Abstract
Activity-dependent alterations in the levels of synaptic AMPA receptors (AMPARs) within the postsynaptic density (PSD) is thought to represent a cellular mechanism for learning and memory. Palmitoylation regulates localization and function of many synaptic proteins including AMPARs, auxiliary factors and synaptic scaffolds in an activity-dependent manner. We identified the synapse differentiation induced gene (SynDIG) family of four genes (SynDIG1-4) encoding brain-specific transmembrane proteins that associate with AMPARs and regulate synapse strength. SynDIG1 is palmitoylated at two cysteine residues located at positions 191 and 192 in the juxta-transmembrane region important for activity-dependent excitatory synapse development. Here, we describe an innovative biochemical approach, the acyl-PEGyl exchange gel shift (APEGS) assay, to investigate the palmitoylation state of any protein of interest and demonstrate its utility with the SynDIG family of proteins in mouse brain lysates.
Keywords: Biochemistry, Issue 157, brain, synapse, palmitoylation, AMPA receptor, SynDIG1, SynDIG4, Prrt1, Prrt2, APEGS assay
Introduction
S-palmitoylation is a reversible post-translational modification of target proteins that regulates stable membrane association, protein trafficking, and protein-protein interactions1. It involves addition of a 16-carbon palmitate moiety to cysteine residues via thioester linkage catalyzed by palmitoyl acyltransferase (PAT) enzymes. Many synaptic proteins in the brain are palmitoylated, including AMPA-Rs and PSD-95, in an activity-dependent manner to regulate stability, localization, and function2,3,4. Alterations in the levels of synaptic AMPARs in the PSD via interaction of auxiliary factors with synaptic scaffolds such as PSD-95 underlies synaptic plasticity; thus, methods to determine the palmitoylation state of synaptic proteins provides important insight into mechanisms of synaptic plasticity.
Previously, we identified the SynDIG family of four genes (SynDIG1-4) encoding brain-specific transmembrane proteins that associate with AMPARs5. Overexpression or knock-down of SynDIG1 in dissociated rat hippocampal neurons increases or decreases, respectively, AMPA-R synapse size and number by ~50% as detected using immunocytochemistry and electrophysiology5. We utilized the acyl-biotin exchange (ABE) assay to demonstrate that SynDIG1 is palmitoylated at two conserved juxta-transmembrane Cys residues (found in all SynDIG proteins) in an activity-dependent manner to regulate stability, localization, and function6. The ABE assay relies on exchange of biotin on cysteines protected by modification and subsequent affinity purification7. Here, we describe an innovative biochemical approach, the acyl-PEGyl exchange gel shift (APEGS) assay8,9,10,11,12, which does not require affinity purification and instead utilizes changes in gel mobility to determine the number of modifications for a protein of interest. The protocol is described for investigation of endogenous membrane proteins from mouse brain for which suitable antibodies are available.
Protocol
All animal procedures followed guidelines set forth by the National Institutes of Health (NIH) and have been approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
1. Preparation of mouse brain membranes
-
1
Decapitate mouse using a guillotine apparatus and dissect brain out rapidly (in <1 min, if possible, to minimize palmitoylation changes that may occur during dissection procedure). Homogenize immediately in 10 mL of homogenization buffer (Table 1) in a glass homogenizer (~12 strokes) on ice.
-
2
Centrifuge lysates for 15 min at 1,400 x g at 4 °C. Transfer the supernatant to a new tube on ice. Resuspend the pellet (~6 strokes) in an equal volume of homogenization buffer and centrifuge at 710 x g for 10 min at 4 °C.
-
3
Combine the supernatants from step 1.2 and centrifuge at 40,000 x g for 20 min at 4 °C.
-
4
Discard the supernatant (cytosolic fraction) and resuspend the pelleted membrane (P2) fraction in homogenization buffer.
Table 1: Solutions used for APEGS assay.
Homogenization buffer and buffer A can be prepared ahead of time; however, add protease inhibitors and phenylmethylsulfonyl fluoride (PMSF) immediately before use. All other solutions should be prepared fresh.
| Buffer | Components | Working conc. | Comments |
|---|---|---|---|
| Homogenization | 0.32 M sucrose, 1 mM Tris pH 7.2, 1 mM MgCl2 | Add PMSF and protease inhibitors immediately before use. | |
| Buffer A | PBS pH 7.2, 5 mM EDTA, 4% SDS (w/v) | Add PMSF and protease inhibitors immediately before use. | |
| TCEP solution | 500 mM TCEP in ddH2O (pH 7.2) | 25 mM | Add 10 N NaOH to increase pH. |
| NEM solution | 2.0 M NEM in 100% ethanol | 50 mM | Prepare fresh. |
| Buffer H (HAM+) | 1.33 M HAM, pH 7.0, 5 mM EDTA, 0.2% Triton X-100 (w/v) | 1.0 M | Prepare fresh. |
| Buffer T (HAM−) | 1.33 M Tris-HCl, pH 7.0, 5 mM EDTA, 0.2% Triton X-100 (w/v) | ||
| mPEG-5k | 100 mM mPEG-5k in ddH2O | 20 mM | Mix well. Highly viscous. |
NOTE: Samples can be flash frozen and stored at −80 °C for later use.
-
5
Perform a bicinchoninic acid (BCA) assay to quantitate protein levels. For the APEGS assay, begin with ~100–200 mg protein (maximum 250 mg) in a volume of 470 μL of buffer A (Table 1) in a 1.5 mL tube. Sonicate and centrifuge at >13,000 x g for 10 min at 25 °C to remove insoluble protein. Transfer solubilized protein to a new 1.5 mL tube.
2. Acyl-PEGyl exchange gel-shift (APEGS) assay
-
1Disrupt disulfide bonds and block free cysteines.
NOTE: The reaction can be extended overnight.
-
2Perform chloroform methanol (CM) precipitation.
- Transfer the protein solution from 1.5 mL tube to a polypropylene or glass tube that can be centrifuged in a swinging bucket rotor at modest speed. A 5 mL tube is ideal. Add four volumes (2 mL) of methanol (MeOH) and vortex briefly.
- Add two volumes (1 mL) of chloroform and vortex briefly.
- Add three volumes (1.5 mL) of dH2O and vortex briefly.
- Centrifuge the samples at 3,000 x g for 30 min at 25 °C in a swinging bucket rotor.
- Carefully remove and discard the upper phase.
- Add 3 volumes (1.5 mL) of MeOH and mix gently but thoroughly, being careful not to fragment the opaque protein pancake.
- Centrifuge at 3,000 x g for 10 min at 25 °C in a swinging bucket rotor.
- Using a glass serological pipet, remove as much of the top phase as possible without disturbing the protein pancake.
- Carefully rinse the pellet with 1 mL of MeOH.
- Air dry the pellet for at least 10 min.
NOTE: Dried pellet can be stored at −20 °C.
-
3Perform cleavage of palmitoyl-thioester linkages with hydroxylamine (NH2OH, HAM).
- Resuspend protein precipitate in 125 μL of buffer A and sonicate briefly. Centrifuge at >13,000 x g for 10 min at 25 °C to remove insoluble material. Transfer solubilized protein to a new 1.5 mL tube.
-
4
Repeat the CM precipitation described in step 2.2.
NOTE: Dried pellet can be stored at −20 °C.
-
5Add mPEG to unprotected cysteines.
-
1Resuspend pellet in 100 μL of buffer A containing 10 mM TCEP. Transfer to a 1.5 mL tube.
-
1
NOTE: It is normal for significant loss of protein to have occurred during CM precipitation steps. All subsequent steps will be performed in a 1.5 mL tube, constraining the volume of protein to a maximum of ~120–130 μL. This will reduce protein loss during the final CM precipitation.
-
2
Add 20 mM mPEG-5k (25 μL, added from a stock solution; Table 1) to protein sample and mix by pipetting. Incubate for 60 min at 25 °C with end-over-end rotation.
-
3
To remove unincorporated mPEG-5k, perform CM precipitation as described in step 2.2 using these adjusted volumes: 4 volumes of MeOH (500 μL), 2 volumes of chloroform (250 μL), and 3 volumes of dH20 (375 μL). Centrifuge at >13,000 x g for 10 min at 25 °C.
-
4
Carefully remove the upper phase as before, avoiding the thick, flocculent pancake. Add 1 mL of MeOH and mix gently but thoroughly. Centrifuge at >13,000 x g for 10 min at 25 °C.
-
5
Carefully remove the supernatant and rinse the pellet with 1 mL of MeOH. Centrifuge at >13,000 x g for 10 min at 25 °C. Air dry pellet.
NOTE: Dried pellet can be stored at −20 °C.
-
6
Resuspend the dried pellet in 50 μL of buffer A (without TCEP or any reducing agent). Reserve 5 μL for BCA protein quantitation. After quantitation, add an appropriate amount of 4x Laemmli sample buffer and, depending on the protein of interest, potentially heat the sample to 70–100 °C for 10 min.
NOTE: Boiling may cause aggregation of some membrane proteins.
3. Western blot analysis of PEGylated proteins
-
1
Load the sample onto an sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel13.
NOTE: In this protocol 10% polyacrylamide was used.
Representative Results
Immunoblotting with antibodies against the protein of interest reveals the palmitoylated state (non, singly, doubly, etc.) in mouse brain lysates as determined by mobility shift compared with samples in which HAM was not included. Previously, we had demonstrated that SynDIG1 was palmitoylated at two sites using the ABE assay6; however, we could not determine whether both sites were modified in brain tissue. Here we show that SynDIG1, SynDIG4/Prrt1, and Prrt2 are palmitoylated in mouse brain and that they have two potential palmitoylation sites (Figure 1). Interestingly, each protein has a different palmitoylation state in mouse brain based on the signal for the non, singly and doubly palmitoylated protein. Densitometry analysis of blots provides quantitative information about the palmitoylated state.
Figure 1: Detection of PEGylated protein species in mouse membrane preparations.
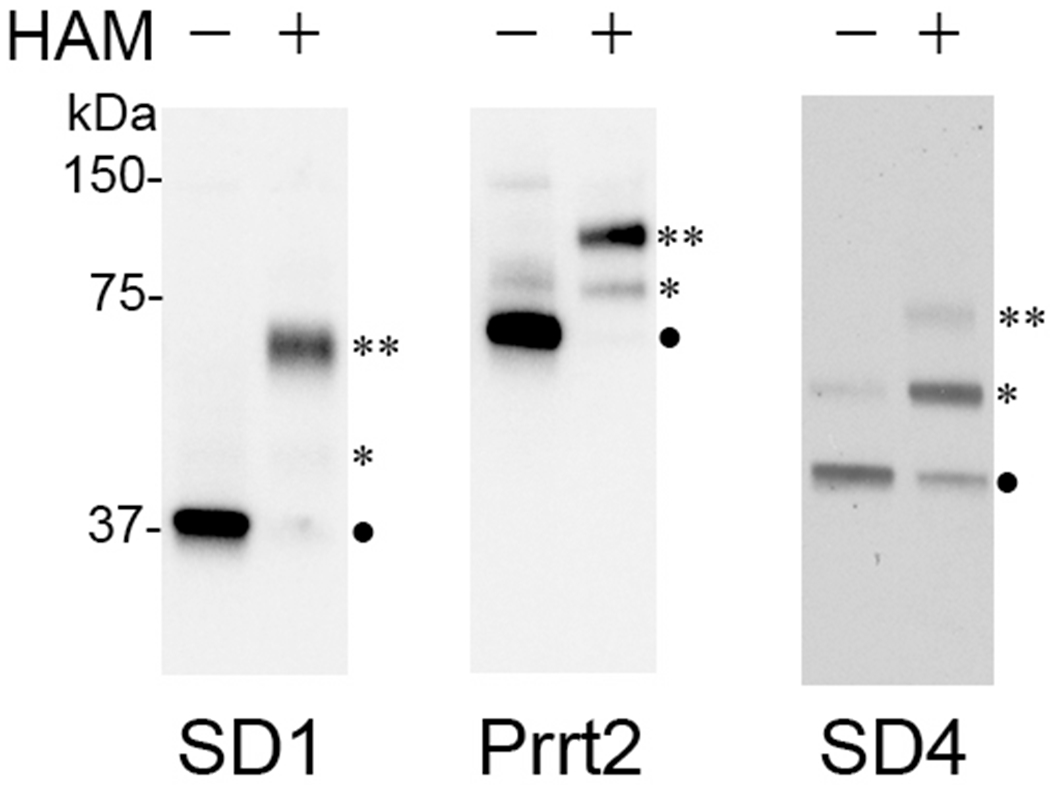
Mouse brains were dissected rapidly and homogenized immediately. P2 membrane fractions were subjected to the APEGs assay as described in this protocol. In this experiment, postnatal day 18 (P18) were separated using a 10% SDS-PAGE gel and immunoblotted and probed with antibodies against SynDIG1 (SD1), Prrt2, and SynDIG4 (SD4). Symbols indicate protein that is not palmitoylated (•), palmitoylated singly (*), or doubly (**). The presence of faint bands in the HAM (−) conditions indicates either an incomplete reduction of disulfide bonds or incomplete blockage of free cysteines by NEM.
Discussion
In our previous work, we utilized the ABE assay to demonstrate that SynDIG1 is palmitoylated at two conserved juxta-transmembrane Cys residues (found in all SynDIG proteins) in an activity-dependent manner to regulate stability, localization, and function6. A limitation is that the ABE assay requires affinity purification with agarose resins conjugated to avidin moieties as the final step in the procedure, resulting in significant loss of signal that complicate quantitative analysis. Furthermore, the ABE assay cannot distinguish if a protein is palmitoylated once or at multiple sites.
Here we use the APEGS assay8,9,10,11,12 to determine the palmitoylation state of the AMPAR auxiliary transmembrane proteins SynDIG1 and the related proteins SynDIG4 (also known as Prrt1) and Prrt2 from mouse brain extracts; however, the assay can be applied to any membrane protein for which a high-quality and specific antibody suitable for western blotting is available. Use of brain membranes from wild type (WT) and knockout (KO) mice provides an ideal control for antibody specificity as we have demonstrated for antibodies against SynDIG115 and SynDIG416.
Performing the APEGS assay with brain lysates from WT and KO mice provides an important control for antibody specificity in this method. This method could also be applied to different membrane fractions obtained via differential centrifugation followed by the APEGS assay to investigate the subcellular localization of the protein of interest.
One critical step of the APEGS assay is CM precipitations, the purpose of which is to remove chemicals (NEM and HAM) from interfering with subsequent downstream reactions. Although it is possible to perform CM precipitations in a 1.5 mL tube, the use of larger tubes and volumes should produce more optimal results. At the same time, in part because of the multiple CM precipitations, one should expect a substantial loss of protein from beginning to end. This might result in apparent variability in total protein levels in the minus and plus HAM conditions.
As illustrated in Figure 1, the addition of mPEG-5k (5 kDa) does not necessarily produce a linear shift on an immunoblot. This can have advantages in resolving proteins which are palmitoylated at multiple sites. Furthermore, the presence of bands at mobility shift locations in the HAM negative conditions indicates either an incomplete reduction of disulfide bonds or incomplete blockage of free cysteines by NEM. Alternatively, apparent shifted bands in the HAM negative condition might indicate that free cysteines within a particular protein are difficult to block. Thus, additional optimization of the blocking step might be required if only a small percentage of total protein is palmitoylated in contrast to SynDIG1, SynDIG4, and Prrt2 in which the majority of the protein is either singly or doubly palmitoylated (Figure 1).
This method is not limited to mouse brain lysates. For example, this method has been used to screen for depalmitoylating enzymes for PSD-95 expressed in heterologous cells9. Expression of proteins in heterologous cells also allows for determination of the exact site of modification via site-directed mutagenesis. This method also informed the role of the synaptic scaffold AKAP150 in synaptic plasticity17,18, demonstrating a broad application in neuroscience.
It is important to note that the APEGS assay is not exclusive to 16-carbon palmitate moieties as the assay will detect other long chain fatty acyl linkages that are subject to cleavage and replacement by mPEG. To establish palmitate modification conclusively requires additional experimentation such as metabolic labeling with tritiated palmitate.
Acknowledgments
The authors thank K. Woolfrey for advice and input on the APEGS assay. These studies were funded by research grants to E.D. from the Whitehall Foundation and the NIH-NIMH (1R01MH119347).
Footnotes
The video component of this article can be found at https://www.jove.com/video/61018/
Disclosures
The authors have nothing to disclose.
References
- 1.Blaskovic S, Blanc M, van der Goot FG What does S-palmitoylation do to membrane proteins? FEBS Journal. 280, 2766–2774 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Fukata Y, Fukata M Protein palmitoylation in neuronal development and synaptic plasticity. Nature Reviews Neuroscience. 11, 161–175 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Globa AK, Bamji SX Protein palmitoylation in the development and plasticity of neuronal connections. Current Opinion in Neurobiology. 45, 210–220 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Thomas GM, Huganir RL Palmitoylation-dependent regulation of glutamate receptors and their PDZ domain-containing partners. Biochemical Society Transactions. 41, 72–78 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalashnikova E et al. SynDIG1: an activity-regulated, AMPA- receptor-interacting transmembrane protein that regulates excitatory synapse development. Neuron. 65, 80–93 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur I et al. Activity-Dependent Palmitoylation Controls SynDIG1 Stability, Localization, and Function. Journal of Neuroscience. 36, 7562–7568 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan J, Roth AF, Bailey AO, Davis NG Palmitoylated proteins: purification and identification. Nature Protocols. 2, 1573–1584 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Howie J et al. Substrate recognition by the cell surface palmitoyl transferase DHHC5. Proceedings of the National Academy of Sciences of the United States of America. 111, 17534–17539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanadome T, Yokoi N, Fukata Y, Fukata M Systematic Screening of Depalmitoylating Enzymes and Evaluation of Their Activities by the Acyl-PEGyl Exchange Gel-Shift (APEGS) Assay. Methods in Molecular Biology. 2009, 83–98 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Percher A et al. Mass-tag labeling reveals site-specific and endogenous levels of protein S-fatty acylation. Proceedings of the National Academy of Sciences of the United States of America. 113, 4302–4307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Percher A, Thinon E, Hang H Mass-Tag Labeling Using Acyl-PEG Exchange for the Determination of Endogenous Protein S-Fatty Acylation. Current Protocols in Protein Science. 89, 14.17.1–14.17.11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoi N et al. Identification of PSD-95 Depalmitoylating Enzymes. Journal of Neuroscience. 36, 6431–6444 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.JoVE Science Education Database. Separating Protein with SDS-PAGE Basic Methods in Cellular and Molecular Biology. JoVE, Cambridge, MA, (2019). [Google Scholar]
- 14.JoVE Science Education Database. The Western Blot Basic Methods in Cellular and Molecular Biology. JoVE, Cambridge, MA, (2019). [Google Scholar]
- 15.Chenaux G et al. Loss of SynDIG1 Reduces Excitatory Synapse Maturation But Not Formation In Vivo. eNeuro. 3 (5), ENEURO.0130-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matt L et al. SynDIG4/Prrt1 Is Required for Excitatory Synapse Development and Plasticity Underlying Cognitive Function. Cell Reports. 22, 2246–2253 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purkey AM et al. AKAP150 Palmitoylation Regulates Synaptic Incorporation of Ca(2+)-Permeable AMPA Receptors to Control LTP. Cell Reports. 25, 974–987. e4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woolfrey KM, Sanderson JL, Dell’Acqua ML The palmitoyl acyltransferase DHHC2 regulates recycling endosome exocytosis and synaptic potentiation through palmitoylation of AKAP79/150. Journal of Neuroscience. 35, 442–456 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


