Supplemental Digital Content is available in the text
Keywords: efficacy, paroxetine, randomized controlled trials, social anxiety disorder, tolerability
Abstract
Objective:
The present study aimed to estimate the comprehensive efficacy and tolerability of paroxetine in adult patients with social anxiety disorder (SAD).
Methods:
We conducted a comprehensive literature review of the PubMed, Embase, Cochrane Central Register of Controlled Trials, Web of Science, and ClinicalTrials databases for eligible randomized controlled trials (RCTs). The efficacy outcome was the mean change of different kinds of scale scores as well as response and remission rates. The secondary outcome was tolerability, defined as the discontinuation rate and the incidence of adverse events (AEs).
Results:
Our meta-analysis included 13 RCTs. Mean changes in the Liebowitz Social Anxiety Scale (LSAS) total score, fear and avoidance subscale of LSAS scores were all significantly greater in patients with SAD that received paroxetine compared to those received placebo (total: MD = 13.46, 95%CI 10.59–16.32, P < .00001; fear: MD = 6.76, 95%CI 4.89–8.62, P < .00001; avoidance: MD = 6.54, 95%CI 4.63–8.45, P < .00001). Response and remission rates were both significantly greater in patients with SAD that received paroxetine compared to those received placebo (response: OR = 3.02, 95%CI 2.30–3.97, P < .00001; remission: OR = 3.14, 95%CI 2.25–4.39, P < .00001). There was no significant difference in discontinuation rate due to any reason between two groups (OR = 1.06, 95%CI 0.81–1.39, P = .65). Discontinuation rate due to AEs was higher in paroxetine than placebo group (OR = 3.41, 95%CI 2.45–4.72, P < .00001) whereas the rate due to lack of efficacy was higher in placebo as compared with paroxetine group (OR = 0.14, 95%CI 0.09–0.22, P < .00001). The incidence of any AE was significantly increased in patients that received paroxetine (OR = 1.83, 95%CI 1.43–2.35, P < .00001).
Conclusion:
Paroxetine was an effective and well-tolerated treatment option for adult patients with SAD.
1. Introduction
Social anxiety disorder (SAD), also known as social phobia, is a common and persistent anxiety disorder with the lifetime prevalence of 13.0% and the 12-month prevalence of 8.0% in adults.[1] The core symptomatology is characterized by an intense fear and avoidance of social and performance situations,[2] leading to considerable functional impairment including occupational, academic, and social dysfunction.[3] SAD is associated with alcohol use disorder, sleep difficulties, avoidant personality disorder, and other impairments.[4] Additionally, it is also related to suicide due to the emotion dysregulation and impaired social function. Typically, it turns to be chronic if no treatment is sought. Considering the chronic and persistent course of SAD together with its adverse long-term consequences, early recognition and proper therapy are of utmost importance. Furthermore, it is critically necessary to develop potential predictors that could identify early symptoms without clinical evidence, classify subtypes and monitor the progression.[5]
Presently, treatment for SAD involve monoamine oxidase inhibitors (MAOIs), benzodiazepines, pregabalin, selective serotonin reuptake inhibitors (SSRIs), and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), and cognitive behavioral therapy (CBT)[6] and guidelines recommend SSRIs and CBT as first-line treatments. However, SSRIs tend to be the most widely pharmacological option.
Paroxetine, an SSRI, has been approved by the Food Administration as an effective agent for SAD.[7] Several randomized controlled trials (RCTs) have also been conducted to examine its efficacy and tolerability, however, the results were inconsistent. Additionally, there was no meta-analysis available to investigate the comprehensive efficacy and tolerability of paroxetine in SAD patients. Thus, we systematically reviewed all published and unpublished RCTs and performed a meta-analysis to explore the efficacy and tolerability of paroxetine in adults with SAD.
2. Methods
This meta-analysis was performed according to the protocol provided as Appendix 1 Supplemental Content and was reported as recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (see Reporting guidelines Checklist).[8] All the included data were based on published studies, and therefore no ethical issues were involved.
2.1. Search strategy
Xinyuan Li and a second author independently searched the PubMed, Embase, Web of Science, and Cochrane Center Register of Controlled Trials (CENTRAL) from inception to December 4, 2017 using the search terms (paroxetine OR paxil OR BRL-29060 OR seroxat OR brisdelle OR LDMP OR pexeva) AND (SAD OR social phobia OR social anxiety). Searches were limited to RCTs and publications in the English language. See Appendix 2 Supplemental Content for the detailed search strategy. Manual searches of the reference lists for all relevant articles were conducted, and corresponding authors of some trials were contacted for missing information. Additionally, we searched ClinicalTrials.gov to identify eligible trials that were registered but not yet published. However, conference abstracts or letters to editor were excluded. The search was updated on January 10, 2017 using the same search strategy.
2.2. Inclusion and exclusion criteria
Inclusion criteria were:
-
1.
population: ≥18 years of age with a diagnosis of SAD according to the Diagnosis and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)[9] or later edition;
-
2.
study design: placebo-controlled RCTs;
-
3.
intervention: paroxetine;
-
4.
outcomes: efficacy and tolerability outcomes.
Trials were excluded if they included patients with:
-
1.
primary diagnosis of Axis I disorders that was clinically predominant other than SAD or avoidant personality disorder within the past 6 months;
-
2.
use of psychotherapy within 6 months or electroconvulsive therapy within the past 3 months of baseline;
-
3.
use of psychotropic medications or antidepressants within 14 days of baseline, MAOIs or fluoxetine within 4 weeks of baseline; depot neuroleptics within 12 weeks of baseline;
-
4.
concomitant therapy with β-adrenergic blockers, MAOIs, benzodiazepines, or other psychoactive medications;
-
5.
history of alcohol or any psychoactive substance abuse or dependence within the past 6 months;
-
6.
risk of suicide;
-
7.
previous treatment with paroxetine before randomization;
-
8.
a history of seizures, schizophrenia, bipolar disorder, body dysmorphic disorder, or any serious medical illness that precluded paroxetine use.
Trials were also excluded if Clinical Global Impression Improvement (CGI-I) scale score ≤ 2 or 17 items of Hamilton Rating Scale for Depression (HAM-D) score ≥ 15.
2.3. Data extraction
Xinyuan Li and Yingying Su independently assessed eligible trials and disagreements were resolved by discussion with a third review author until consensus was reached. The following data were extracted: first author's name, year of publication, study design, patient population, sample, age, sex distribution, intervention, treatment duration, and efficacy and tolerability outcomes.
2.4. Outcomes and definitions
The primary efficacy outcomes were mean changes in the Liebowitz Social Anxiety Scale (LSAS) total score (a 24-question assessment of fear and avoidance of public and social situations), the fear and avoidance subscale of LSAS, Clinical Global Impression Severity of Illness (CGI-S) scale score, the Social Avoidance and Distress Scale (SADS) (a measure of social avoidance and discomfort) and the Sheehan Disability Scale (SDS) for work, social, and family items (a measure of quality of life) from baseline to endpoint. The key secondary efficacy parameters were response and remission rates. The response was defined as very much improved (score = 1) or much improved (score = 2) and remission was defined as very much improved (score = 1) according to CGI-I scale. With regard to tolerability outcomes, the primary tolerability outcomes were rates of overall discontinuation, discontinuation due to adverse events (AEs), and discontinuation due to lack of efficacy. The secondary tolerability outcome was the incidence of any AEs and most commonly treatment-emergent adverse events (TEAEs).
2.5. Quality assessment
Two authors independently assessed the risk of bias in included trials using the Cochrane Collaboration's Risk of Bias Tool[10] and disagreements were resolved by discussion with Xinyuan Li until consensus was reached. Reviewers examined seven domains including random sequence generation, allocation concealment, blinding of outcome assessment, blinding of participants and personnel, incomplete outcome data, selective reporting, and other bias. Risk of bias was categorized as low, high, or unclear.
2.6. Statistical analysis
The statistical analysis was performed using Review Manager 5.3 (Cochrane Collaboration, London, UK). All analyses were conducted on the intent-to-treat (ITT) populations. Mean differences (MDs) with 95% confidence intervals (CIs) were calculated for continuous variables, and odds ratios (ORs) with 95% CIs were calculated for dichotomous variables. The significance of the pooled estimates was determined by the Z statistic; statistical significance was set at P < .05.[11] A random-effects model was used to pool studies with substantial heterogeneity, as determined by the chi-squared test (P < .05) and the inconsistency index (I2 ≥ 50%).[12,13] Publication bias was assessed with funnel plots and the Begg/Egger's test using Stata 12.0 software.[14] We also conducted sensitivity analyses to evaluate the stability of the outcomes.
3. Results
3.1. Characteristics of included studies
The searches identified a total of 448 articles. The titles and abstracts were screened then 415 articles were excluded. Among these, 143 were irrelevant, 230 were duplicates, and 42 were reviews. The full text of 33 articles was examined, and 22 articles were excluded due to inappropriate study design, study population, or other reasons. Finally, 11 articles that described 13 RCTs were considered eligible for inclusion in our meta-analysis (Fig. 1).
Figure 1.
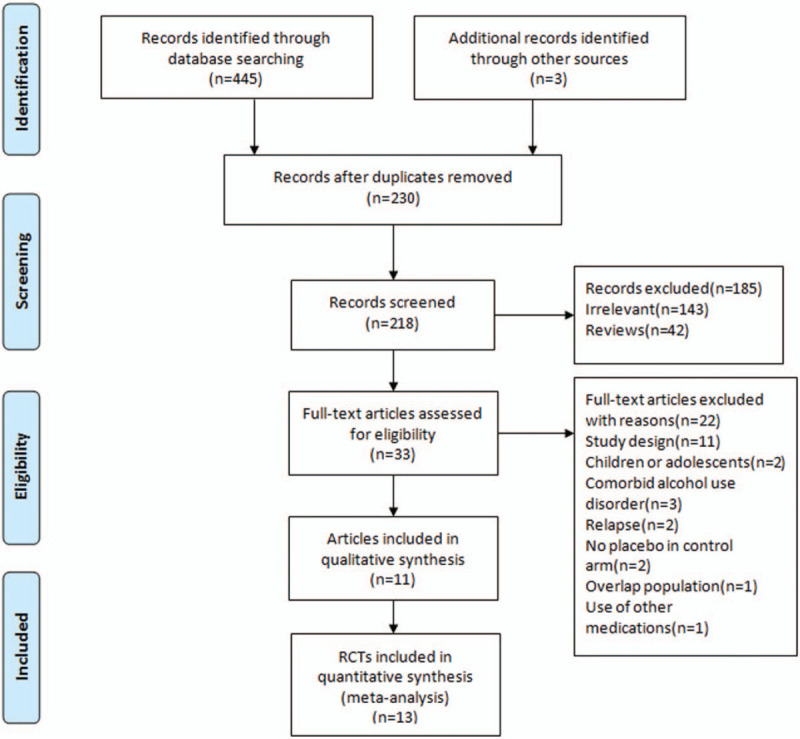
PRISMA flow diagram. RCTs = randomized controlled trials.
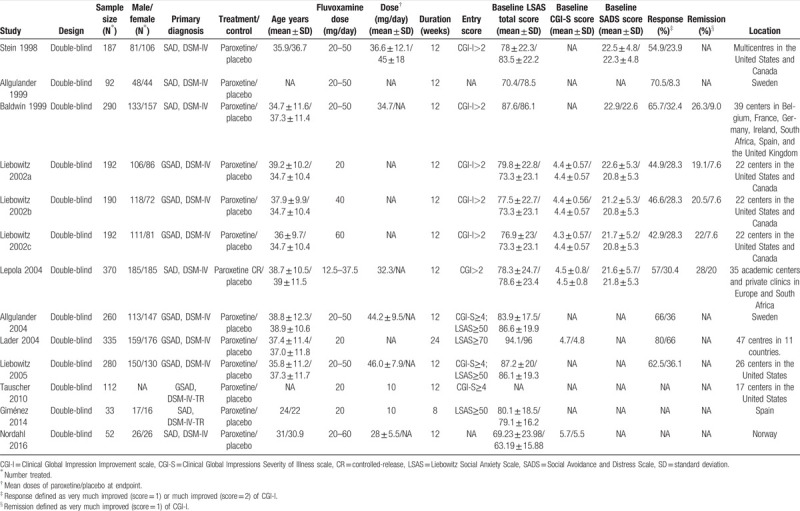
The characteristics of the included trials were shown in Table 1. The RCTs were conducted from 1998 to 2016. The trials included 2593 adult patients with SAD (paroxetine: 1281; placebo: 1312). Among the included trials, eleven trials lasted 12 weeks; one trial lasted 8 weeks, and one trial lasted 24 weeks. Six trials administered fixed doses of paroxetine at 20, 40, and 60 mg/day; five trials administered flexible doses of 20 to 50 mg/day; one trial administered a flexible dose of 20 to 60 mg/day, and the remaining one administered a flexible dose of 12.5 to 37.5 mg/day.
Table 1.
Characteristics of the included studies.
3.2. Quality assessment
Overall, risk of bias in the included RCTs was shown in Figure 2. Risk of bias across studies was shown in Figure 2A and risk of bias in individual studies was shown in Figure 2B.
Figure 2.
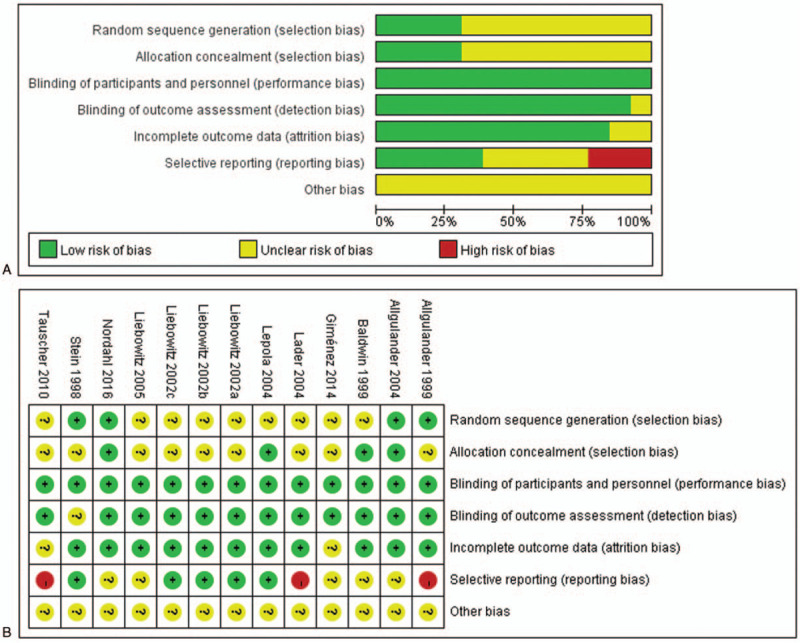
Risk of bias in the included RCTs. (A) risk of bias graph; (B) risk of bias summary. “-” = high risk, “?” = unclear risk, “+”=low risk.
3.3. Publication bias
Visual inspection of the funnel plot as well as the results of Egger's test both revealed there was no significant publication bias (P = .662) (Fig. 3).
Figure 3.
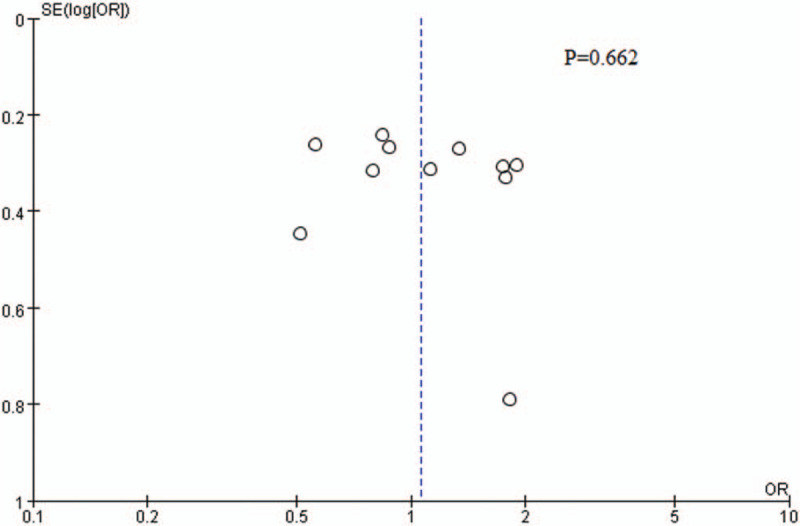
Funnel plot of publication bias.
3.4. Outcomes
3.4.1. Primary efficacy outcomes
Mean change in LSAS total score from baseline to endpoint was reported in 10 trials.[15–22] There was no significant difference in baseline LSAS total score between paroxetine and placebo groups (MD = 0.63, 95%CI −1.40 to 2.66, P = .54). However, change in the LSAS total score was significantly greater in patients with SAD that received duloxetine compared to those received placebo (MD = 13.46, 95%CI 10.59–16.32, P < .00001) (Fig. 4). No evidence of significant heterogeneity was found (P = .53, I2 = 0%).
Figure 4.
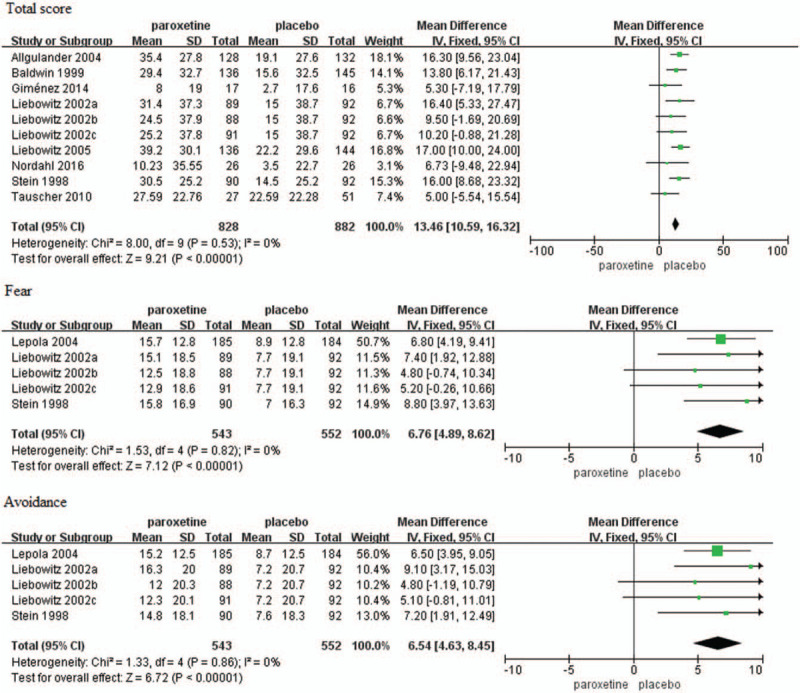
Mean changes in the LSAS total score, fear and avoidance subscale of LSAS scores. CI = confidence interval, SD = standard deviation.
Mean changes in the fear and avoidance subscale of LSAS score from baseline to endpoint were reported in five trials.[18,22,23] The baseline scores had no significant difference between paroxetine and placebo groups (fear: MD = 0.90, 95%CI −0.71 to 2.50, P = .27; avoidance: MD = 1.32, 95%CI −1.77 to 4.41, P = .40). Changes in the fear and avoidance subscale of LSAS score were both significantly higher in the paroxetine group as compared with placebo group (fear: MD = 6.76, 95%CI 4.89–8.62, P < .00001; avoidance: MD = 6.54, 95%CI 4.63–8.45, P < .00001) (Fig. 4). However, there was no evidence of significant heterogeneity (fear: P = .82, I2 = 0%; avoidance: P = .86, I2 = 0%).
Mean change in CGI-S score from baseline to endpoint was reported in five trials.[16,18,23] The baseline score had no significant difference (MD = −0.00, 95%CI −0.16 to 0.16, P = .99). Change in the CGI-S score was significantly greater in patients with SAD that received paroxetine compared to those received placebo (MD = 0.62, 95%CI 0.48–0.76, P < .00001) (Fig. 5). No significant heterogeneity was identified (P = .62, I2 = 0%).
Figure 5.
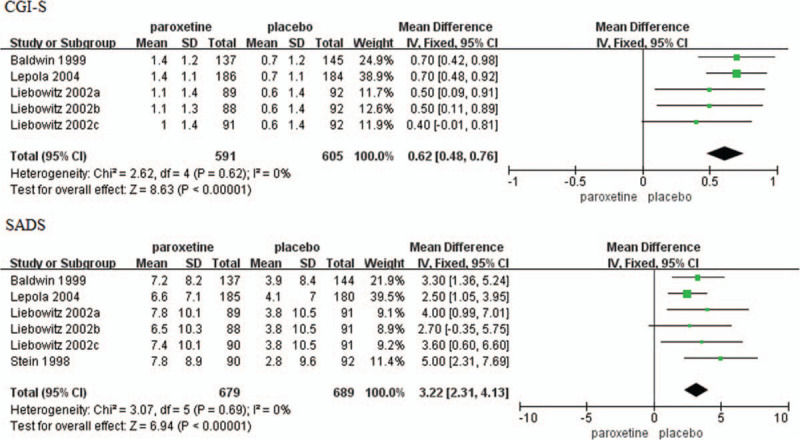
Mean changes in CGI-S score and SADS total score. CGI-S = Clinical Global Impression Severity of Illness, CI = confidence interval, SADS = Social Avoidance and Distress Scale, SD = standard deviation.
Mean change in SADS total score from baseline to endpoint was reported in six trials.[16,18,21,23] The baseline score had no significant difference (MD = 0.49, 95%CI −0.14 to 1.11, P = .13). Change was significantly greater in patients with SAD that received paroxetine compared to those received placebo (MD = 3.22, 95%CI 2.31–4.13, P < .00001) (Fig. 5). No significant heterogeneity was identified (P = .69, I2 = 0%).
Mean changes in work, social, and family items of SDS scores from baseline to endpoint were reported in six trials.[18,21,23] The baseline scores had no significant difference, but changes in work, social, and family items of SDS scores were all significantly higher in the paroxetine group as compared with placebo group (work item: MD = 0.93, 95%CI 0.56–1.29, P < .00001; social item: MD = 1.13, 95%CI 0.77–1.48, P < .00001; family item: MD = 0.53, 95%CI 0.22–0.83, P = .0006) (Fig. 6).
Figure 6.
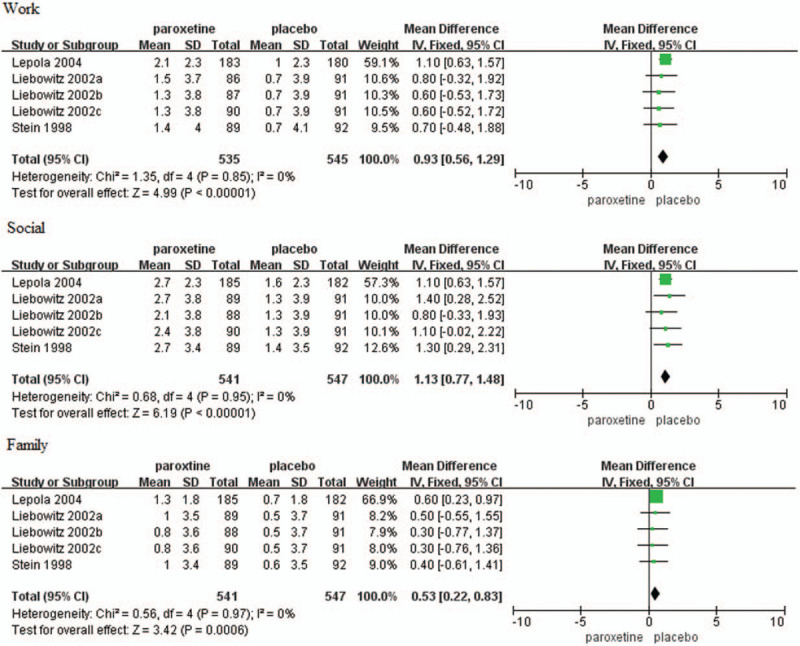
Mean changes in work, social and family items of SDS scores. CI = confidence interval, SD = standard deviation.
3.4.2. Secondary efficacy outcomes
Response rate was reported in 10 trials[15–16,18–19,21,23–25] and the result was significantly greater in patients with SAD that received paroxetine compared to those received placebo (OR = 3.02, 95%CI 2.30–3.97, P < .00001) (Fig. 7). There was significant heterogeneity (P = .01, I2 = 57%).
Figure 7.
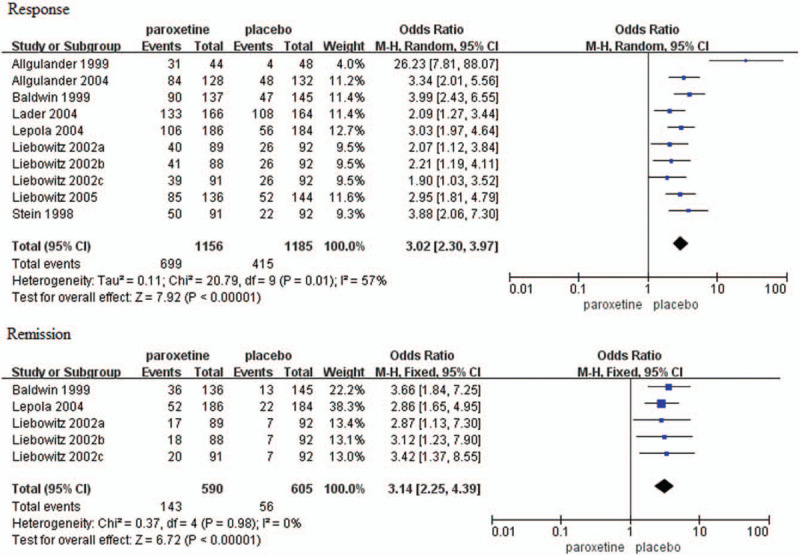
Forest plots of response and remission rates. CI = confidence interval.
Remission rate was reported in five trials[16,18,23] and the result was significantly greater in patients with SAD that received paroxetine compared to those that received placebo (OR = 3.14, 95%CI 2.25–4.39, P < .00001) (Fig. 7). There was no evidence of significant heterogeneity (P = .98, I2 = 0%).
3.4.3. Primary tolerability outcomes
Primary tolerability outcomes comprised the discontinuation rates due to any reason, AEs, and lack of efficacy. There was no significant difference in discontinuation rate due to any reason between two groups (OR = 1.06, 95%CI 0.81–1.39, P = .65) (Fig. 8). Heterogeneity was detected (P = .02, I2 = 52%), thus, a random-effects model was used. Discontinuation rate due to AEs was higher in paroxetine group than placebo group (OR = 3.41, 95%CI 2.45–4.72, P < .00001) whereas the rate due to lack of efficacy was higher in placebo group as compared with paroxetine group (OR = 0.14, 95%CI 0.09–0.22, P < .00001) (Fig. 8). There was no substantial heterogeneity (AEs: P = .59, I2 = 0%; lack of efficacy: P = .76, I2 = 0%).
Figure 8.
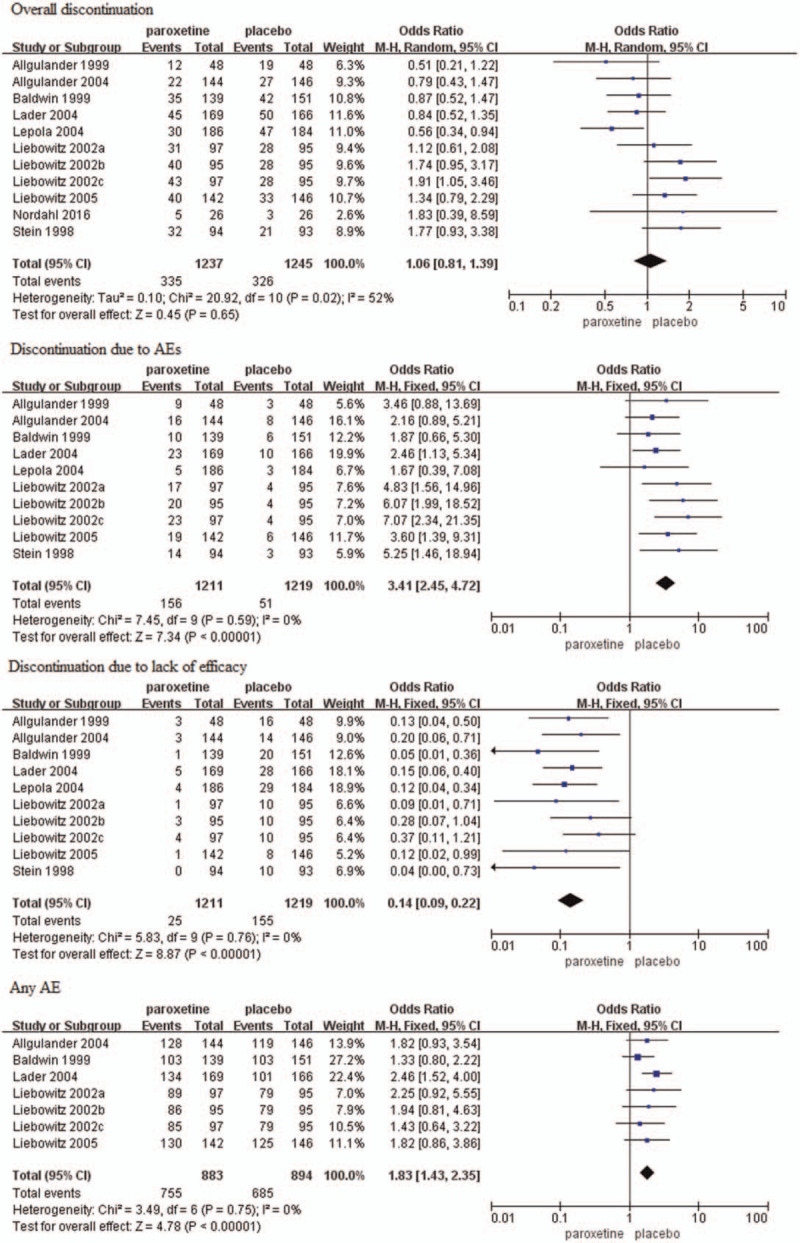
Forest plots of rates of overall discontinuation, discontinuation due to AEs, discontinuation due to lack of efficacy, and the incidence of any AE. AE = adverse event, CI = confidence interval.
3.4.4. Secondary tolerability outcomes
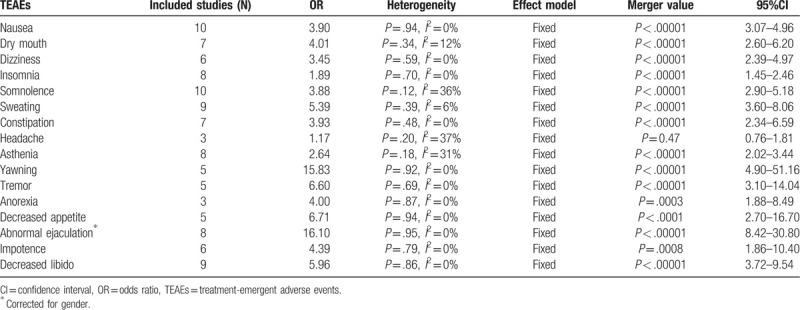
The incidence of overall AEs (any AE) was reported in seven trials[15–16,18–19,25] and the result was significantly higher in paroxetine than placebo group (OR = 1.83, 95%CI 1.43–2.35, P < .00001) (Fig. 8). There was no substantial heterogeneity (P = .75, I2 = 0%). Among all the included studies, the most frequently reported TEAEs were nausea, dry mouth, dizziness, insomnia, somnolence, sweating, constipation, headache, asthenia, yawning, tremor, anorexia, decreased appetite, and sexual problems. See Table 2 for the analysis of all common frequently TEAEs and most were higher in patients that received paroxetine than those received placebo. However, it should be noted that there was no significant difference in the incidence of headache.
Table 2.
Meta-analysis of the most frequent treatment-emergent adverse events.
3.5. Sensitivity analysis
We conducted sensitivity analyses of the main outcomes and the results were robust and stable (see Appendix 3 Supplemental Content).
4. Discussion
Our meta-analysis investigated the efficacy and tolerability of paroxetine in adults with SAD. We found that mean change in the LSAS total score, the fear and avoidance subscale of LSAS, CGI-S, SADS, and work, social and family items of SDS scores were all significantly greater in patients with SAD that received paroxetine compared to those received placebo. In addition, response and remission rates were both higher in paroxetine group as compared with placebo group. These results were consistent with previous reviews indicating that SSRIs were the most widely effective treatment choices in treating SAD.[4,26–28] There was no significant difference in discontinuation rates due to any reason between two groups. Discontinuation rate due to AEs, most of commonly reported TEAEs were higher in paroxetine group as compared with placebo group, whereas discontinuation rate due to lack of efficacy was higher in placebo group as compared with paroxetine group. It was worth mentioning that the incidence of headache had no significant difference between paroxetine and placebo groups and it was considered probably to be unrelated to paroxetine. However, further studies were warranted to be conducted to explore this event.
To the best of our knowledge, this was the first meta-analysis which evaluated the efficacy and tolerability of paroxetine in adult patients with SAD. Although there were some meta-analyses for efficacies of pharmacotherapy including paroxetine in SAD, there has been no meta-analysis estimating the comprehensive efficacy and tolerability of paroxetine in SAD patients. In addition, evaluation of tolerability or safety of paroxetine in those mixed pharmacotherapy meta-analyses was not clear. However, selection of an effective pharmacologic agent is dependent on efficacy as well as tolerability or safety. Therefore, our meta-analysis provided more evidence for the SAD treatment landscape, showing that paroxetine was an effective and well-tolerated treatment option for adults with SAD.
Furthermore, cost-effectiveness is another aspect which should be taken into consideration when selecting a pharmacologic agent. A previous economic analysis suggested that paroxetine was more cost-effective than fluvoxamine, pregabalin, and venlafaxine while paroxetine was less cost-effective than escitalopram/citalopram and sertraline.[29] Moreover, a mixed treatment meta-analysis showed that there was no significant difference in different drugs in terms of effectiveness.[3,30] However, additional network meta-analyses were warranted to clearly contrast efficacies of different drugs.
The present analysis had some strength. First, the analysis of 13 RCTs owned large sample size of 2593 patients. Second, the trials included in this analysis that was all multicentered, randomized, double-blind, and placebo-controlled trials proved to be of high quality. Third, we conducted manual searches of the reference lists and ClinicalTrials.gov to identify eligible trials that were registered for missing information.
Some limitations of this meta-analysis should be taken into account. First, no restriction was imposed on paroxetine dose or treatment duration, which may increase heterogeneity among the included trials. We attempted to overcome this limitation using sensitivity analyses that showed robust results. Secondly, some trials were excluded from several outcome analyses as data were not available (e.g., only three studies were included in the analysis of the incidence of headache). Finally, the severity (mild, moderate, or severe) of SAD was not predefined.
In conclusion, this was the first meta-analysis to evaluate the efficacy and tolerability of paroxetine in treating SAD. The results showed that paroxetine was effective in relieving the symptoms of fear and avoidance and improving social participation. In terms of tolerability or acceptance, paroxetine was proved to be generally well-tolerated despite higher discontinuation rate due to AEs in comparison with placebo, Besides, the incidence of serious AEs should be further assessed.
Acknowledgments
Xinyuan Li made the greatest contribution to this work in all aspects among all the authors. Besides, we would like to thank Dong An who assisted with the preparation of figures and tables.
Author contributions
Conceptualization: Xinyuan Li.
Data curation: Xinyuan Li, Yanbo hou, Yingying Su, Beilin Zhang.
Formal analysis: Xinyuan Li.
Investigation: Xinyuan Li, Yanbo hou, Beilin Zhang.
Methodology: Xinyuan Li, Yanbo hou, Yingying Su.
Project administration: Xinyuan Li.
Resources: Xinyuan Li, Hongping Liu.
Software: Yanbo hou, Yingying Su, Beilin Zhang.
Supervision: Xinyuan Li, Shaokuan fang.
Validation: Xinyuan Li, Yanbo hou.
Visualization: Xinyuan Li, Hongping Liu.
Writing – original draft: Xinyuan Li.
Writing – review & editing: Xinyuan Li, Shaokuan fang.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AE = adverse event, CBT = cognitive behavioural therapy, CENTRAL = Cochrane Central Register of Controlled Trials, CGI-I = Clinical Global Impression Improvement, CGI-S = Clinical Global Impression Severity of Illness, CI = confidence interval, DSM-IV = Diagnosis and Statistical Manual of Mental Disorders, Fourth Edition, HAM-D = Hamilton Rating Scale for Depression, ITT = intent-to-treat, LSAS = Liebowitz Social Anxiety Scale, MD = mean difference, MOAIs = monoamine oxidase inhibitors, OR = odds ratio, PRISMA = Preferred Reporting Items for Systematic Review and Meta-Analyses, RCTs = randomized controlled trials, SAD = social anxiety disorder, SD = standard deviation, SE = standard error, SSRIs = selective serotonin reuptake inhibitors, TEAE = treatment-emergent adverse event.
How to cite this article: Li X, Hou Y, Su Y, Liu H, Zhang B, Fang S. Efficacy and tolerability of paroxetine in adults with social anxiety disorder: A meta-analysis of randomized controlled trials. Medicine. 2020;99:14(e19573).
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
XYL and YBH contributed equally to this work.
This work was supported by the National Natural Science Foundation of China (81873794).
The authors have no conflict of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci 2015;17:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed.Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- [3].de Menezes GB, Coutinho ES, Fontenelle LF, et al. Second-generation antidepressants in social anxiety disorder: meta-analysis of controlled clinical trials. Psychopharmacology (Berl) 2011;215:1–1. [DOI] [PubMed] [Google Scholar]
- [4].Davis ML, Smits JA, Hofmann SG. Update on the efficacy of pharmacotherapy for social anxiety disorder: a meta-analysis. Expert Opin Pharmacother 2014;15:2281–91. [DOI] [PubMed] [Google Scholar]
- [5].Girardi P, Pompili M, Innamorati M, et al. Duloxetine in acute major depression: review of comparisons to placebo and standard antidepressants using dissimilar methods. Hum Psychopharmacol 2009;24:177–90. [DOI] [PubMed] [Google Scholar]
- [6].Williams T, Hattingh CJ, Kariuki CM, et al. Pharmacotherapy for social anxiety disorder (SAnD). Cochrane Database Syst Rev 2017;10:CD001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Germann D, Ma G, Han F, et al. Paroxetine hydrochloride. Profiles Drug Subst Excip Relat Methodol 2013;38:367–406. [DOI] [PubMed] [Google Scholar]
- [8].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4 ed.Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- [10].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moraros J, Nwankwo C, Patten SB, et al. The association of antidepressant drug usage with cognitive impairment or dementia, including Alzheimer disease: a systematic review and meta-analysis. Depress Anxiety 2017;34:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li X, Zhu L, Su Y, et al. Short-term efficacy and tolerability of venlafaxine extended release in adults with generalized anxiety disorder without depression: a meta-analysis. PLoS One 2017;12:e0185865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Swartzman S, Booth JN, Munro A, et al. Posttraumatic stress disorder after cancer diagnosis in adults: a meta-analysis. Depress Anxiety 2017;34:327–39. [DOI] [PubMed] [Google Scholar]
- [14].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [15].Allgulander C, Mangano R, Zhang J, et al. Efficacy of Venlafaxine ER in patients with social anxiety disorder: a double-blind, placebo-controlled, parallel-group comparison with paroxetine. Hum Psychopharmacol 2004;19:387–96. [DOI] [PubMed] [Google Scholar]
- [16].Baldwin D, Bobes J, Stein DJ, et al. Paroxetine in social phobia/social anxiety disorder. Randomised, double-blind, placebo-controlled study. Paroxetine Study Group. Br J Psychiatry 1999;175:120–6. [DOI] [PubMed] [Google Scholar]
- [17].Giménez M, Ortiz H, Soriano-Mas C, et al. Functional effects of chronic paroxetine versus placebo on the fear, stress and anxiety brain circuit in Social Anxiety Disorder: initial validation of an imaging protocol for drug discovery. Eur Neuropsychopharmacol 2014;24:105–16. [DOI] [PubMed] [Google Scholar]
- [18].Liebowitz MR, Stein MB, Tancer M, et al. A randomized, double-blind, fixed-dose comparison of paroxetine and placebo in the treatment of generalized social anxiety disorder. J Clin Psychiatry 2002;63:66–74. [DOI] [PubMed] [Google Scholar]
- [19].Liebowitz MR, Gelenberg AJ, Munjack D. Venlafaxine extended release vs placebo and paroxetine in social anxiety disorder. Arch Gen Psychiatry 2005;62:190–8. [DOI] [PubMed] [Google Scholar]
- [20].Nordahl HM, Vogel PA, Morken G, et al. Paroxetine, cognitive therapy or their combination in the treatment of social anxiety disorder with and without avoidant personality disorder: a randomized clinical trial. Psychother Psychosom 2016;85:346–56. [DOI] [PubMed] [Google Scholar]
- [21].Stein MB, Liebowitz MR, Lydiard RB, et al. Paroxetine treatment of generalized social phobia (social anxiety disorder): a randomized controlled trial. JAMA 1998;280:708–13. [DOI] [PubMed] [Google Scholar]
- [22].Tauscher J, Kielbasa W, Iyengar S, et al. Development of the 2nd generation neurokinin-1 receptor antagonist LY686017 for social anxiety disorder. Eur Neuropsychopharmacol 2010;20:80–7. [DOI] [PubMed] [Google Scholar]
- [23].Lepola U, Bergtholdt B, St Lambert J, et al. Controlled-release paroxetine in the treatment of patients with social anxiety disorder. J Clin Psychiatry 2004;65:222–9. [DOI] [PubMed] [Google Scholar]
- [24].Allgulander C. Paroxetine in social anxiety disorder: a randomized placebo-controlled study. Acta Psychiatr Scand 1999;100:193–8. [DOI] [PubMed] [Google Scholar]
- [25].Lader M, Stender K, Bürger V, et al. Efficacy and tolerability of escitalopram in 12- and 24-week treatment of social anxiety disorder: randomised, double-blind, placebo-controlled, fixed-dose study. Depress Anxiety 2004;19:241–8. [DOI] [PubMed] [Google Scholar]
- [26].Blanco C, Bragdon LB, Schneier FR, et al. The evidence-based pharmacotherapy of social anxiety disorder. Int J Neuropsychopharmacol 2013;16:235–49. [DOI] [PubMed] [Google Scholar]
- [27].Curtiss J, Andrews L, Davis M, et al. A meta-analysis of pharmacotherapy for social anxiety disorder: an examination of efficacy, moderators, and mediators. Expert Opin Pharmacother 2017;18:243–51. [DOI] [PubMed] [Google Scholar]
- [28].Stein DJ, Baldwin DS, Bandelow B, et al. A 2010 evidence-based algorithm for the pharmacotherapy of social anxiety disorder. Curr Psychiatry Rep 2010;12:471–7. [DOI] [PubMed] [Google Scholar]
- [29].Mavranezouli I, Mayo-Wilson E, Dias S, et al. The cost effectiveness of psychological and pharmacological interventions for social anxiety disorder: a model-based economic analysis. PLoS One 2015;10:e0140704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hansen RA, Gaynes BN, Gartlehner G, et al. Efficacy and tolerability of second-generation antidepressants in social anxiety disorder. Int Clin Psychopharmacol 2008;23:170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


