Supplemental Digital Content is available in the text
Keywords: bacteremia, cancer, Staphylococcus aureus
Abstract
Susceptibility to infectious disease may be a marker of immunodeficiency caused by unrecognized cancer. To test the hypothesis, the risk of incident primary cancer was estimated among survivors of Staphylococcus aureus bacteremia (SAB) and compared to a random population cohort.
Nation-wide population-based matched cohort study. Cases of SAB were identified from a national database and incident primary cancers were ascertained by record linkage. Incidence rate (IR) and ratio (IRR) with 95% confidence interval (CI) of 27 cancers was calculated by Poisson regression.
During the first year of follow-up, 165 and 943 incident cases of cancer occurred in the case cohort (n = 12,918 (1.3%)) and the population cohort (n = 117,465 (0.8%)) for an IR of 3.78 (3.22–4.40) and 2.28 (2.14–2.43) per 100,000 person-years. The IRR was 1.65 (1.40–1.95). Of 27 cancers, 7 cancers occurred more frequently amongst cases than controls: cervical cancer (IRR 37.83 (4.23–338.47)), multiple myeloma (IRR 6.31 (2.58–15.44)), leukemia (IRR 4.73 (2.21–10.10)), sarcoma (IRR 4.73 (1.18–18.91)), liver cancer (IRR 3.64 (1.30–10.21)), pancreatic cancer (IRR 2.8 (1.27–6.16)), and urinary tract cancer (IRR 2.58 (1.23–5.39)). Compared to the control population, the risk of cancer was higher for those without comorbidity and with younger age. The overall risk of cancer during 2 to 5 years of follow-up was not increased (IRR 0.99 (95% CI: 0.89–1.11). However, the risk of pharyngeal cancer was increased (IRR 1.88 (1.04–3.39)) and the risk of liver cancer remained increased (IRR 3.93 (2.36–6.55)).
The risk of primary incident cancer was 65% higher in the SAB cohort compared to the population cohort during the first year of follow-up and included 7 specific cancers. The risk was higher for those without comorbidity and with younger age. Screening for these specific cancers in selected populations may allow for earlier detection.
1. Introduction
More than 1 in 3 persons will be diagnosed with cancer during a lifetime and cancer ranks as 1 of the 2 most frequent causes of death in high-income countries.[1] Early detection is essential to improve outcome of cancer. Up to 20% of all cancers are associated with microbial coinfection, suggesting that immunity and inflammation play a role in cancer development.[2–5] The association of impaired immune function, microbial coinfection and cancer is well described in the context of HIV infection and organ transplantation.[6] Non-microbial–associated tumors, such as skin and thyroid cancer, have been associated with iatrogenic immunosuppression.[7]
Based on these observations, we hypothesized that a developing cancer may be preceded by an increased susceptibility to invasive bacterial infection due to impending immunodeficiency. In a recent study, we showed that individuals with Staphylococcus aureus bacteremia (SAB) were more likely to die from cancer than the general population during long-term follow-up, supporting the concept that SAB may be an early indicator of cancer.[8] To test our hypothesis, we used a nationwide matched cohort study to estimate rates of 27 types of cancer occurring after SAB and compared these to a population cohort without SAB.
2. Methods
2.1. Study design
A nation-wide population-based matched cohort study consisting of a cohort of survivors of SAB and a comparison cohort of random population control. Cases of SAB were identified from the Danish Staphylococcal Bacteremia Database[9] and incident primary cancers were ascertained by record linkage to the Danish Cancer Registry.[10,11] The case cohort comprised individuals at risk of incident cancer, i.e. alive, without a cancer diagnosis, and discharged after a first episode of SAB. The comparison cohort included up to 10 population controls that were randomly selected from the Civil Registration System matched to the corresponding case on age and sex. Controls had to be alive and without a cancer diagnosis on the date of the cases discharge. Individuals were excluded from both cohorts if they died, emigrated, were lost to follow-up or were diagnosed with cancer within the first 90 days of discharge.
Follow-up time was calculated from 91 days after discharge and censored on the date of emigration, loss to follow-up, date of diagnosed cancer or January 19, 2015, whichever came first. Population controls were assigned the index date of their corresponding case. The study was conducted in accordance with the STROBE statement.[12]
2.2. Setting
All regions of Denmark from January 1992 to October 2014. All cases of SAB during the study period were included.
2.3. Data sources
The Civil Registration System,[13] the Danish Staphylococcal Bacteremia Database[9] and the Danish National Patient Register[14] have been described previously.[8] Prevalent and incident cancers were identified using the Danish Cancer Registry that registers 98% of all cancers in Denmark.[10,11] Since 1987, all physicians in hospitals and private practices have been required by law to report all diagnoses of cancer to the registry. All diagnoses are assigned based on histologic examination by a fully trained pathologist. The registry uses a conservative strategy to avoid misclassification of relapse of the first cancer and records only true occurrence of a second primary cancer of the same type.[15] We classified diagnoses into 27 categories according to the ICD 7th edition codes 140.0–207.0 for the period 1992–2003, and ICD 10th edition codes C00.0–C96.0 and D00.0–D09.0 for the period 2004–2014 (Supplementary Table 1).[16]
2.4. Statistics
Statistical analyses were performed using R software version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
A modified Charlson Comorbidity Index (mCCI) score (cancer excluded) was constructed using all discharge data prior to hospitalization for SAB.[17,18] The score considers both the number and severity of comorbid disease. Each of the 15 categories of CCI could only contribute once to the overall index. We defined 3 levels of comorbidity: None, intermediate (1–2) and high (>2).
Crude incidence rate was estimated as the number of incident cancer divided by person years (PYs) at risk with corresponding 95% confidence interval (CI), computed using a Poisson distribution. Incidence rate (IR) and ratio (IRR) with 95% CI was estimated by Poisson regression.
To assess the competing event of death, survival curves were estimated by the cumulative incidence function. In order to account for inverse causality, the case and population cohorts were left-censored for the first 3 months.
2.5. Ethical considerations
The study was approved by the Danish Data Protection Agency (record no. 2014–41–3376). Danish legislation does not require informed consent for register-based studies.
3. Results
3.1. Characteristics of the study populations
Of the 27,901 SAB cases that were discharged from January 1, 1992 to October 31, 2014, 2046 were excluded due to an invalid sample date or identifier, 6767 cases were diagnosed with cancer before or during admission for SAB and 6170 died or had a cancer diagnosis within 90 days of discharge. Of the corresponding comparison cohort, 230 controls were excluded due to an invalid sample date, 52,728 controls were excluded because their corresponding case had a cancer diagnosis before, during admission, or within the first 90 days of admission, and 358 controls had a cancer diagnosis within 90 days after the corresponding cases discharge. Thus, 12,918 cases and 11,7465 population controls were included in the analysis (Table 1 and Fig. 1).
Table 1.
Characteristics of the Staphylococcus aureus bacteremia and population cohorts.
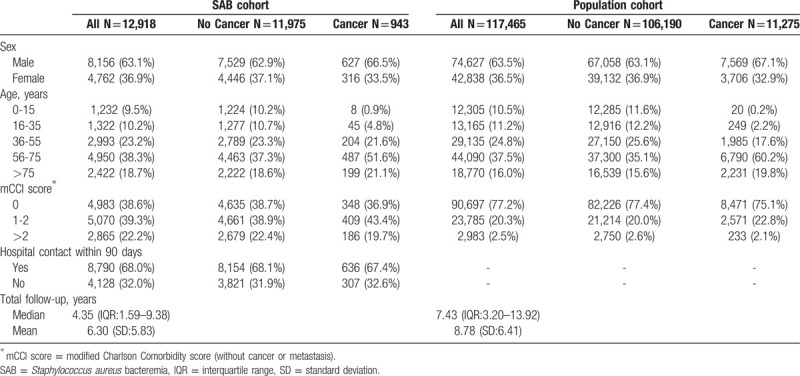
Figure 1.

Patient flow diagram.
The majority of the case cohort was male (63.1%), older than 55 years (57.0%) and had had a hospital contact within 90 days before SAB (68.0%). The case cohort had more comorbidity compared to the comparison cohort (Table 1).
The median follow-up time was 4.35 (IQR 1.59–9.38) years for the SAB cohort and 7.43 (IQR 3.20–13.92) years for the population cohort.
3.2. Risk of incident cancer
During the first year of follow-up, 165 (1.3%) cases (IR 3.78 (3.22–4.40) per 100,000 PYs) and 943 (0.8%) controls (IR 2.28 (2.14–2.43) per 100,000 PYs) were diagnosed with incident cancer corresponding to a 65% increased risk (IRR 1.65 (1.40–1.95)) (Table 2, Fig. 2). Of 27 types of cancer, 7 cancers occurred more frequently amongst cases than controls: cervical cancer (IRR 37.83 (4.23–338.47)), multiple myeloma (IRR 6.31 (2.58–15.44)), leukemia (IRR 4.73 (2.21–10.10)), sarcoma (IRR 4.73 (1.18–18.91)), liver cancer (IRR 3.64 (1.30–10.21)), pancreatic cancer (IRR 2.8 (1.27–6.16)), and urinary tract cancer (IRR 2.58 (1.23–5.39)).
Table 2.
Incident cancer during the first year of follow-up after Staphylococcus aureus bacteremia compared to a population cohort without Staphylococcus aureus bacteremia.

Figure 2.
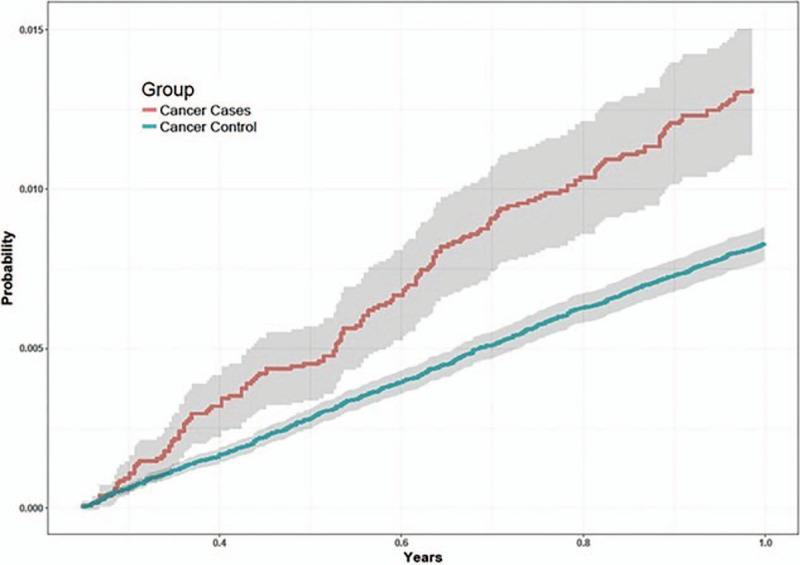
Cumulative incidence of cancer in the case and population cohorts with 95%CI.
3.3. Subsequent follow-up
From 2 to 5 years of follow-up, the overall risk of cancer was comparable in the SAB (IR 2.39 (95% CI: 2.15–2.65)) and population cohort (IR 2.40 (95% CI: 2.33–2.48)) for an IRR of 0.99 (95% CI: 0.89–1.11) (Table 3). However, the risk of pharyngeal cancer was increased (IRR 1.88 (1.04–3.39)) and the risk of liver cancer remained increased (IRR 3.93 (2.36–6.55)).
Table 3.
Incident cancer from 2 to 5 years of follow-up after Staphylococcus aureus bacteremia compared to a population cohort without Staphylococcus aureus bacteremia.

3.4. Effect of sex, age, and comorbidity on risk of cancer
The risk of cancer during the first year of follow-up was higher for individuals with SAB, no prior comorbidity and for all age groups (Table 4). Individuals with prior comorbidity had similar incidences of cancer in the SAB and population cohorts (Fig. 3A-C). Sex did not affect the incidence of cancer for the SAB cohort compared to the population cohort. After adjustment for age, sex and Charlson comorbidity index, the risk of cancer remained increased for the SAB cohort compared to the comparator cohort (IRR 1.84 (95% CI: 1.17–2.89).
Table 4.
Incidence rate and ratio of cancer for individuals with and without Staphylococcus aureus.

Figure 3.

A-C. Cumulative incidence of cancer in the case and population cohorts according to the level of prior comorbidity. 2A: no comorbidity; 2B: intermediate level of comorbidity; and 2C: high level of comorbidity.
4. Discussion
4.1. Main findings
In this large nationwide population-based cohort study, we found that SAB was a marker of cancer. In particular, we found an increased risk of 7 specific cancers during the first year after hospitalization for SAB. After the first year, overall risk of cancer was comparable in the case and population cohorts.
4.2. Comparison with other studies
Few population-based studies have investigated the risk of cancer following invasive bacterial disease. Søgaard et al showed an increased risk of mainly gastrointestinal and genitourinary cancer in a cohort of individuals with Gram negative bacteremia.[19] The incidence of any cancer between 6 and 12 months after bacteremia was estimated to be 1.46 higher than for controls; a rate comparable to the 1.65 in our study. Similarly, Thomsen et al showed an increased risk of any cancer of 1.75-fold from 4 to 12 month after a diagnosis of endocarditis.[20] The latter study found an increased risk of leukemia and myeloma but only during the first 3 months after endocarditis while Søgaard et al did not find any association between Gram negative bacteremia and hematological cancer except non-Hodgkin lymphoma during the first 6 month. These discrepancies suggest that different bacteria may confer different risks of malignant disease. Several studies suggest that this may be the case. The association of Helicobacter pylori and gastrointestinal lymphoma is well documented.[21] Associations between non-hemolytic streptococci, Bacillus fragilis, Fusobacterium nucleatum, Peptostreptococcus species, Clostridium species and colorectal cancer[22–24] have been shown as well as between Salmonella typhi infection and gallbladder cancer,[25] between Chlamydia pneumoniae and lung cancer,[26] between Bartonella species and vascular tumors,[27] and between Gram negative bacteria and prostate cancer.[28] A recent study of elderly adults with sepsis of any cause found increased risk of 7 cancers including liver, cervix, and leukemia.[29] The study differs in design and analysis from our study; microbiological etiologies of sepsis were not reported and individual follow-up was left-censored for the first year, i.e., the study reported cancers that occurred more than 12 months after the diagnosis of sepsis. In our cohort, the risk of cancer beyond 1 year was comparable to the comparison cohort. However, our study did show an almost 4-fold late increased risk of liver cancer similar to Liu et al. To our knowledge no other study has described an association between S. aureus bacteremia and risk of cervical cancer, multiple myeloma, leukemia, sarcoma, liver cancer, pancreatic cancer, or urinary tract cancer.
4.3. Implication and explanation of findings
Several mechanisms may explain the increased risk of cancer after SAB. Firstly, cancer-related immune suppression may occur through either general immune hyporesponsiveness induced by the cancer per se or by malignant transformation of immune cells. In support of the latter, we found that the risk of leukemia and multiple myeloma were increased. The previously described association between an increased risk of bacteremia in patients with hematological malignancy as well as between invasive pneumococcal disease and subsequent multiple myeloma is consistent with our findings.[30–32] However, to our knowledge this is the first report of an increased risk of hematological malignancy following SAB. While the pathophysiology is unknown it is obvious to speculate that the risk may be caused by a failing production of immune effector cells and immunoglobulin.
4.4. Strengths and limitations
Our study has several strengths including the nationwide population-based design with complete follow-up, its size, and the unique Danish registration system that enables linkage of data for each individual in the study to a randomly selected and matched population comparison cohort. Potential limitations include reliance on register data that may contain errors but misclassification would occur in both cases and controls, and, thus, be non-differential. Infection following invasive diagnostic procedures or detection due to increased diagnostic work-up related to SAB could explain part of the increased risk associations observed. In fact, an increased risk in the case cohort was observed for 14 of the 27 cancer groups in the first 3 months after discharge for SAB. In order to account for detection bias the case and population cohorts were left-censored for the first 3 months. However, the risk differed substantially among various cancer types arguing against increased general diagnostic surveillance as being the main explanation for our findings. Our dataset did not include information on lifestyle factors such as smoking and alcohol use or socioeconomics that affect life expectancy and susceptibility to infection or cancer. Residual confounding related to these and other factors cannot be excluded.
4.5. Conclusion, recommendation, and future directions
Our study aimed to clarify the role of SAB as a marker of occult cancer. The impact of the increased risk in absolute numbers is small, and therefore further work-up to diagnose cancer of patients in general after SAB may not be cost-effective. The results suggest, however, that diagnostic work-up for an occult cancer may be appropriate for some patients based on an individualized assessment. Specifically, this may include screening of individuals with concomitant anemia or hypercalcaemia by either determining a white blood cell differential or protein electroforesis that may permit earlier detection of hematological malignancy. Further, younger individuals without prior comorbidity who develop SAB may be candidates for extended follow-up.
The risk of primary incident cancer was 65% higher in the SAB cohort compared to the population cohort during the first year of follow-up and included 7 specific cancers. Screening for these specific cancers in selected populations may allow for earlier detection.
Finally, our findings should be interpreted with caution because we are unable to exclude reverse causation due to the study design.
Author contributions
Data curation: Niels Mejer, Thomas Benfield.
Formal analysis: Nanja Gotland, Håkon Sandholdt.
Investigation: Nanja Gotland.
Methodology: Thomas Benfield.
Project administration: Nanja Gotland.
Writing – original draft: Nanja Gotland.
Writing – review & editing: Nanja Gotland, Marie-Louise Uhre, Håkon Sandholdt, Niels Mejer, Lene Fogt Lundbo, Andreas Petersen, Anders Rhod Larsen, Thomas Benfield.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, IR = incidence rate, IRR = incidence rate ratio, mCCI = modified Charlson Comorbidity Index, PYs = person years, SAB = Staphylococcus aureus bacteremia.
How to cite this article: Gotland N, Uhre M, Sandholdt H, Mejer N, Lundbo L, Petersen A, Larsen A, Benfield T. Increased risk of incident primary cancer after Staphylococcus aureus bacteremia: a matched cohort study. Medicine. 2020;99:17(e19984).
The study was funded by an unrestricted grant from Anna and Preben Simonsen's Foundation.
All authors report no conflict of interest.
Supplemental Digital Content is available for this article.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.;
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med 2000;248:171–83. [DOI] [PubMed] [Google Scholar]
- [3].Elinav E, Nowarski R, Thaiss CA, et al. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer 2013;13:759–71. [DOI] [PubMed] [Google Scholar]
- [4].Pardoll D. Cancer and the immune system: basic concepts and targets for intervention. Semin Oncol 2015;42:523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Plummer M, de MC, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016;4:e609–16. [DOI] [PubMed] [Google Scholar]
- [6].Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59–67. [DOI] [PubMed] [Google Scholar]
- [7].Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA 2006;296:2823–31. [DOI] [PubMed] [Google Scholar]
- [8].Gotland N, Uhre ML, Mejer N, et al. Long-term mortality and causes of death associated with Staphylococcus aureus bacteremia. A matched cohort study. J Infect 2016;73:346–57. [DOI] [PubMed] [Google Scholar]
- [9].Jessen O, Rosendal K, Bülow P, et al. Changing staphylococci and staphylococcal infections. A ten-year study of bacteria and cases of bacteremia. N Engl J Med 1969;281:627–35. [DOI] [PubMed] [Google Scholar]
- [10].Storm HH, Michelsen EV, Clemmensen IH, et al. The Danish Cancer Registry--history, content, quality and use. Dan Med Bull 1997;44:535–9. [PubMed] [Google Scholar]
- [11].Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health 2011;39:42–5. [DOI] [PubMed] [Google Scholar]
- [12].Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2–10. [DOI] [PubMed] [Google Scholar]
- [13].Pedersen CB, Gotzsche H, Moller JO, et al. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull 2006;53:441–9. [PubMed] [Google Scholar]
- [14].Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health 2011;39:30–3. [DOI] [PubMed] [Google Scholar]
- [15].Storm HH, Lynge E, Osterlind A, et al. Multiple primary cancers in Denmark 1943-80; influence of possible underreporting and suggested risk factors. Yale J Biol Med 1986;59:547–59. [PMC free article] [PubMed] [Google Scholar]
- [16].Nielsen SF, Nordestgaard BG, Bojesen SE. Associations between first and second primary cancers: a population-based study. CMAJ 2012;10:E57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sundararajan V, Henderson T, Perry C, et al. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 2004;57:1288–94. [DOI] [PubMed] [Google Scholar]
- [18].Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173:676–82. [DOI] [PubMed] [Google Scholar]
- [19].Sogaard KK, Farkas DK, Sogaard M, et al. Gram-negative bacteremia as a clinical marker of occult malignancy. J Infect 2017;74:153–62. [DOI] [PubMed] [Google Scholar]
- [20].Thomsen RW, Farkas DK, Friis S, et al. Endocarditis and risk of cancer: a Danish nationwide cohort study. Am J Med 2013;126:58–67. [DOI] [PubMed] [Google Scholar]
- [21].Palli D, Masala G, Del GG, et al. CagA+ Helicobacter pylori infection and gastric cancer risk in the EPIC-EURGAST study. Int J Cancer 2007;120:859–67. [DOI] [PubMed] [Google Scholar]
- [22].McKenna AJ, O’Donnell ME, McMullan R, et al. Long-term gastrointestinal outcomes after Streptococcus bovis bacteraemia. Int J Clin Pract 2011;65:1203–5. [DOI] [PubMed] [Google Scholar]
- [23].Boleij A, van Gelder MM, Swinkels DW, et al. Clinical Importance of Streptococcus gallolyticus infection among colorectal cancer patients: systematic review and meta-analysis. Clin Infect Dis 2011;53:870–8. [DOI] [PubMed] [Google Scholar]
- [24].Kwong TNY, Wang X, Nakatsu G, et al. Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology 2018;155:383–90. [DOI] [PubMed] [Google Scholar]
- [25].Nath G, Gulati AK, Shukla VK. Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J Gastroenterol 2010;16:5395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhan P, Suo LJ, Qian Q, et al. Chlamydia pneumoniae infection and lung cancer risk: a meta-analysis. Eur J Cancer 2011;47:742–7. [DOI] [PubMed] [Google Scholar]
- [27].Vogelmann R, Amieva MR. The role of bacterial pathogens in cancer. Curr Opin Microbiol 2007;10:76–81. [DOI] [PubMed] [Google Scholar]
- [28].Hayes RB, Pottern LM, Strickler H, et al. Sexual behaviour, STDs and risks for prostate cancer. Br J Cancer 2000;82:718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Liu Z, Mahale P, Engels EA. Sepsis and risk of cancer among elderly adults in the United States. Clin Infect Dis 2019;68:717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gregersen H, Pedersen G, Svendsen N, et al. Multiple myeloma following an episode of community-acquired pneumococcal bacteraemia or meningitis. APMIS 2001;109:797–800. [PubMed] [Google Scholar]
- [31].Roed C, Engsig FN, Omland LH, et al. Long-term mortality in patients diagnosed with pneumococcal meningitis: a Danish nationwide cohort study. Am J Epidemiol 2010;172:309–17. [DOI] [PubMed] [Google Scholar]
- [32].Norgaard M, Larsson H, Pedersen G, et al. Risk of bacteraemia and mortality in patients with haematological malignancies. Clin Microbiol Infect 2006;12:217–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


