were eventually enrolled in this study, including 104 males and 102 females, aged 23 to 90 years, median age of 59 years.
2.2. Clinical data and follow-up
Clinical data were totally collected from 206 patients with colorectal carcinoma. The clinical data, including gender, age, results of electronic colonoscopy, pathological results, preoperative serum CA125, CEA, CA19-9, hemoglobin (HGB), lymphocyte and post-operative chemotherapy were collected from the electronic medical records of the Second Hospital of Lanzhou University. The follow-up period was from the operative date to the death date, the follow-up period was 5-years post-surgery, and no patients died during follow-up.
2.3. Statistical analysis
SPSS 22.0 statistical software was used to analyze all data. Kaplan-Meier survival estimates model were used to assess the correlation of the progression survival (PFS) and overall survival (OS) with gender, age, CEA, CA125, CA199, HGB, white blood cell, lymphatic metastasis, tumor node metastasis stage (TNM stage) and chemotherapy regimen. Finally, Subgroup analysis was used to assess the correlation between the expression of CEA and CA125 and chemotherapy. P < .05 was considered statistically significant.
3. Results
3.1. Figures of electronic colonoscopy and pathology from typical cases and Statistical results
Most of patients come to the hospital for bloody stool. For instance, a patient was detected by colonoscopy for hematochezia, and the result showed that ascending colon tumor, local necrosis, hemorrhage, ulceration (Fig. 1A). The result of pathological biopsy: Moderately differentiated adenocarcinoma (Fig. 1 B); Another case who suffered from hematochezia was detected by colonoscopy, the result showed that rectal malignant tumor (about 5 cm from anal verge), it is circumferential hyperplasia with ulceration and local hemorrhage (Fig. 1 C). The result of pathological biopsy: Poorly differentiated adenocarcinoma (Fig. 1 D). The correlations between the OS and age, gender, lymphatic metastasis, TNM stage, pathology grade, chemotherapy regimen, HGB, white blood cell, percentage of neutrophils, percentage of lymphocyte, the expression of CA125, CA199, and CEA in serum of patients with colorectal carcinoma before operation were analyzed by Log-rank test. Results showed that the expression of CA125 and CEA, Lymphatic metastasis, TNM stage, pathology grade, Chemotherapy regimen were significantly associated with the PFS, but it is not related to other clinical factors (Table 1, Fig. 2); and found that the expression of CA125 and CEA, Lymphatic metastasis, TNM stage, pathology grade, Chemotherapy regimen were significantly associated with the OS, but it is not related to other clinical factors (Table 2, Fig. 3).
Figure 1.
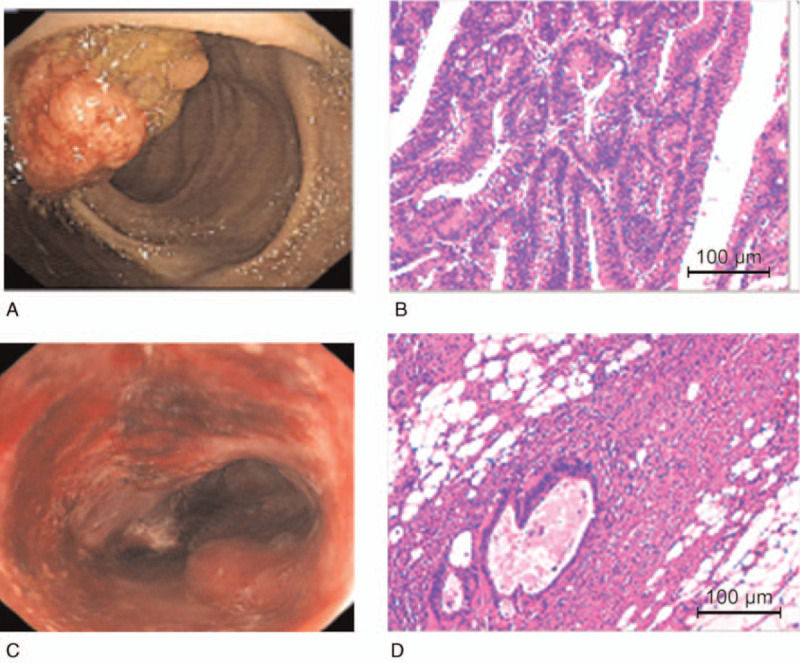
Figures of electronic colonoscopy (Fig. 1A 1Aand C) and pathology (Fig. 1B and D) from typical cases.
Table 1.
The correlation between clinical data and PFS of patients with colorectal carcinoma were analyzed by Log-rank test.
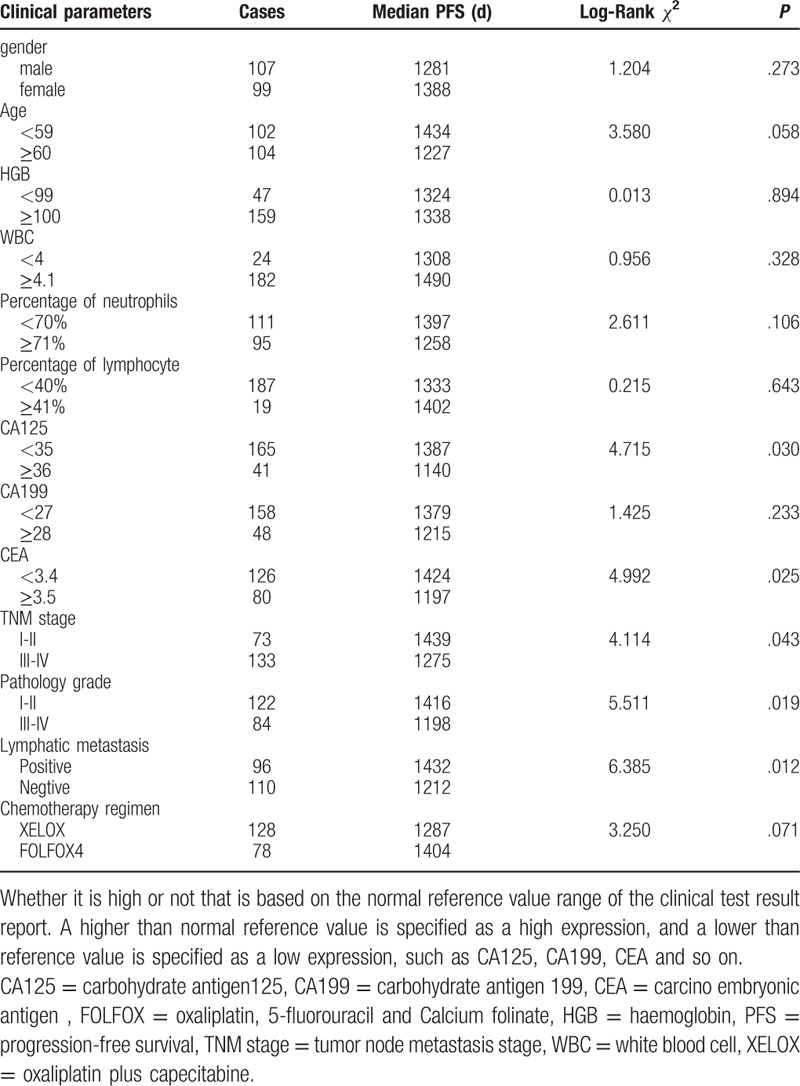
Figure 2.
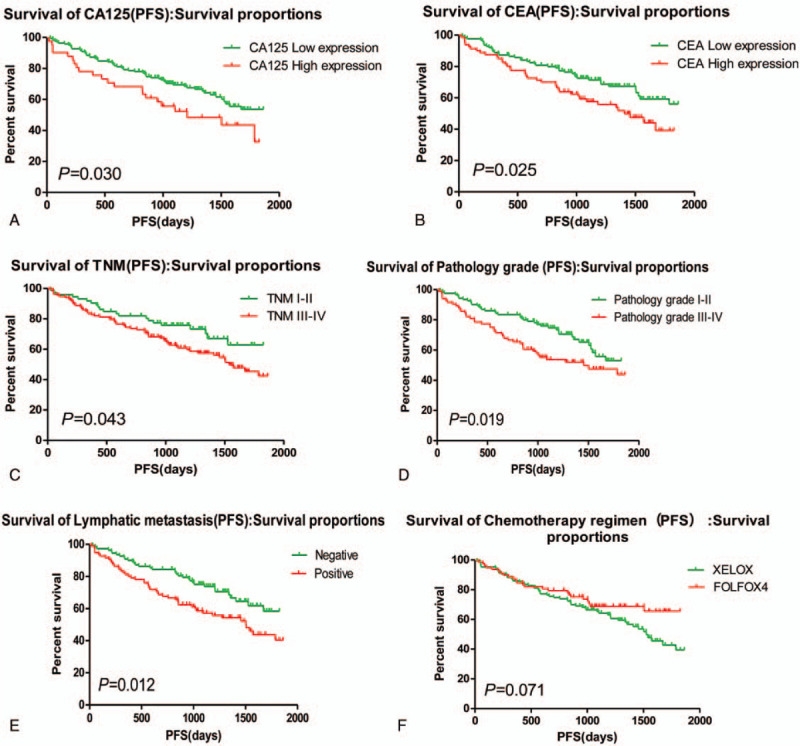
Clinical factors affecting the PFS of patients. PFS = progression-free survival.
Table 2.
The correlation between clinical data and OS of patients with colorectal carcinoma were analyzed by Log-rank test.
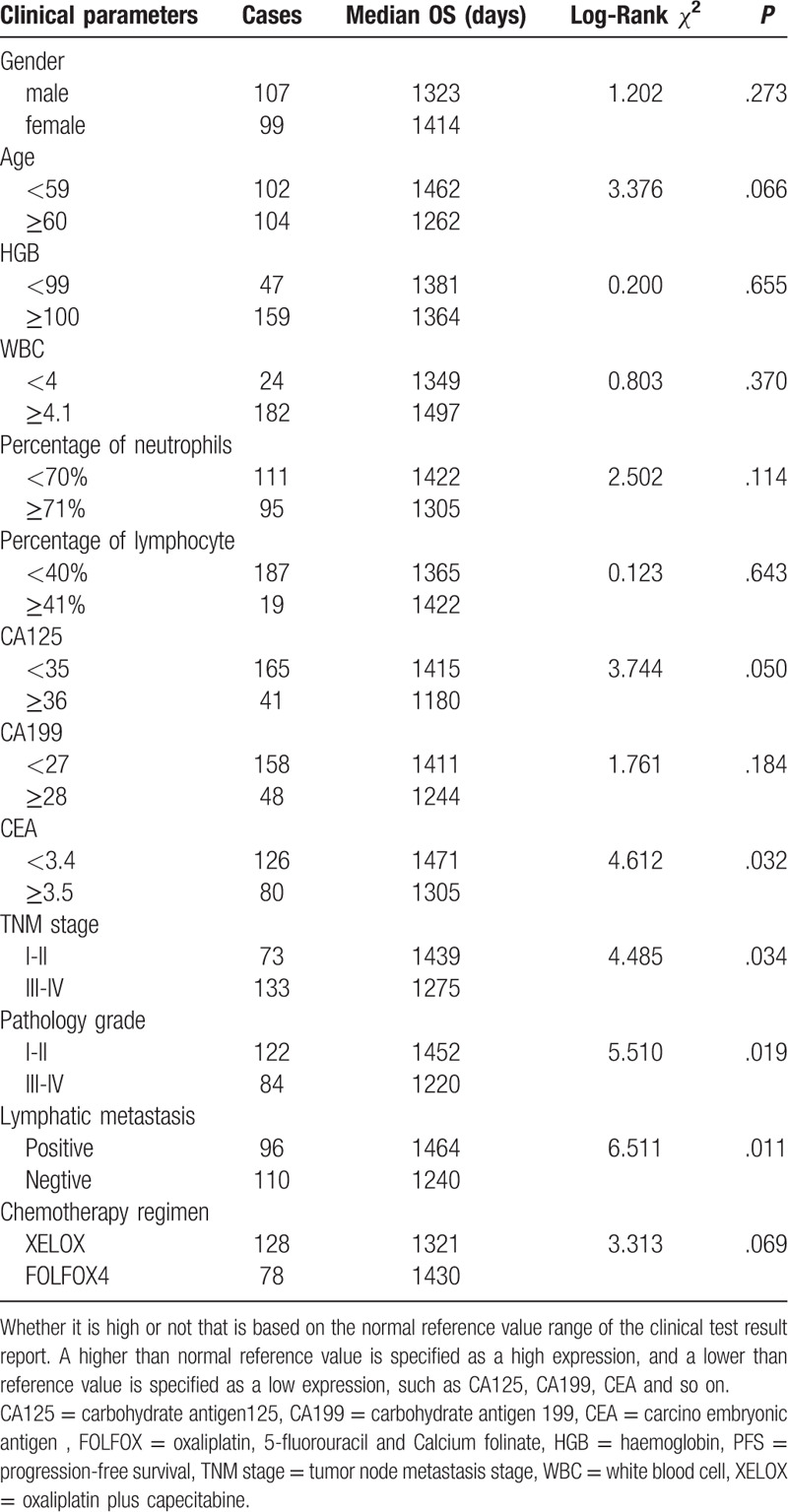
Figure 3.
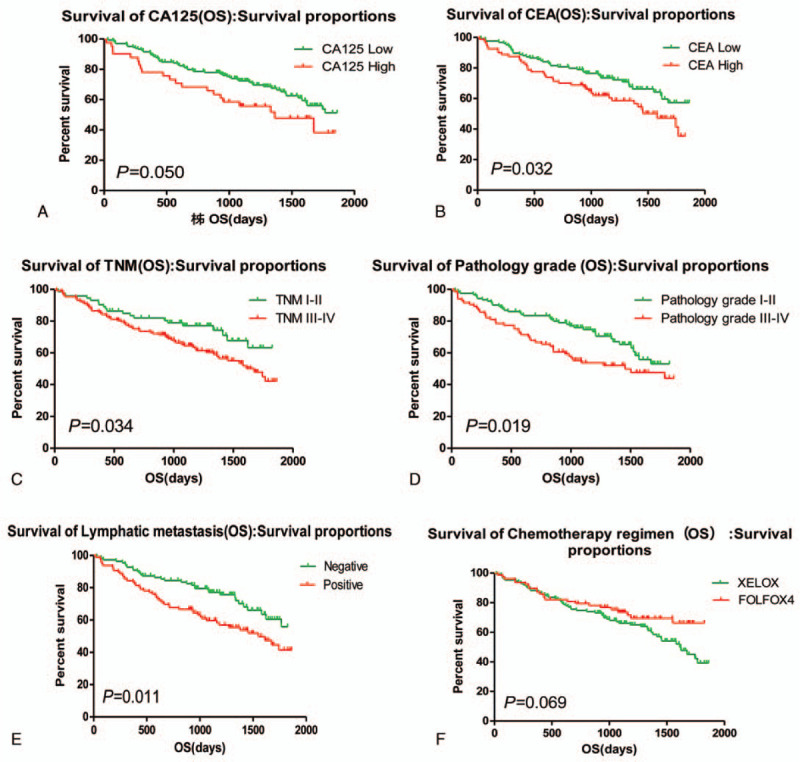
Clinical factors affecting the OS of patients. OS = overall survival.
3.2. Hierarchical analysis for the correlation between the PFS, OS, and chemotherapy regimen and expression of CA125 and CEA in serum of patients with colorectal carcinoma before operation
The statistical results showed that CA125 and or CEA expression was negatively related to the PFS of the post-operative patients, Median PFS was 1162 days in the patients with CA125 and or CEA high expression, and Median PFS was 1505 days in the patients with CA125 and CEA expression (χ2 = 13.346, P = .001); In patients with low expression of CA125 and CEA, comparing with patients treated with oxaliplatin plus capecitabine (XELOX) and FOLFOX4 chemotherapy, there was no statistical difference about the PFS of patients with colorectal carcinoma. Interestingly, the Median PFS was 1459 days in the patients treated with XELOX, the Median PFS was 1520 days in the patients treated with FOLFOX4 (χ2 = 0.038, P = .845). However, In patients with high expression of CA125 and or CEA, comparing with patients treated with XELOX and FOLFOX4 chemotherapy, the Median PFS was 1082 days in the patients treated with XELOX, the Median PFS was 1335 days in the patients treated with FOLFOX4 (χ2 = 4.547, P = .033), the PFS of patients with colorectal carcinoma was statistical difference. It was also found that patients with TNM III-IV were better treated with FOLFOX4 regimen. (Table 3, Fig. 4). Moreover, in patients with low expression of CA125 and CEA, comparing with patients treated with XELOX and FOLFOX4 chemotherapy, there was no statistical difference about the OS of patients with colorectal carcinoma. In patients with high expression of CA125 and CEA, the Median OS was 1457 days in the patients treated with XELOX, the Median PFS was 1531 days in the patients treated with FOLFOX4 (χ2 = 0.020, P = .889). In patients with high expression of CA125 and or CEA, comparing with patients treated with XELOX and FOLFOX4 chemotherapy, the Median PFS was 1140 days in the patients treated with XELOX, the Median PFS was 1372 days in the patients treated with FOLFOX4 (χ2 = 4.349, P = .037), the PFS of patients with colorectal carcinoma was statistical difference. It was also found that patients with TNM III-IV were better treated with FOLFOX4 regimen. (Table 4, Fig. 5).
Table 3.
The correlation between the PFS and the expression of CA125 and CEA in the patients with different chemotherapy regimen.
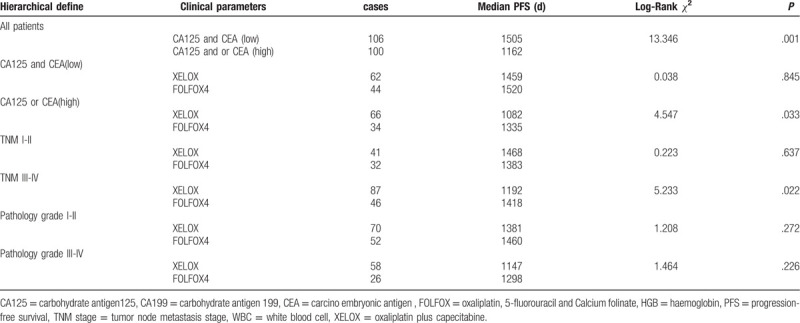
Figure 4.
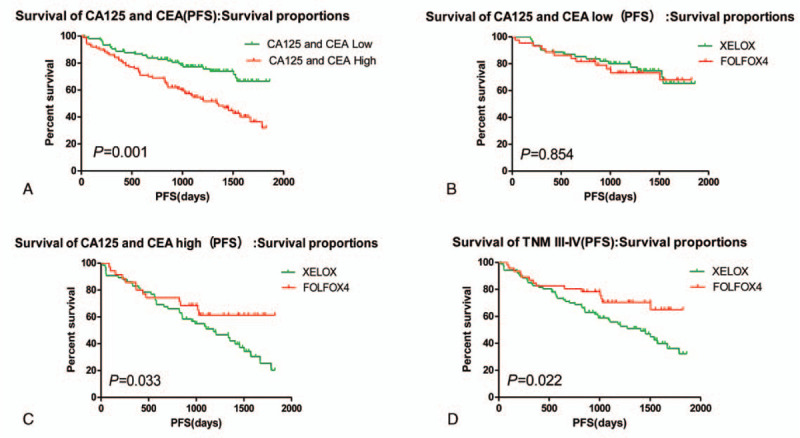
Subgroup analysis: The effect of CA125 and CEA expression on chemotherapy efficacy. CA125 = Carbohydrate antigen125, CEA = carcino embryonic antigen.
Table 4.
The correlation between the OS and the expression of CA125 and CEA in the patients with different chemotherapy regimen.
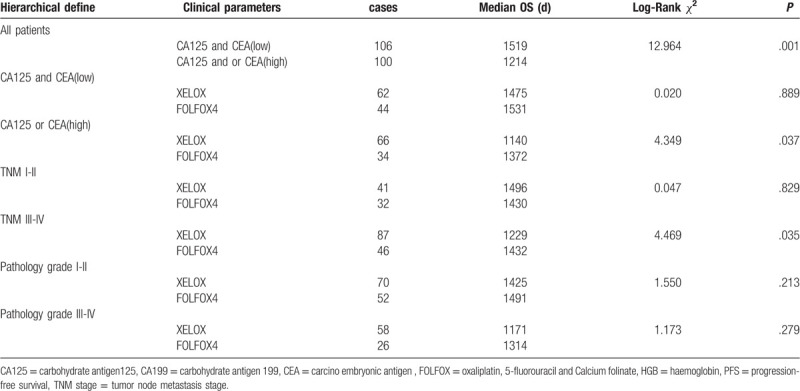
Figure 5.
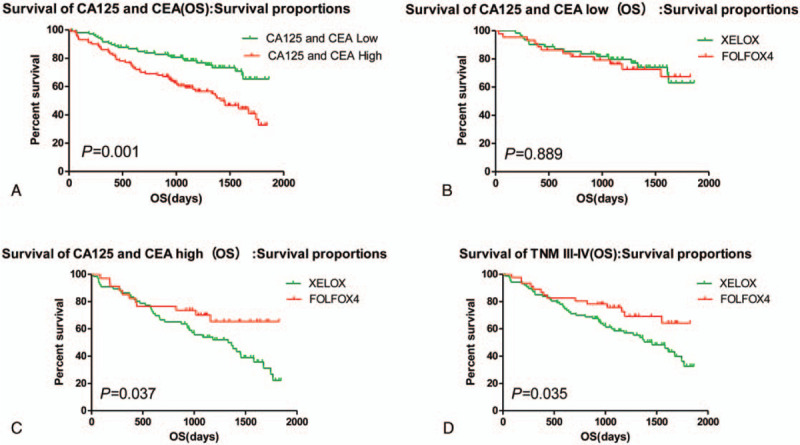
Subgroup analysis: The effect of CA125 and CEA expression on chemotherapy efficacy. CA125 = Carbohydrate antigen125, CEA = carcino embryonic antigen.
3.3. The results of Cox regression
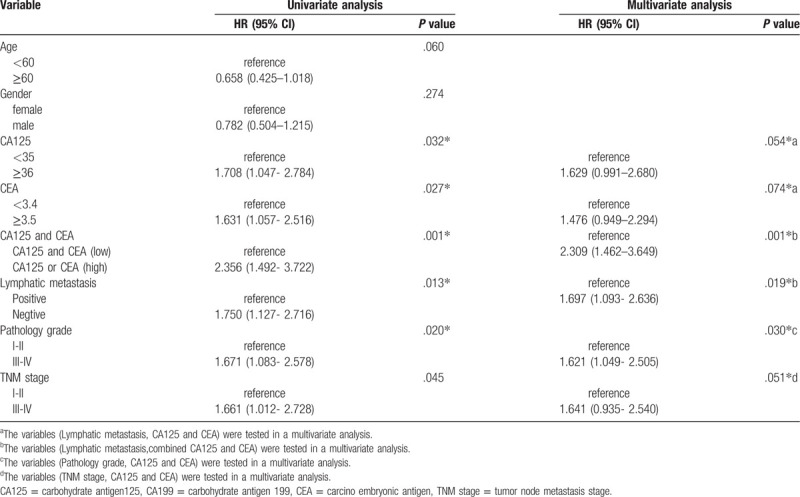
Cox regression is used to analyze the factors that affect the risk of tumor progression and evaluate whether they are independent prognostic factors. Univariate analysis revealed that comparing with the patients with CA125 low, patients with CA125 high had higher tumor progression risk (HR = 1.708, 95% CI: 1.047- 2.784, P = .32) and comparing with the patients with CEA low, patients with CEA high had higher tumor progression risk (HR = 1.631, 95% CI: 1.057- 2.516, P = .27). The result also showed that comparing with the patients with CA125 and CEA low, patients with CA125 and or CEA high had higher tumor progression risk (HR = 2.356, 95% CI: 1.492–3.722, P = .001); patients with negative lymphatic metastasis vs patients with and positive lymphatic metastasis, the latter is at higher risk of tumor progression (HR = 1.750, 95% CI: 1.127–2.716, P = .013), patients with pathology grade I-II colorectal carcinoma vs patients with pathology grade III-IV colorectal carcinoma, the latter is at higher risk of tumor progression (HR = 1.671, 95% CI: 1.083–2.578, P = .020), patients with TNM I-II colorectal carcinoma vs patients with TNM III-IV colorectal carcinoma, the latter is at higher risk of tumor progression (HR = 1.661, 95% CI: 1.012- 2.728, P = .045); Multivariate analysis showed that CA125 and CEA are not an independent prognostic factor (HR = 1.629, 95% CI: 0.991–2.680, P = .54 for CA125; HR = 1.476, 95% CI: 0.949–2.294, P = .74 for CEA), When CA125 and CEA are combined, their predictive effect is enhanced, and it is an independent prognostic factor (HR = 2.309, 95% CI: 1.462–3.649, P = .001). and also found that Lymphatic metastasis, TNM stage and pathology grade are an independent prognostic factors (HR = 1.697, 95% CI: 1.093- 2.636, P = .19 for Lymphatic metastasis; HR = 1.621, 95% CI: 1.049- 2.505, P = .30 for pathology grade; HR = 1.641, 95% CI: 0.935- 2.540, P = .51 for TNM stage). (Table 5).
Table 5.
The results of Cox regression.
4. Discussion
At present, the treatment of colorectal cancer has made some progress. However, the mortality of patients with colorectal carcinoma is still high. Therefore, it is urgent to optimize the current treatment methods to reduce the mortality of patients with colorectal carcinoma. Research on tumor markers is also a means to improve the efficiency of tumor diagnosis and treatment in clinical work.[18] The application of molecular markers means that tumor prognosis assessment is no longer a limit to clinical pathological parameters, research on biomarkers has main 2 parts, including the prognosis of patient with carcinoma and guiding the treatment. The intervention of some tumor related markers could promote anti-tumor effects, this is so-called “targeted therapy” which provides a new method for tumor management.[9] Study showed that CA125 and CEA play an important role in the diagnosis and treatment of colorectal carcinoma.[19] The results of this study also showed that the expression of CA125and CEA had a negative correlation with the OS of patients with colorectal carcinoma.
Recently, CEA and CA125 are widely used in clinical laboratory, but they are mainly used to monitor the recurrence of tumors. There are few reports on the application of CEA and CA125 in clinical chemotherapy. The results of this study showed that the prognosis of patients with colorectal carcinoma was poor, especially these patients with high expression of CEA and CA125 in serum. In patients with normal CA125 and CEA, there was no significant difference in OS, whether it is A or B regimen. However, the median OS was shorter in patients receiving XELOX chemotherapy, and longer in patients receiving FOLFOX chemotherapy than in patients with CA125 or (and) CEA over-expression. The result of this study has some guiding significance for the prognosis evaluation and chemotherapy plan of postoperative patients with colorectal carcinoma.
Author contributions
Conceptualization: Jie Mao, Peng Du, Zhi-Bin Cheng.
Data curation: Peng Du, Han-teng Yang, Huan Hu,Shi-Yao Wang, Xia Wu.
Formal analysis: Jie Mao, Peng Du, Huan Hu, Shi-Yao Wang.
Funding acquisition: Jie Mao, Zhi-Bin Cheng.
Methodology: Peng Du, Han-teng Yang, Huan Hu, Shi-Yao Wang.
Writing – original draft: Peng Du.
Writing – review & editing: Jie Mao, Zhi-Bin Cheng.
Footnotes
Abbreviations: CA125 = carbohydrate antigen125, CA199 = carbohydrate antigen 199, CEA = carcino embryonic antigen , FOLFOX = oxaliplatin, 5-fluorouracil and Calcium folinate, HGB = hemoglobin, OS = overall survival, PFS = progression-free survival, TNM stage = tumor node metastasis stage, XELOX = oxaliplatin plus capecitabine.
How to cite this article: Mao J, Du P, Yang Ht, Hu H, Wang SY, Wu X, Cheng ZB. Prognostic value of carbohydrate antigen125 and carcino embryonic antigen expression in patients with colorectal carcinoma and its guiding significance for chemotherapy. Medicine. 2020;99:14(e19420).
JM and PD contributed equally to this work.
This work was supported by grants from Lanzhou Science and Technology Bureau Project (No. 2017-RC-64); Cuiying Scientific, Technological Innovation Program of Lanzhou University Second Hospital (CY2017-BJ09); Project of Gansu Provincial Administration of Traditional Chinese Medicine(GZK-2019–47); The scientific research project of Gansu health industry (No. GSWST2013–03).
The authors have no conflicts of interest to disclose.
References
- [1].Katchman BA, Smith JT, Obahiagbon U, et al. Application of flat panel OLED display technology for the point-of-care detection of circulating cancer biomarkers. Sci Rep 2016;6: (29057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang XF, Wu YH, Wang MS, et al. CEA, AFP, CA125, CA153 and CA199 in malignant pleural effusions predict the cause. Asian Pac J Cancer Prev 2014;15:363–8. [DOI] [PubMed] [Google Scholar]
- [3].Li Y, Li DJ, Chen J, et al. Application of Joint Detection of AFP, CA19-9, CA125 and CEA in Identification and Diagnosis of Cholangiocarcinoma. Asian Pac J Cancer Prev 2015;16:3451–5. [DOI] [PubMed] [Google Scholar]
- [4].He CZ, Zhang KH, Li Q, et al. Combined use of AFP, CEA, CA125 and CAl9-9 improves the sensitivity for the diagnosis of gastric cancer. BMC Gastroenterol 2013;87:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shomaf M, Yousef AL, Ababna N, et al. Cyclooxygenase-2 (COX2) gene polymorphisms and the risk of sporadic colorectal cancer and polyps among Jordanian population. Turk J Gastroenterol 2015;26:154–8. [DOI] [PubMed] [Google Scholar]
- [6].Costabile V, Duraturo F, Delrio P, et al. Lithium chloride induces mesenchymaltoepithelial reverting transition in primary colon cancer cell cultures. Int J Oncol 2015;46:1913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu X, George GC, Tsimberidou AM, et al. Retreatment with anti-EGFR based therapies in metastatic colorectal cancer: impact of intervening time interval and prior anti-EGFR response. BMC Cancer 2015;15(713.): [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lee HS, Soh JS, Lee S, et al. Clinical features and prognosis of resectable primary colorectal signet-ring cell carcinoma. Intest Res 2015;13:332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Guo H, Zhou X, Lu Y, et al. Translational progress on tumor biomarkers. Thorac Cancer 2015;6:665–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Morrow GR, Bellg AJ. Behavioral science in translational research and cancer control. Cancer 1994;74: 4 Suppl: 1409–17. [DOI] [PubMed] [Google Scholar]
- [11].Normanno N, Pinto C, Castiglione F, et al. The Italian external quality assessment for RAS testing in colorectal carcinoma identifies methods-related inter-laboratory differences. J Transl Med 2015;13:287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kraus S, Hummler S, Toriola AT, et al. Impact of genetic polymorphisms on adenoma recurrence and toxicity in a COX2 inhibitor (celecoxib) trial: results from a pilot study. Pharmacogenet Genomics 2013;23:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ge L, Pan B, Song F, et al. Comparing the diagnostic accuracy of five common tumour biomarkers and CA19-9 for pancreatic cancer: a protocol for a network meta-analysis of diagnostic test accuracy. BMJ Open 2017;7:e018175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bergquist JR, Ivanics T, Storlie CB, et al. Implications of CA19-9 elevation for survival, staging, and treatment sequencing in intrahepatic cholangiocarcinoma: a national cohort analysis. J Surg Oncol 2016;114:475–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Stojkovic Lalosevic M, Stankovic S, Stojkovic M, et al. Can preoperative CEA and CA19-9 serum concentrations suggest metastatic disease in colorectal cancer patients? Hell J Nucl Med 2017;20:41–5. [DOI] [PubMed] [Google Scholar]
- [16].Sherbet GV, Patil D. Genetic abnormalities of cell proliferation, invasion and metastasis, with special reference to gynaecological cancers[J]. Anticancer Res 2003;23(2B):1357–71. [PubMed] [Google Scholar]
- [17].General Assembly of the World Medical A. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent 2014;81:14–18. [PubMed] [Google Scholar]
- [18].Turkington RC, Coyle VM, Johnston PG, et al. Predictive and prognostic markers in colorectal cancer[J]. Per Med 2007;4:295–306. [DOI] [PubMed] [Google Scholar]
- [19].Gao Y, Wang J, Zhou Y, et al. Evaluation of Serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep 2018;8:2732. [DOI] [PMC free article] [PubMed] [Google Scholar]


