Supplemental Digital Content is available in the text
Keywords: ABO blood type, adenosquamous carcinoma, carbohydrate antigen 19-9, pancreatic ductal adenocarcinoma, postoperative chemotherapy, prognosis
Abstract
The aim of this observational study was to test whether ABO blood type was a prognostic factor for pancreatic ductal adenocarcinoma (PDAC) patients and whether other risk factors could influence pancreatic cancer patients’ survival. This study included 610 patients who were diagnosed as pancreatic cancer and had undergone radical surgery. Patients’ characteristics included age, gender, tumor stage, tumor grade, adenosquamous carcinoma (ASC) status, preoperative serum carbohydrate antigen 19-9 (CA19-9) levels, preoperative serum carcinoembryonic antigen (CEA) levels, ABO blood type, smoking status, and drinking status were analyzed in this study. Cox proportional hazards regression model and Kaplan–Meier method were used to evaluate the role of prognostic factors. For pancreatic cancer patients undergoing radical surgery, the overall survival was worse for ASC patients than PDAC patients (Log-rank = 11.315, P < .001). Compared with ASC patients (Log-rank < 0.001, P = .996), PDAC patients can benefit from chemotherapy (Log-rank = 17.665, P < .001). For PDAC patients, O blood type had better overall survival than non-O blood type (Log-rank = 4.153, P = .042). Moreover, the group with higher serum levels of CA19-9 had poor prognosis compared to another group with low serum CA19-9 (Log-rank = 4.122, P = .042). Higher CEA levels indicated poor prognosis (Log-rank = 13.618, P < .001). In conclusion, ASC status was associated with overall survival of pancreatic cancer patients and cannot benefit from postoperative chemotherapy. Non-O blood type was a prognostic factor for PDAC patients.
1. Introduction
Pancreatic cancer is the fourth leading cause of cancer death in western countries.[1] Overall mortality from pancreatic cancer is expected to be the second leading cause of cancer-related deaths by 2030.[2] Pancreatic cancer is usually diagnosed at the late stage. Most pancreatic cancer patients have initial metastasis and lose the opportunity of surgical treatment. The 5-year survival rate of pancreatic cancer patients is only 7%.[3–5] Therefore, it is important to understand the factors that affect the prognosis of pancreatic ductal adenocarcinoma (PDAC) in order to improve the prognosis and survival of pancreatic cancer patients.
There are many risk factors for pancreatic cancer, such as age, tobacco smoking, heavy alcohol consumption, obesity, low physical activity, chronic pancreatitis, long-standing type 2 diabetes, ABO blood type, and family history.[6] Among these risk factors, Chen et al[7] had reported that cigarette smoking was associated with the poor prognosis of pancreatic cancer, and that smoking cessation can improve the survival of pancreatic cancer patients. Long-standing diabetes was also associated with worse prognosis of pancreatic cancer patients.[8] Heavy drinking for 30 or more years was found to be a negative prognostic factor for PDAC.[9] On the other hand, it is controversial for evaluating the impact of obesity on overall survival in patients with pancreatic cancer. Li et al[10] reported that obesity was associated with worse clinical outcome, while another study found that obesity had no significant influence on survival of pancreatic cancer patients.[11]
The ABO gene is located on chromosome 9q34. By coding N-acetylgalactosamine transferase or galactotransferase, the A and B gene could convert H antigen to A antigen or B antigen, respectively. A frequent ABO variant could not induce the activatity of encoded acetylggalactosamine transferase, which chould not convert H antigen and lead to O blood type.[12] several studies identified the relationship between ABO blood type and various types of cancers.[13,14] For example, the ABO blood type was associated with the overall survival of renal carcinoma patients who had underwent radical operation.[15] Furthermore, compared with O blood type, non-O blood type had worse overall survival for patintes with hepatocellular carcinoma.[16] ABO blood type can be used as a risk factor for recurrence for ovarian cancer and vulvar cancer.[17] ABO blood type could influence the risk for PDAC in China population[18] and studies reported that the overall survival was poor in the non-O blood type group than in the O blood type group, and that non-O blood type indicated poor prognosis.[19–21] However, in the sudy of Wang et al there was no significant correlation between ABO blood type and prognosis of patients with PDAC.[22] Thus, it is urgent to explore whether ABO blood type was correlated with the prognosis of PDAC patients, and whether other risk factors could influence the overall survival of PDAC patients.
2. Methods
2.1. Patient samples
A total of 492 patients with pancreatic cancer in West China Hospital of Sichuan University and 118 patients with pancreatic cancer in the First Affiliated Hospital of Xi’an Jiaotong University Medical College were eligible for this study between June 2010 and December 2018. Inclusion criteria include pancreatic cancer patients undergoing radical surgery. Excluded criteria include pancreatic cancer patients who had neoadjuvant radiotherapy and chemotherapy or had a previous history of cancer. According to the American Joint Committee on cancer (AJCC), all cancer patients were confirmed histopathologically. All patients were followed up until August 2019. Overall survival referd to the time from cancer diagnosis to August 2019 or death. The study acquired ethical approval from the ethics committee of West China Hospital of Sichuan University and the ethics committee of Xi’an Jiaotong University. The collection information is shown in supplementary Table 1.
2.2. Statistical analysis
SPSS version 22.0 was used for all statistical analyses. The association of ASC, ABO blood type, cigarette smoking, drinking, diabetes, serum CA19–9 levels, and serum CEA levels with overall survival were evaluated by Kaplan–Meier survival curve analysis. The univariate and multivariate Cox regression were used to analyze the relationship between each factor and prognosis.
3. Results
3.1. Clinical characteristics and prognostic value of pancreatic cancer
A total of 610 primary pancreatic cancer patients (492 patients in West China Hospital of Sichuan University and 118 pancreatic cancer patients in First Affiliated Hospital of Xi’an Jiaotong University) who had underwent radical surgery from Jun 2010 to December 2018 were included for analyses. The average age was 60.2 years old, and 60.7% of them were men. Among the 610 pancreatic cancer patients, the ages ranged from 31 to 84 years old. Patients were divided into three groups according to AJCC stage, including stage I (n = 46), stage II (n = 537), stage III patients (n = 24), and missing data (N = 3). All PDAC patients were further divided into three groups according to the pathological type which included highly differentiated (n = 9), moderately differentiated (n = 252), minimally differentiated (n = 328) and missing data (n = 21). According to the serum CA19–9 levels, pancreatic cancer patients were divided into two groups (patients with CA19–9 value >37 U/mL and patients with CA19–9 value ≤37 U/mL). Total bilirubin was the sum of direct bilirubin and indirect bilirubin, which was recognized as abnormal when it was higher than 34.2 μmol/L. The ABO blood type was divided into four groups which included O blood type (n = 181), A blood type (n = 193), B blood type (n = 175) and AB blood type (n = 61). All PDAC patients were RH positive. Other factors such as smoking status, drinking status were shown in Supplemental Digital Content 1.
According to postoperative pathology, pancreatic cancer patients were divided into ASC (N = 30) and PDAC (N = 580). Survival outcomes were compared between PDAC group and ASC group. The median overall survival was 9 months for ASC patients and 16 months for the PDAC patients (Log-rank = 11.315, P < .001) (Fig. 1A), which indicated ASC was related to poor prognosis.
Figure 1.
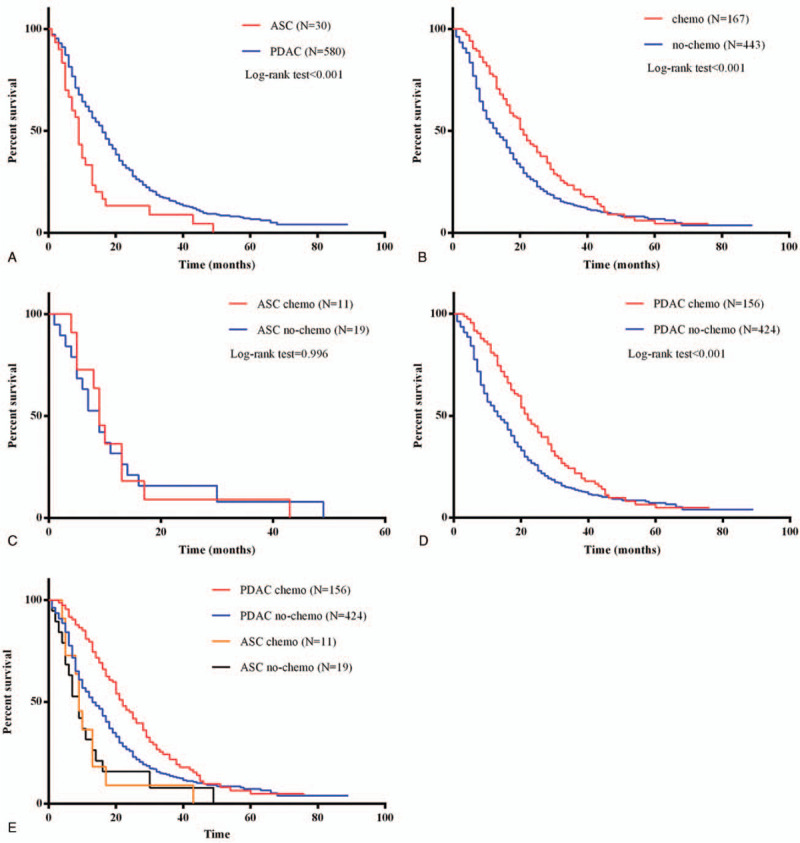
Survival outcomes in pancreatic cancer patients with ASC or PDAC status treated with postoperative chemotherapy. (A) Survival curves of patients among the ASC or PDAC status. (B) Survival curves of all pancreatic cancer patients with or without postoperative chemotherapy. (C) Survival curves of ASC patients with or without postoperative chemotherapy. (D) Survival curves of PDAC patients with or without postoperative chemotherapy. (E) Impact of postoperative chemotherapy introduction on overall survival was also illustrated separately in terms of ASC or PDAC in all patients.
For investigating the survival outcomes, the pancreatic cancer patients were separated into two groups according to the chemotherapy usage. Most importantly, the overall survival time was higher in patients with chemotherapy treatment (Log-rank = 15.839, P < .001) (Fig. 1B). Compared with ASC group (Log-rank < 0.001, P = .996) (Fig. 1C), PDAC patients can benefit from chemotherapy (Log-rank = 17.665, P < .001) (Fig. 1D and E). Univariate and multivariate survival analyses were conducted, indicating ASC status and chemotherapy were independent prognostic factors for pancreatic cancer patients (Supplemental Digital Content 2).
3.2. Prognostic value of ABO blood type for PDAC patients
Since postoperative chemotherapy and PDAC status were correlated with prognosis of pancreatic cancer. We analyzed the prognosis of 424 PDAC patients without postoperative chemotherapy. Clinical characteristics of 424 participants were shown in Table 1.
Table 1.
Clinical characteristics of 424 PDAC patients.

Whether ABO blood was a prognostic factor for PDAC remains unclear. Cox proportional hazards regression model and Kaplan–Meier survival curve were performed to determine the relationship between ABO blood type and prognosis of PDAC patients. As shown in Table 2, ABO blood type was an independent prognostic factor for PDAC patients (Log-rank = 1.358, 95% CI = 1.054–1.749, P = .018). The median overall survival time was 15 months for O blood type and 13 months for non-O blood type, which indicated non-O blood type related to worse prognosis for PDAC patients (Log-rank = 4.153, P = .042) (Fig. 2A). PDAC patients with O blood type have a higher overall survival time than B blood type (Log-rank = 3.894, P = .048) (Fig. 2C), while there were no significant differences in overall survival between the O blood type with the other blood types (Log-rank = 3.023, 0.387; P = .082, .534; compared with A, AB blood type, respectively) (Fig. 2B and D). There was also no significant difference between A blood type and B (Log-rank = 0.098, P = .754) as well as A and AB blood type (Log-rank = 0.317, P = .573) (Fig. 2E and F). There was no difference between B blood type and AB blood type (Log-rank = 0.7053, P = .401) (Fig. 2G).
Table 2.
Cox regression analysis for 424 PDAC patients.

Figure 2.
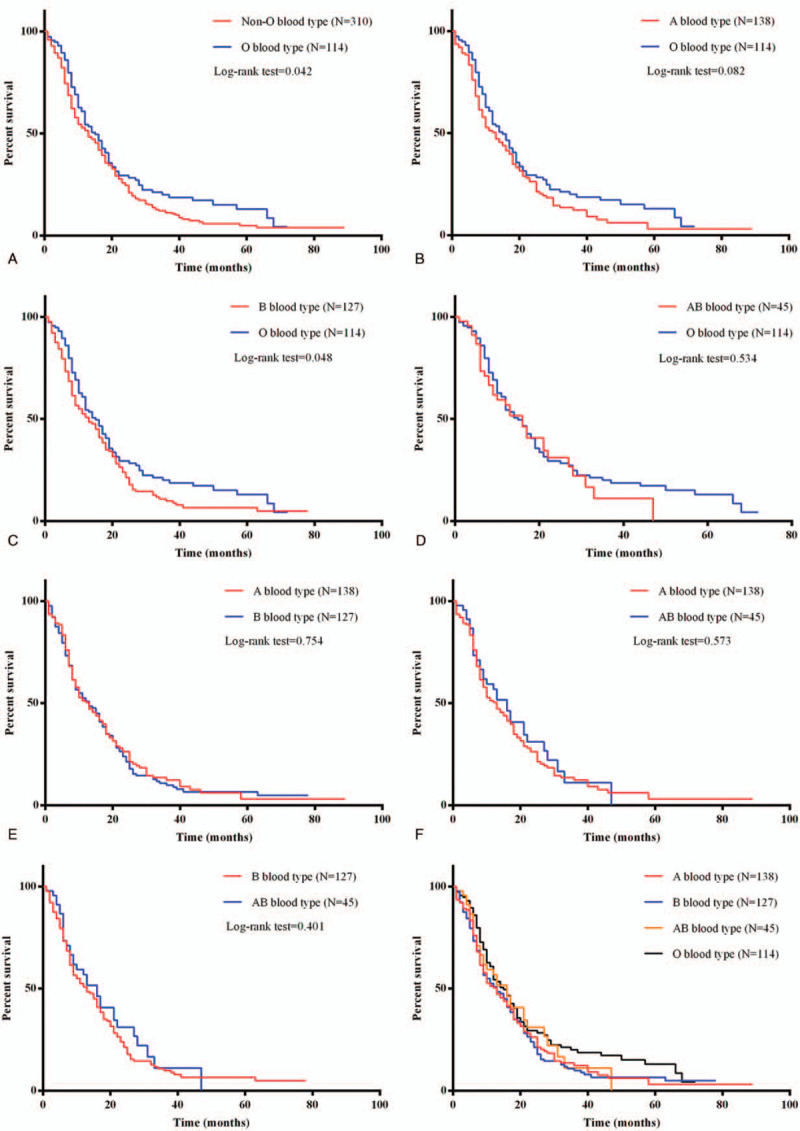
Kaplan–Meier analysis of overall survival of PDAC patients. (A) Kaplan–Meier analysis of overall survival of O blood type with non-O blood type PDAC patients. (B) Kaplan–Meier analysis of A blood type with O blood type PDAC patients. (C) Kaplan–Meier analysis of overall survival of B blood type with O blood type PDAC patients. (D) Kaplan–Meier analysis of A blood type with B blood type PDAC patients. (E) Kaplan–Meier analysis of A blood type with AB blood type PDAC patients. (F) Kaplan–Meier analysis of B blood type with AB blood type PDAC patients.
3.3. Combined CA19–9 and CEA levels improve prognostic prediction of PDAC patients
The role of serum CEA levels in pancreatic cancer patients is poorly understood. Although many studies reported the relationship between serum CEA levels and pancreatic cancer, the results were unclear. Therefore, Cox proportional hazards regression model and Kaplan–Meier survival curve were performed to analyze whether the serum CEA levels and the combined serum CA19–9 with CEA levels could evalute the prognosis of PDAC patients. Among the 424 PDAC patients, 333 patients had higher serum CA9–9 levels (>37 U/mL) and 116 had higher serum CEA levels (>5 ng/mL). Positive rate of abnormal serum CA19–9 and CEA were 78.5% and 27.4%, respectively.
Compared to the 17 months for group with lower CA19–9 levels, the median overall survival was 13 months for the group with higher CA19–9 levels. Kaplan–Meier analysis showed those who had higher serum CA19–9 levels had worse overall survival (Log-rank = 4.122, P = .042) (Fig. 3A). In addition, higher CEA levels indicated poor prognosis for PDAC patients (Log-rank = 13.618, P < .001) (Fig. 3B). This result was consistent with the previous study.[23]
Figure 3.
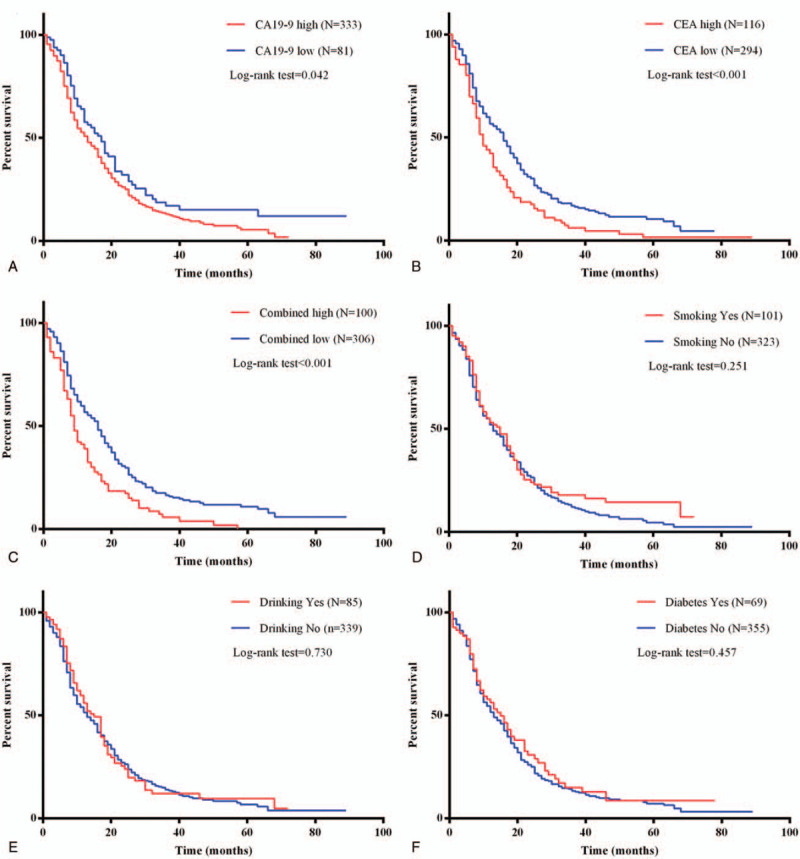
Kaplan–Meier analysis of overall survival of PDAC patients. (A) Kaplan–Meier analysis of overall survival of PDAC patients with serum CA19-9 levels. (B) Kaplan–Meier analysis of overall survival of PDAC patients with serum CEA levels. (C) Kaplan–Meier analysis of overall survival of PDAC patients with combined serum CA19-9 and CEA levels. (D) Kaplan–Meier analysis of overall survival of PDAC patients with smoking status. (E) Kaplan–Meier analysis of overall survival of PDAC patients with drinking status. (F) Kaplan–Meier analysis of overall survival of PDAC patients with diabetes.
Pearson correlation analysis showed that serum CA19–9 levels were slightly associated with serum CEA levels (P < .001, r = 0.220) (data not show). The combined positive was defined when serum CA19–9 was higher than 37 U/mL and CEA was higher than 5 ng/mL, otherwise was defined as combined negative. When combined CA19–9 with CEA levels, the median survival time was 9 months for positive group but 16 months for negative group (Log-rank = 17.776, P < .001) (Fig. 3C). This indicated combined serum CA19–9 with CEA levels was a prognostic factor for PDAC patients.
3.4. Prognostic value of other factors for PDAC patients
Smoking status, drinking status and diabetes were previously reported risk factors for PDAC, furthermore, we tested whether these factors correlated with the prognosis of PDAC patients. As shown in Table 2, univariate Cox regression analysis indicated that smoking (HR = 0.868, 95% CI = 0.676–1.113, P = .265), drinking status (HR = 0.956, 95% CI = 0.736–1.243, P = .737), diabetes (HR = 0.899, 95% CI = 0.674–1.199, P = .470), total bilirubin (HR = 0.915, 95% CI = 0.741–1.131, P = .412) and ALT with AST (HR = 0.815, 95% CI = 0.653–1.017, P = .070) were not associated with the prognosis of PDAC patients. The results showed smoking status (Log-rank = 1.316, P = .251), drinking status (Log-rank = 0.119, P = .730) and diabetes (Log-rank = 0.552, P = .457) were not associated with the overall survival of the PDAC patients (Fig. 3D–F).
4. Discussion
Pancreatic cancer is one of most fatal cancers and the 5-year survival rate of pancreatic cancer was only 7%.[3] As one of the pancreatic cancer subtypes, ASC was enriched for TP53 mutation, upregulation of the TP63ΔN transcriptional network and key factors involved in metastasis,[24] which may cause poor prognosis. In the study of Hoshimoto et al, the overall survival was comparable between the ASC and PDAC patients after surgical resection.[25] Boyd et al also found there was no difference for overall survival between ASC and PDAC patients.[26] However, Hester et al reported that the ASC patients had worse overall survival than PDAC patients.[27] In this study, we divided the patients into two groups, and found that the ASC status is associated with overall survival of pancreatic cancer patients. ASC indicated poor prognosis, which was supported by the study of Hester et al.[27] Considering the different response to postoperative chemotherapy for ASC and PDAC patients, the pancreatic cancer patients were divided into ASC and PDAC groups and the result showed PDAC but not ASC patients can benefit from postoperative chemotherapy. It is helpful of us to consider the postoperative chemotherapy usage for PDAC but not ASC patients.
Among the risk factors of PDAC, smoking status,[7] diabetes[8] and heavy drinking[9] were correlated with the prognosis of PDAC patients. However, the impact of ABO blood on overall survival in patients with PDAC remains controversial. The relationship between ABO blood type and cancer survival can be summarized as follows:
-
1.
chronic inflammation, the single nucleotide polymorphism of ABO site is related to some inflammatory factors, such as tumor necrosis factor α[28] and intercellular adhesion molecule 1,[29] which were known to modulate inflammatory responses. And inflammation was a hallmark of cancer and was linked with tumor progression.[30] These results indicated the mechanism by which ABO blood type influence tumor survival.
-
2.
Immune surveillance, soluble intercellular adhesion molecule-1 (sICAM-1) was associated with single nucleotide polymorphism at the ABO locus and played an important role in immune surveillance and tumor progression, which was another mechanism for ABO blood type correlated with tumor survival.[15]
In this study, we focused on the relationship between ABO blood type and overall survival in PDAC patients who had undergone radical surgery. For PDAC patients, non-O blood type had poor overall survival time than O blood type group (Log-rank = 4.153, P = .042), which was supported by the previous study (Jellas et al).[18] The A blood type, B blood type, and AB blood type had similar overall survival.
In addition, our study indicated serum CA199 levels, CEA levels and combined CA19–9 with CEA levels were prognostic factors for PDAC patients. In contrast to previously reported studies,[7–9] we found no association between smoking status, drinking status, or diabetes and overall survival in PDAC patients who had undergone radical surgery. The possible reasons for our findings were as follows: first, some PDAC patients only quit smoking and drinking for a short time while some patients quit smoking and drinking for a long time when data was collected. Therefore, the effects of smoking and drinking were complex and cannot be analyzed by this way. Secondly, there is a significant crosstalk between smoking and drinking, which could influence the association with overall survival of pancreatic cancer patients. In contrast, the relationship between diabetes and pancreatic cancer is complex. Type 2 diabetes mellitus was a risk for pancreatic cancer.[31,32] Furthermore, long-standing diabetes was a prognostic factor for pancreatic cancer patients while recent-onset diabetes had little impact on pancreatic cancer patients’ survival.[8] In this study, there was no difference in the overall survival between patients with or without diabetes. The possible explanation for this result was that some new-onset pancreatic cancer patients might not know that they have diabetes. Thus, we cannot distinguish between new diabetics and long-standing diabetics. Additionally, the sample size was too small to distinguish statistical differences. Further research is needed in the future.
In this study, we also found that the serum ALT or AST (before surgery) levels were not correlated with the overall survival of PDAC patients who had undergone radical surgery. This consist with findings by Zhang[33] reported that ALT or AST levels were not associated with overall survival for stage III patients, but correlated with worse survival for stage IV patients. In this study, there was no relationship between overall survival of PDAC patients and preoperative total bilirubin. This was likely that radical surgery relieved the compression of the tumor to the head of the pancreas and eliminated the jaundice, which in turn reduced total bilirubin levels.
Limitations of our study also require consideration. Our results showed that TNM staging had no influence on the prognosis of PDAC patients (HR = 0.994, 1.258; 95% CI = 0.670–1.473, 0.659–2.399; P = .974, .487; respectively). The possible reason was that there were only 33 patients in stage I and 16 patients in stage III. Information on body mass index, low density lipoprotein, high density lipoprotein, total cholesterol, tumor location, and size were not analyzed. Meanwhile, information about smoking, drinking, and diabetes were not detailed enough. Family history of chronic pancreatitis was not considered and it was another limitation of the present study.
In summary, our results indicated that ASC status is associated with overall survival of pancreatic cancer patients. ASC patients indicated poor prognosis and cannot benefit from postoperative chemotherapy. Non-O blood type indicated poor prognosis for PDAC patients.
Acknowledgments
The authors would like to thank Liu-yun Gong and Tian-tian Lei yu for their help in collecting the patient’ clinical data.
Author contributions
Qing Zhu and Cheng Yi designed the study. Shuang-Shuang Li, Cong-Ya Zhou, Rong Liao, Lai Xiong performed the data analysis. Ning-Na Weng, Ya-Qin Zhao and Hong-Feng Gou collected the clinical data for pancreatic cancer patients and follow up patients. Clifford Mason modified the article.
Qing Zhu orcid: 0000-0002-6905-2517.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, ASC = Adenosquamous carcinoma, CA19-9 = carbohydrate antigen 19-9, CEA = carcinoembryonic antigen, PDAC = pancreatic ductal adenocarcinoma.
How to cite this article: Li SS, Zhou CY, Liao R, Xiong L, Weng NN, Zhao YQ, Mason C, Gou HF, Yi C, Zhu Q. ABO blood type, smoking status, other risk factors and prognosis of pancreatic ductal adenocarcinoma. Medicine. 2020;99:14(e19413).
SL and CZ conserve the same work.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
This study was approved by 1·3·5 Project for Disciplines of Excellence, West China Hospital (ZYGD18003), Sichuan University for J. Han.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. [DOI] [PubMed] [Google Scholar]
- [3].Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v56–68. [DOI] [PubMed] [Google Scholar]
- [4].Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers 2016;2:16022. [DOI] [PubMed] [Google Scholar]
- [5].Vincent A, Herman J, Schulick R, et al. Pancreatic cancer. Lancet 2011;378:607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yuan C, Morales-Oyarvide V, Babic A, et al. Cigarette smoking and pancreatic cancer survival. J Clin Oncol 2017;35:1822–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yuan C, Rubinson DA, Qian ZR, et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J Clin Oncol 2015;33:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang S, Wang C, Huang H, et al. Effects of alcohol drinking and smoking on pancreatic ductal adenocarcinoma mortality: a retrospective cohort study consisting of 1783 patients. Sci Rep 2017;7:9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009;301:2553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dandona M, Linehan D, Hawkins W, et al. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas 2011;40:931–7. [DOI] [PubMed] [Google Scholar]
- [12].Yamamoto F. Molecular genetics of the ABO histo-blood group system. Vox Sang 1995;69:1–7. [DOI] [PubMed] [Google Scholar]
- [13].Franchini M, Lippi G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med 2015;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Franchini M, Liumbruno GM, Lippi G. The prognostic value of ABO blood group in cancer patients. Blood Transfus 2016;14:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kaffenberger SD, Morgan TM, Stratton KL, et al. ABO blood group is a predictor of survival in patients undergoing surgery for renal cell carcinoma. BJU Int 2012;110(11 Pt B):E641–6. [DOI] [PubMed] [Google Scholar]
- [16].Li Q, Wu T, Ma XA, et al. Prognostic role of ABO blood group in patients with unresectable hepatocellular carcinoma after transarterial chemoembolization. Ther Clin Risk Manag 2018;14:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sartorius CM, Schoetzau A, Kettelhack H, et al. ABO blood groups as a prognostic factor for recurrence in ovarian and vulvar cancer. PLoS One 2018;13:e0195213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li X, Xu HQ, Gao PJ. ABO blood group and diabetes mellitus influence the risk for pancreatic cancer in a population from China. Med Sci Monitor 2018;24:9392–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].El Jellas K, Hoem D, Hagen KG, et al. Associations between ABO blood groups and pancreatic ductal adenocarcinoma: influence on resection status and survival. Cancer Med 2017;6:1531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Rahbari NN, Bork U, Hinz U, et al. AB0 blood group and prognosis in patients with pancreatic cancer. BMC Cancer 2012;12:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ben Q, Wang K, Yuan Y, et al. Pancreatic cancer incidence and outcome in relation to ABO blood groups among Han Chinese patients: a case-control study. Int J Cancer 2011;128:1179–86. [DOI] [PubMed] [Google Scholar]
- [22].Wang DS, Wang ZQ, Zhang L, et al. Are risk factors associated with outcomes in pancreatic cancer? PLoS One 2012;7:e41984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Imaoka H, Mizuno N, Hara K, et al. Prognostic impact of carcinoembryonic antigen (CEA) on patients with metastatic pancreatic cancer: a retrospective cohort study. Pancreatology 2016;16:859–64. [DOI] [PubMed] [Google Scholar]
- [24].Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hoshimoto S, Hoshi N, Hishinuma S, et al. Clinical implications of the proliferative ability of the squamous component regarding tumor progression of adenosquamous carcinoma of the pancreas: a preliminary report. Pancreatology 2017;17:788–94. [DOI] [PubMed] [Google Scholar]
- [26].Boyd CA, Benarroch-Gampel J, Sheffield KM, et al. 415 patients with adenosquamous carcinoma of the pancreas: a population-based analysis of prognosis and survival. J Surg Res 2012;174:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hester CA, Augustine MM, Choti MA, et al. Comparative outcomes of adenosquamous carcinoma of the pancreas: an analysis of the National Cancer Database. J Surg Oncol 2018;118:21–30. [DOI] [PubMed] [Google Scholar]
- [28].Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 2001;104:487–501. [DOI] [PubMed] [Google Scholar]
- [29].Yazer MH. What a difference 2 nucleotides make: a short review of ABO genetics. Transfus Med Rev 2005;19:200–9. [DOI] [PubMed] [Google Scholar]
- [30].Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- [31].Bosetti C, Rosato V, Li D, et al. Diabetes, antidiabetic medications, and pancreatic cancer risk: an analysis from the International Pancreatic Cancer Case-Control Consortium. Ann Oncol 2014;25:2065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang C, Dong S, Wang L, et al. Prognostic values of common clinical parameters in advanced pancreatic ductal adenocarcinoma: a large multicenter cohort study of ten years. Discov Med 2018;25:91–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


