Abstract
Although banned from production for decades, PCBs remain a significant risk to human health. A primary target of concern is the developing brain. Epidemiological studies link PCB exposures in utero or during infancy to increased risk of neuropsychiatric deficits in children. Nonclinical studies of legacy congeners found in PCB mixtures synthesized prior to the ban on PCB production suggest that non-dioxin-like (NDL) congeners are predominantly responsible for the developmental neurotoxicity associated with PCB exposures. Mechanistic studies suggest that NDL PCBs alter neurodevelopment via ryanodine receptor-dependent effects on dendritic arborization. Lightly chlorinated congeners, which were not present in the industrial mixtures synthesized prior to the ban on PCB production, have emerged as contemporary environmental contaminants, but there is a paucity of data regarding their potential developmental neurotoxicity. PCB 11, a prevalent contemporary congener, is found in the serum of children and their mothers, as well as in the serum of pregnant women at increased risk for having a child diagnosed with a neurodevelopmental disorder (NDD). Recent data demonstrates that PCB 11 modulates neuronal morphogenesis via mechanisms that are convergent with and divergent from those implicated in the developmental neurotoxicity of legacy NDL PCBs. This review summarizes these data and discusses their relevance to adverse neurodevelopmental outcomes in humans.
Keywords: Axonal outgrowth, calcium signaling, CREB, dendritic arborization, neuronal morphogenesis, neurodevelopmental disorders, persistent organic pollutants, ryanodine receptor
1. Introduction
Polychlorinated biphenyls (PCBs) are a class of 209 structurally related chemicals, or congeners, comprised of a biphenyl with a variable number of chlorine substitutions in varying positions on the benzene rings. PCBs are broadly categorized as dioxin-like (DL) or non-dioxin-like (NDL) congeners based on their three-dimensional structure and affinity for the arylhydrocarbon receptor (AhR). DL congeners are co-planar and bind to the AhR with moderate-high affinity, whereas the NDL congeners are non-coplanar with negligible to no binding affinity for the AhR [reviewed in (Pessah et al. 2010)] (Figure 1).
Figure 1. Examples of non-dioxin-like (NDL) and dioxin-like (DL) PCB congeners.
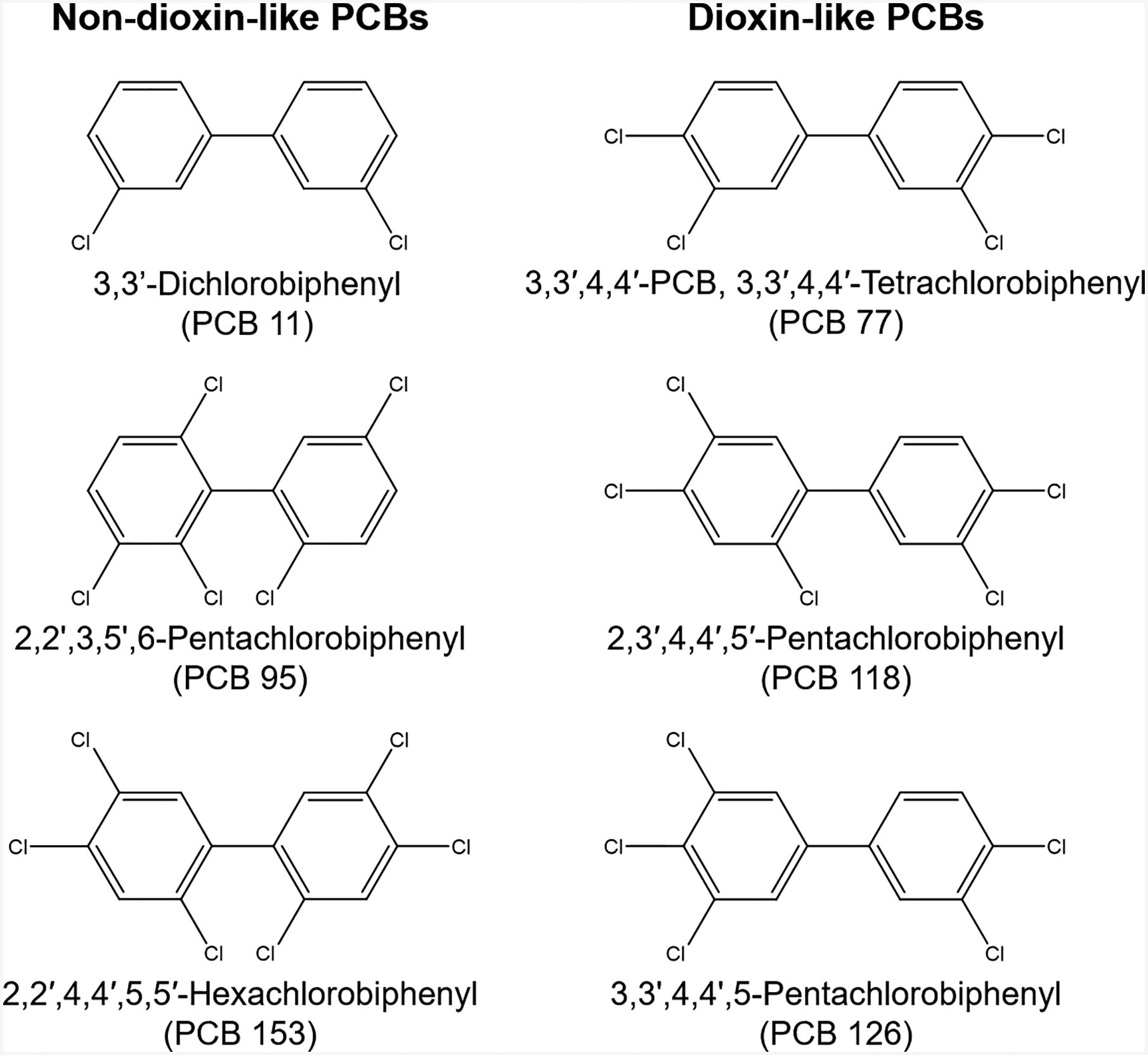
The higher chlorinated, legacy NDL PCBs have more than > 1 ortho-substituted chlorine, which creates steric hindrance, thereby preventing these congeners from assuming a coplanar configuration. NDL congeners have negligible or no activity at the AhR. Lightly chlorinated, non-Aroclor or “contemporary” PCBs like PCB 11 also have no activity at the AhR. In contrast, DL PCB congeners typically have ≤ 1 chlorine at the ortho positions of the biphenyl and are at their lowest energy state when lying in a coplanar configuration, like dioxin. Similar to dioxin, these congeners also have activity at the arylhydrocarbon receptor (AhR).
During the 20th century, PCBs were synthesized and sold globally as commercial mixtures (Aroclor®, Clophen®, Phenclor®, or Kanechlor®) that varied by the percentage of chlorine by mass. While these mixtures contained both DL and NDL congeners, the specific congener profile varied between Aroclor mixtures. The chemical stability of the higher-chlorinated PCBs that predominated in these commercial mixtures made them desirable for numerous industrial and commercial applications, but also conferred resistance to environmental degradation. This environmental persistence combined with their lipophilic nature resulted in significant bioaccumulation of PCBs in food webs, including human food supplies (McIntyre and Beauchamp 2007). The realization in the 1960s that PCBs were pervasive pollutants (Jensen 1972), coupled with growing concern regarding human cancer risks associated with PCB exposure, led the United States Congress to ban PCB production in the United States in 1979. The Stockholm Convention on Persistent Organic Pollutants (POPs) instituted a more global ban on PCB production in 2001 (Carpenter 2006; White and Birnbaum 2009). Following these regulatory efforts, environmental levels of these “legacy” PCBs present in commercial mixtures steadily decreased. However, these higher-chlorinated legacy PCBs remain a risk to human health due to continued use of old equipment containing PCBs, leaching of PCBs from hazard waste sites, and off-gassing of PCBs from aging construction materials (Consonni et al. 2012; Hopf et al. 2009; Koh et al. 2015).
Emerging evidence indicates that a significant component of contemporary human PCB exposures includes PCB congeners not present in Aroclors and other “legacy” commercial mixtures. Data collected over the last decade have documented increasing levels of “non-legacy” or “contemporary” PCB congeners in various environmental media (Hornbuckle and Robertson 2010; Hu and Hornbuckle 2010; Koh et al. 2015), including indoor and outdoor air (Hu et al. 2008) and human foods (Chen et al. 2017). These contemporary PCB congeners, many of which are more lightly chlorinated than the legacy congeners, are inadvertent byproducts of current pigment manufacturing processes. For example, PCB 11, a lightly chlorinated congener not found in Aroclors or other commercial mixtures, is generated during the synthesis of paint pigments, particularly azo/diarylide (yellow) and phthalocyanine (blue, green) pigments (Guo et al. 2014; Hu and Hornbuckle 2010; Shang et al. 2014). While congeners associated with the legacy PCB mixtures are also detected in various pigments produced using contemporary manufacturing processes, including the DL PCBs 77, 114, and 123, and the NDL PCB 95, the congeners detected with the greatest frequency in many pigments are PCB 11 and other lightly chlorinated congeners (Hu and Hornbuckle 2010). These pigments are used extensively to not only color paint, but also inks, paper, textiles, leather, plastics, and even cosmetics and food products (Gregory 2000; Stolz 2001). Of concern, studies in the United States have documented exposure to these lightly chlorinated PCBs in children and their mothers living in urban and rural areas of the Midwest (Koh et al. 2016; Koh et al. 2015; Marek et al. 2013). These contemporary PCB congeners have also been detected in the serum of pregnant women living in Northern California who are at increased risk of having a child diagnosed with a NDD (Granillo et al. 2019; Sethi et al. 2017a).
Here, we review the data associating PCBs with adverse neurodevelopmental outcomes, which is a primary endpoint of human health concern for these POPs [reviewed in (Berghuis et al. 2015; Pessah et al. 2019)]. This review primarily summarizes work from our laboratory that we presented at the 10th International PCB Workshop in Krakow, Poland. We examine the experimental evidence demonstrating that NDL legacy PCBs and the contemporary pollutant, PCB 11, disrupt neuronal morphogenesis via divergent and convergent mechanisms. We also discuss the relevance of these findings to human NDDs, and identify critical data gaps in the PCB developmental neurotoxicity literature.
2. PCB developmental neurotoxicity
PCBs first gained attention as developmental neurotoxicants following two accidental human poisonings with cooking oil contaminated with PCBs: the Yusho incident in Japan in 1968 (Mitoma et al. 2015) and the Yu-Cheng incident in Taiwan in 1979 (Hsu et al. 1985). Infants born to women who ingested PCB-contaminated cooking oil while pregnant had a significantly increased incidence and severity of cognitive and psychomotor deficits. While these incidents involved high-level PCB exposures, subsequent epidemiological studies of infants and children exposed to lower, environmentally relevant levels of PCBs during development further suggested that PCBs are developmental neurotoxicants [reviewed in (Pessah et al. 2019)]. Multiple reviews have concluded that the epidemiological literature generally supports the hypothesis that exposure to PCBs during critical developmental periods increases the risk of adverse neuropsychological function in children, evidenced as impairments in executive function, psychomotor function, attention, learning, and memory (Berghuis et al. 2015; Pessah et al. 2019; Schantz et al. 2003). More recently, in utero exposure to PCBs have been positively associated with increased risk of NDDs, including attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) (Cheslack-Postava et al. 2013; Eubig et al. 2010; Granillo et al. 2019; Lyall et al. 2017; Pessah et al. 2019; Rosenquist et al. 2017; Sagiv et al. 2010).
A question of critical importance to assessing the risks of developmental PCB exposure is whether developmental neurotoxicity is generalizable to all PCB congeners. Exposure studies suggest that NDL PCB congeners predominate in human samples, including umbilical cord blood, breast milk, and post-mortem brain [reviewed in (Pessah et al. 2019)]. However, because a variety of analytical techniques are used for detection of PCBs in human tissues and differing PCB congener profiles are often analyzed across cohorts, it has been difficult to discern whether adverse neurodevelopmental outcomes are predominantly associated with specific subsets of PCB congeners. Nonclinical studies, which have largely focused on the legacy PCB congeners, suggest that NDL congeners mediate much of the developmental neurotoxicity associated with the legacy Aroclors and other industrial mixtures [reviewed in (Pessah et al. 2010; Pessah et al. 2019; Sable and Schantz 2006; Schantz et al. 2003; Winneke 2011)]. The question of whether DL congeners are directly neurotoxic to the developing brain remains controversial [reviewed in (Pessah et al. 2019)], although data from animal models indicates that deficits in cognitive function do not appear to be directly driven by DL PCBs [reviewed in (Sable and Schantz 2006)]. However, other nonclinical studies suggest that DL PCBs can influence neurotoxic outcomes of NDL PCBs by inducing expression of cytochrome P450 enzymes that subsequently metabolize NDL PCBs (Curran et al. 2012; Giera et al. 2011; Klinefelter et al. 2018).
It is widely posited that PCBs alter the normal trajectory of neurodevelopment by potentially several different mechanisms, including thyroid hormone (TH) disruption, altered γ-aminobutyric acid (GABA) signaling, or interference with intracellular Ca2+ dynamics [reviewed in (Pessah et al. 2010; Pessah et al. 2019; Winneke 2011)]. Below, we briefly summarize the evidence of PCB action on TH and GABA signaling before providing a more extensive review of the data causally linking PCB effects on Ca2+-dependent signaling to endpoints of direct relevance to human NDDS, specifically altered neuronal morphogenesis.
3. PCB effects on TH and GABA signaling
The scientific premise underlying the TH hypothesis of PCB developmental neurotoxicity is based on epidemiological evidence demonstrating that marked TH deficiency interferes with normal neurodevelopment [reviewed in (Rovet 2014)] and data indicating that developmental exposures to PCBs can decrease serum TH levels in both human and animal models (Hagmar 2003; Martin and Klaassen 2010; Zoeller et al. 2002). There is nonclinical evidence linking the effects of PCBs on circulating TH levels to developmental neurotoxicity. For example, TH supplementation was found to prevent motor and auditory deficits induced by developmental exposure to Aroclor 1254 (Goldey and Crofton 1998). Additionally, in vitro data support a key role for TH disruption in mediating PCB effects on oligodendrocyte maturation and myelination (Nave and Werner 2014).
However, recent epidemiologic studies suggest that developmental PCB exposure in humans is not consistently associated with decreased serum TH levels (Itoh et al. 2018; Li et al. 2018), and nonclinical studies suggest that the cognitive deficits associated with developmental PCB exposure occur independent of decreased TH levels. For example, developmental exposures to Aroclor 1254 at 8 mg/kg/d that significantly reduced serum T4 levels were not associated with learning and memory deficits in rats assessed using the Morris water maze or T-maze (Zahalka et al. 2001). Conversely, developmental exposure to Aroclor 1254 at 1 mg/kg/d caused performance deficits in the Morris water maze in the absence of significantly decreased serum T3 or T4 levels (Yang et al. 2009). Studies examining hippocampal neurogenesis following developmental exposure to Aroclor 1254 at 6 mg/kg/d in the maternal diet observed no effect on neuronal progenitor cell proliferation and survival despite a significant reduction in maternal serum TH (Naveau et al. 2014; Parent et al. 2016). The relevance of the doses of A1254 used in these nonclinical studies to human exposures is suggested by data indicating that total brain PCB levels in weanling rats exposed to Aroclor 1254 in at 1.0 mg/kg/d in the maternal diet ranged from 0.5 to 3.0 ng/g wet weight (Yang et al. 2009). This is well within the range of total PCB levels measured in human postmortem brain, which range from approximately 66 ng/g wet weight in samples from Greenland (Dewailly et al. 1999) to 1.5 ng/g wet weight (range < LOD to 18.5 ng/g ww) in postmortem samples from the United States (Mitchell et al. 2012).
These observations do not rule out thyroid hormone-dependent mechanisms other than PCB-induced hypothyroidism (Pinson et al. 2016; Wadzinski et al. 2014; Zoeller 2007). For example, gestational exposure of rats to Aroclor 1254 at 1 or 4 mg/kg/d in the maternal diet increased expression of TH-responsive genes in the fetal cortex despite significantly reducing maternal levels of serum TH, suggesting direct effects of PCBs TH receptors in the fetal brain (Bansal et al. 2005; Gauger et al. 2004). However, others observed no effect of developmental exposure to Aroclor 1254 at 6 mg/kg/d on brain TH gene expression or genes related to TH function (Royland and Kodavanti 2008). Consistent with the latter observation, recent in vitro studies of PCBs abundant in the serum of pregnant women found no significant agonistic or antagonistic interactions with canonical TH receptors expressed in a TH reporter cell line when exposed to these PCBs singly or in combination over a wide range of concentrations (Sethi et al. 2019). This same study also saw no effect of the hydroxylated or sulfated metabolites of PCB 11 and PCB 52 on TH receptor activity. These observations are consistent with an earlier study that failed to detect a direct interaction between Aroclor 1254 and the THR (Gauger et al. 2004), and screening studies of different PCBs than those tested by Sethi et al. that used reporter cell lines expressing only the THR alpha isoform (Pencikova et al. 2018; Takeuchi et al. 2017). In contrast, other in vitro studies using TH reporter cell lines that expressed only the TH receptor β1 isoform observed agonistic activity of μM concentrations of hydroxylated PCB metabolites (Iwasaki et al. 2002; Miyazaki et al. 2008). There is also evidence that PCBs may affect TH signaling via mechanisms upstream of the TH receptor, such as disruption of the hypothalamic-pituitary-adrenal (HPA) axis (Zimmer et al. 2009) or modulation of crosstalk between TH and other endocrine hormones and nuclear receptors (Kouidhi and Clerget-Froidevaux 2018). However, to date, there are no experimental data directly linking PCB effect on TH signaling to effects of developmental PCB exposure on cognitive function or on neurodevelopmental processes of direct relevance to NDDs.
Experimental evidence suggests that lightly chlorinated NDL PCBs may also cause developmental neurotoxicity via allosteric modulation of the GABAA receptor. An in vitro study using Xenopus oocytes discovered that PCB 28 and PCB 52 interact with the GABAA receptor to potentiate GABA-induced ion current in a concentration-dependent manner at concentrations ≥ 0.3 μM or 10 μM, respectively (Antunes Fernandes et al. 2010b). PCBs 101, 138, 153, or 180 had no effect on GABA-mediated currents, suggesting this mechanism may be unique to lightly chlorinated PCBs. Interestingly, PCB 153 partially attenuated the effect of PCB 28 on GABAA receptor activity, suggesting potential mixture effects. This same group also found that the PCBs 19, 47, 51, and 100 are able to directly activate the GABAA receptor in the absence of GABA, with modulation of ion current depending on the chlorination pattern of the congeners tested (Antunes Fernandes et al. 2010a; Hendriks et al. 2010). In vivo studies demonstrated that while developmental exposure to PCB 52, 138, or 180 at 1 mg/kg/d during gestation and lactation via the maternal diet caused learning and motor deficits in rats, only PCB 52 exposure significantly increased extracellular GABA levels in the cerebellum (Boix et al. 2010).
Collectively, the experimental data identify TH and GABAergic signaling as potential targets of PCBs, but underscore the importance of congener-specific effects and potential interactions between congeners. However, TH and GABAergic signaling may not be the most sensitive mechanisms by which PCBs cause developmental neurotoxicity, as discussed below.
4. NDL PCBs alter synaptic connectivity via Ca2+-dependent mechanisms
The spatiotemporal patterning of cytoplasmic Ca2+ is tightly regulated during normal neurodevelopment [reviewed in (Berridge 2006; Brini et al. 2014)]. Structure-activity relationship (SAR) studies have demonstrated that NDL PCBs (Kodavanti and Tilson 2000; Yang and Kodavanti 2001), but not DL PCBs (Do and Lee 2012), increase intracellular Ca2+ levels and alter Ca2+ signaling in primary neuronal cell cultures. As demonstrated using pharmacologic tools that block specific Ca2+ channels, NDL PCBs can increase levels of intracellular Ca2+ in neurons by activating NMDA receptors or L-type voltage-sensitive Ca2+ channels in the plasma membrane (Inglefield and Shafer 2000; Mundy et al. 1999), and by sensitizing ryanodine receptors (RyR) [reviewed in (Pessah et al. 2010)] and inositol 1,4,5-trisphosphate receptors (Inglefield et al. 2001) in the endoplasmic reticulum. Of these various mechanisms, the most sensitive is RyR sensitization. Thus, long-term exposure (10–13 d) of primary cerebellar neurons to NDL PCB 52 at micromolar (μM) concentrations or to NDL PCBs 138, 153, or 180 at high nanomolar (nM) concentrations disrupted the glutamate-nitric oxide-cGMP pathway via activation of NMDA receptors (Llansola et al. 2010; Llansola et al. 2009). In contrast, NDL PCBs interact directly with RyRs to stabilize these channels in the open configuration over concentrations ranging from picomolar (pM) to nanomolar (nM) to micromolar (μM), depending on the potency of the RyR congener (Holland et al. 2017; Samso et al. 2009). As determined using electrophysiological, biochemical, and cellular approaches, the interaction of NDL PCBs with RyRs exhibits a stringent SAR, including stereoselectivity (Feng et al. 2017; Fritsch and Pessah 2013; Holland et al. 2017; Yang et al. 2014).
Sensitization of the RyR by NDL PCBs increases the frequency and amplitude of Ca2+ oscillations in the somatodendritic domain of primary rat hippocampal neurons in dissociated culture (Wayman et al. 2012a), and alters the plasticity of hippocampal CA1 neurons in acute slice culture (Wong et al. 1997). In primary mouse cortical neurons, the hydroxylated metabolite of the higher chlorinated NDL PCB 106 increases intracellular Ca2+ oscillations at μM concentrations, and pharmacological blockade of the RyR prevents this effect (Londono et al. 2010). In vitro studies using primary rat hippocampal neurons demonstrate that RyR sensitization by pM to nM concentrations of PCB 95, a NDL congener with potent RyR activity, activates two Ca2+-dependent signaling pathways that mediate activity-dependent dendritic arborization and synapse formation during normal neurodevelopment (Wayman et al. 2008; Wayman et al. 2006) (Figure 2). In the first signaling pathway, PCB 95 sensitization of RyRs sequentially activates CaMKK, CaMKIα/γ, MEK/ERK and CREB to increase transcription of Wnt2, which acts as an autocrine factor to promote dendritic growth (Wayman et al. 2012a). In the second pathway, PCB 95 activates CREB to upregulate transcription of miR132, which then suppresses translation of p250GAP mRNA. The resulting decrease in p250GAP promotes synaptogenesis, evident as increased density of dendritic spines and increased frequency of miniature excitatory post-synaptic currents (Lesiak et al. 2014).
Figure 2. Schematic illustrating PCB effects on dendritic arborization.
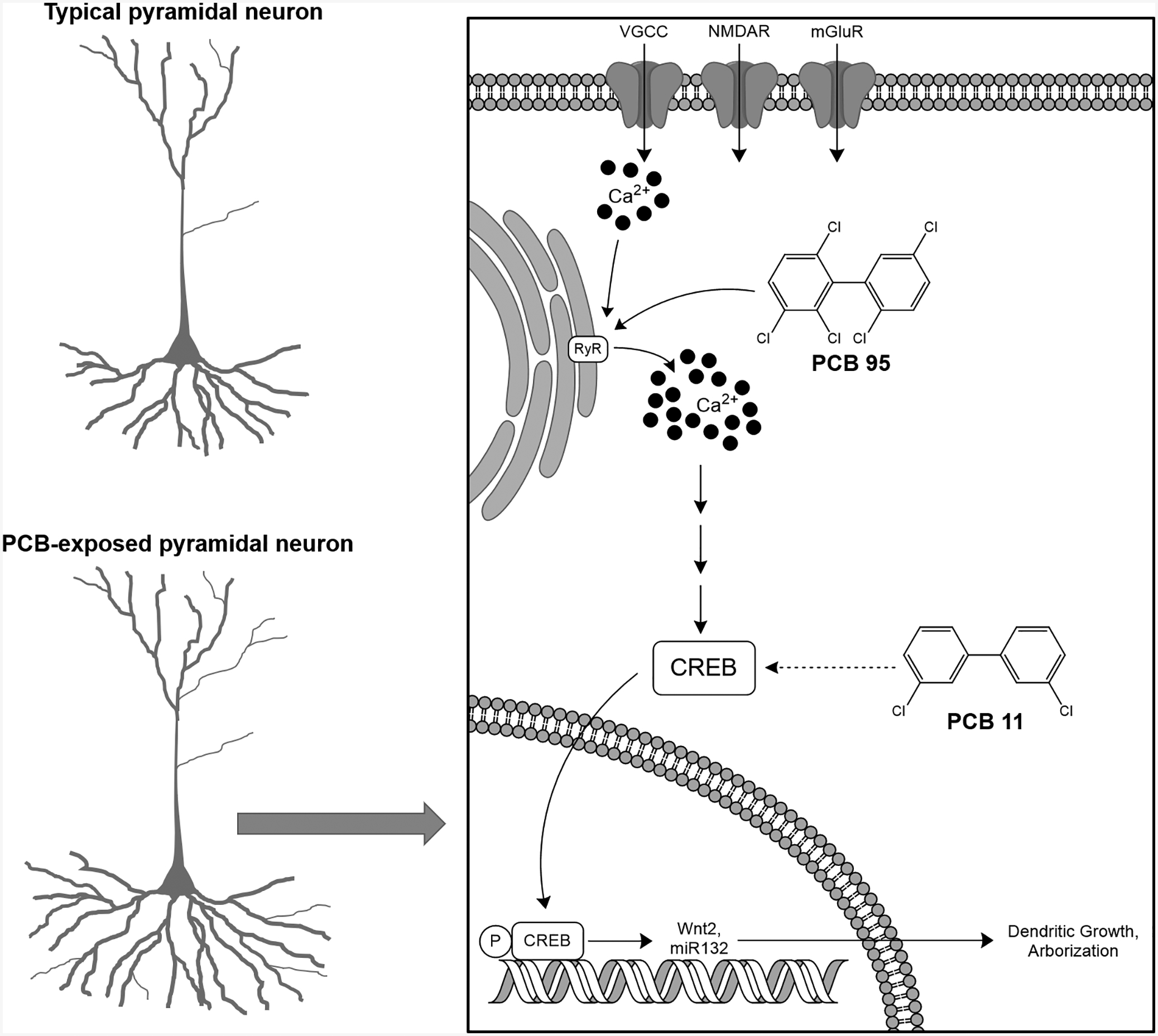
Data available in the peer-reviewed literature indicate that developmental exposure to higher chlorinated NDL PCB congeners, exemplified by PCB 95, or the lightly chlorinated contemporary congener, PCB 11, enhances dendritic arborization in pyramidal neurons of the hippocampus and cortex. PCB 95 and 11 act on different proximal molecular targets, but converge on the CREB signaling pathway. Abbreviations: Ca2+ = calcium, CREB = cAMP response element binding protein; RyR = ryanodine receptor, VDCC = voltage dependent calcium channel.
Several lines of evidence support a causal link between NDL PCB effects on RyR sensitization and promotion of dendritic growth. First, RyR-active PCBs 95 and 136 promote dendritic arborization and synaptogenesis in primary neurons; in contrast, PCB 66, which has physicochemical properties similar to that of PCB 95, but lacks RyR activity, has no significant effect on dendritic morphology (Wayman et al. 2012b; Yang et al. 2014). Second, siRNA knockdown or pharmacological blockade of RyRs inhibits the dendrite and spine enhancing activity of PCBs 95 and 136 (Lesiak et al. 2014; Wayman et al. 2012b; Yang et al. 2014). Third, several RyR-active PCBs are chiral, including PCB 136. PCB 136 atropselectively sensitizes RyRs (Pessah et al. 2009) and demonstrates the same atropselectivity with respect to its effects on dendritic arborization in vitro (Yang et al. 2014). RyR-active NDL PCBs promote dendritic growth in not only rat hippocampal neurons but also mouse hippocampal neurons, and cortical neurons derived from mice or rats (Keil et al. 2019; Wayman et al. 2012b). Furthermore, the morphogenic effects of NDL PCBs are dendrite-selective in they have not been observed to alter axonal growth (Yang et al. 2014). In vitro studies of PCB-induced dendritic growth have consistently revealed a non-monotonic or inverted U-shaped concentration-effect relationship, with dendrite-promoting activity observed in the pM to nM range but not at femtomolar (fM) or micromolar (μM) concentrations (Wayman et al. 2012b; Yang et al. 2014). The biological mechanism(s) contributing to this non-linear concentration-effect relationship are unknown, but are not due to cytotoxicity at the higher concentrations (Wayman et al. 2012b; Yang et al. 2014).
Changes in dendritic growth in response to neural activity (aka “experience”), are considered the biological substrate of associative learning (Pittenger and Kandel 2003). Altered patterns of dendritic arborization during development are associated with neurobehavioral deficits in animal models (Berger-Sweeney and Hohmann 1997) and in humans (Copf 2016; Penzes et al. 2011; Supekar et al. 2013). Thus, it is biologically plausible that PCB effects on dendritic arborization contribute to PCB developmental neurotoxicity. In support of this hypothesis, learning and memory is impaired in rats exposed to Aroclor 1254 at 1, but not 6, mg/kg in the maternal diet (Yang et al. 2009). These behavioral deficits coincided with increased RyR activity, increased basal dendritic arborization and altered dendritic plasticity in brain regions known to be important for performance in the Morris water maze (Yang et al. 2009). This study also demonstrated similar non-monotonic dose-response relationships for the behavioral effects, dendritic effects and RyR sensitization of developmental Aroclor exposure. However, Aroclor effects on serum levels of TH or sex steroids exhibited distinctly different dose-response relationships (Yang et al. 2009). In a separate study, developmental exposure to PCB 95 in the maternal diet similarly enhanced the dendritic arborization of hippocampal CA1 pyramidal neurons in a non-monotonic dose-related manner (Wayman et al. 2012b).
5. PCB 11 modulates neuronal morphogenesis via CREB-dependent mechanism(s)
In contrast to legacy NDL PCBs, there is a paucity of data regarding the potential developmental neurotoxicity of the contemporary lightly chlorinated NDL PCBs. This gap is significant in light of data from recent studies which found mothers who are at increased risk of having a child diagnosed with a NDD (Hertz-Picciotto et al. 2018) had elevated levels of lightly-chlorinated PCBs in their serum (Granillo et al. 2019; Sethi et al. 2018). In one of these studies, the lightly chlorinated contemporary congener, PCB 11, was detected in all 241 women enrolled in the study at concentrations ranging from 0.005 ng/mL to 1.717 ng/mL. It ranked as second in abundance to PCB 28, another lower chlorinated PCB, and together, PCB 11 and PCB 28 constituted more than 50% of the total PCB mass in these samples (Sethi et al. 2019).
In vitro studies of primary hippocampal and cortical neurons revealed that PCB 11 and its hydroxylated and sulfated metabolites, which are found in human serum (Grimm et al. 2015; Grimm et al. 2017), significantly alter neuronal morphogenesis in a species-, sex-, and brain region-specific manner (Sethi et al. 2017b; Sethi et al. 2018). Similar to the legacy NDL PCBs, PCB 11 enhanced dendritic arborization; however, in contrast to the legacy NDL PCBs, PCB 11 also promoted axonal growth. PCB 11 elicited these morphogenic effects at concentrations as low as 1 fM (approximately 0.22 ng/mL), which is within the range of PCB 11 concentrations observed in the serum of pregnant women. The data from the Sethi et al. (2018) study suggested PCB 11 is more potent than the higher chlorinated legacy congeners PCB 95 or PCB 136 in promoting dendritic arborization, since the lowest concentrations at which these legacy congeners significantly enhanced dendritic arborization (low pM range) were an order of magnitude above the lowest observed effect level for PCB 11. These studies also suggested that the sensitivity to the morphogenic effects of PCB 11 and its metabolites varied between neuronal cell types. For example, the axon-promoting activity of OH-PCB 11 and the dendrite-promoting activity of PCB 11 were significant at lower concentrations in cortical than hippocampal neurons, whereas PCB 11-induced axonal growth was significant at lower concentrations in hippocampal neurons relative to cortical neurons. In general, the PCB 11 sulfate metabolite was more potent than either PCB 11 or hydroxylated PCB 11, suggesting that PCB 11 metabolism is as much a toxifying as detoxifying mechanism.
Mechanistic studies of PCB 11-induced dendritic growth revealed that PCB 11 does not modulate dendritic arborization via interaction with canonical molecular targets of legacy DL or NDL PCBs. Specifically, PCB 11 did not activate the AhR (Sethi et al. 2018), the TH receptor (Sethi et al. 2019), or the RyR (Holland et al. 2017). Moreover, pharmacologic blockade of AhR, THR, or RyR did not inhibit the dendrite-promoting effects of PCB 11 (Sethi et al. 2018). Pharmacologic block of L-type Ca2+ channels or the IP3 receptor also had no significant effect on dendritic growth in primary neurons exposed to PCB 11 (Sethi et al. 2018). While PCBs have been shown to increase intracellular levels of reactive oxygen species (ROS) [reviewed in (Winneke 2011)], and ROS is known to modulate dendritic arborization (Chandrasekaran et al. 2015), antioxidants did not inhibit PCB 11-induced dendritic growth in vitro (Sethi et al. 2018). However, siRNA knockdown or pharmacologic inhibition of CREB significantly decreased PCB 11-induced dendritic arborization (Sethi et al. 2018) (Figure 2). The molecular initiating event(s) of PCB 11-induced dendritic growth upstream of CREB activation remain to be determined.
While it has yet to be determined whether developmental exposures to PCB 11 similarly modulate dendritic arborization in vivo, these findings suggest that the higher chlorinated legacy NDL PCBs and the lightly chlorinated contemporary PCB congeners, at least as exemplified by PCB 11, have shared (dendrite-promoting) and unique (axon-promoting) effects on neuronal morphogenesis (Table 1). Interestingly, while the mechanism(s) mediating the dendrite-promoting activity of legacy NDL PCBs and contemporary lower chlorinated PCBs converge on CREB signaling, the upstream signaling events that link PCBs to CREB activation are divergent with legacy NDL PCBs triggering CREB-dependent signaling via RyR-dependent mechanisms and PCB 11 activating CREB via RyR-independent mechanisms (Figure 2 and Table 1).
Table 1.
Comparison between legacy and contemporary PCB effects and mechanisms.
| Parameter | PCB 95 | PCB 11 |
|---|---|---|
| Present in serum of women at risk for having a child with a NDD | ✓ | ✓ |
| Effects on dendritic arborization in primary neurons of the developing brain | ↑ | ↑ |
| Effects on axonal outgrowth in primary neurons of the developing brain | No effect | ↑ |
| Ryanodine receptor activity | +++ | Negligible activity |
| CREB-dependent effects on dendritic growth | ✓ | ✓ |
CREB = cAMP response element-binding protein
6. The relevance of PCB effects on neuronal morphogenesis to human NDDs
The organizational patterning of synaptic connections that occurs during development is a critical determinant of cognitive function later in life [reviewed in (Copf 2016)]. Synaptic connectivity is determined in part by the rate and extent of dendritic and axonal growth [reviewed in (Libersat and Duch 2004; Scott and Luo 2001)], and disruptions in the timing or magnitude of axonal and dendritic growth can perturb the pattern of connections formed between neurons (Berger-Sweeney and Hohmann 1997). Moreover, altered dendritic and axonal morphology are consistent pathologic correlates of the clinical symptoms associated with diverse NDDs [reviewed in (Copf 2016; Engle 2010; Penzes et al. 2011; Supekar et al. 2013)]. Thus, synaptic connectivity likely represents a convergence point in pathogenic mechanisms that confer NDD risk (Stamou et al. 2013) (Figure 3).
Figure 3. Factors that influence the risk and/or severity of NDDs.
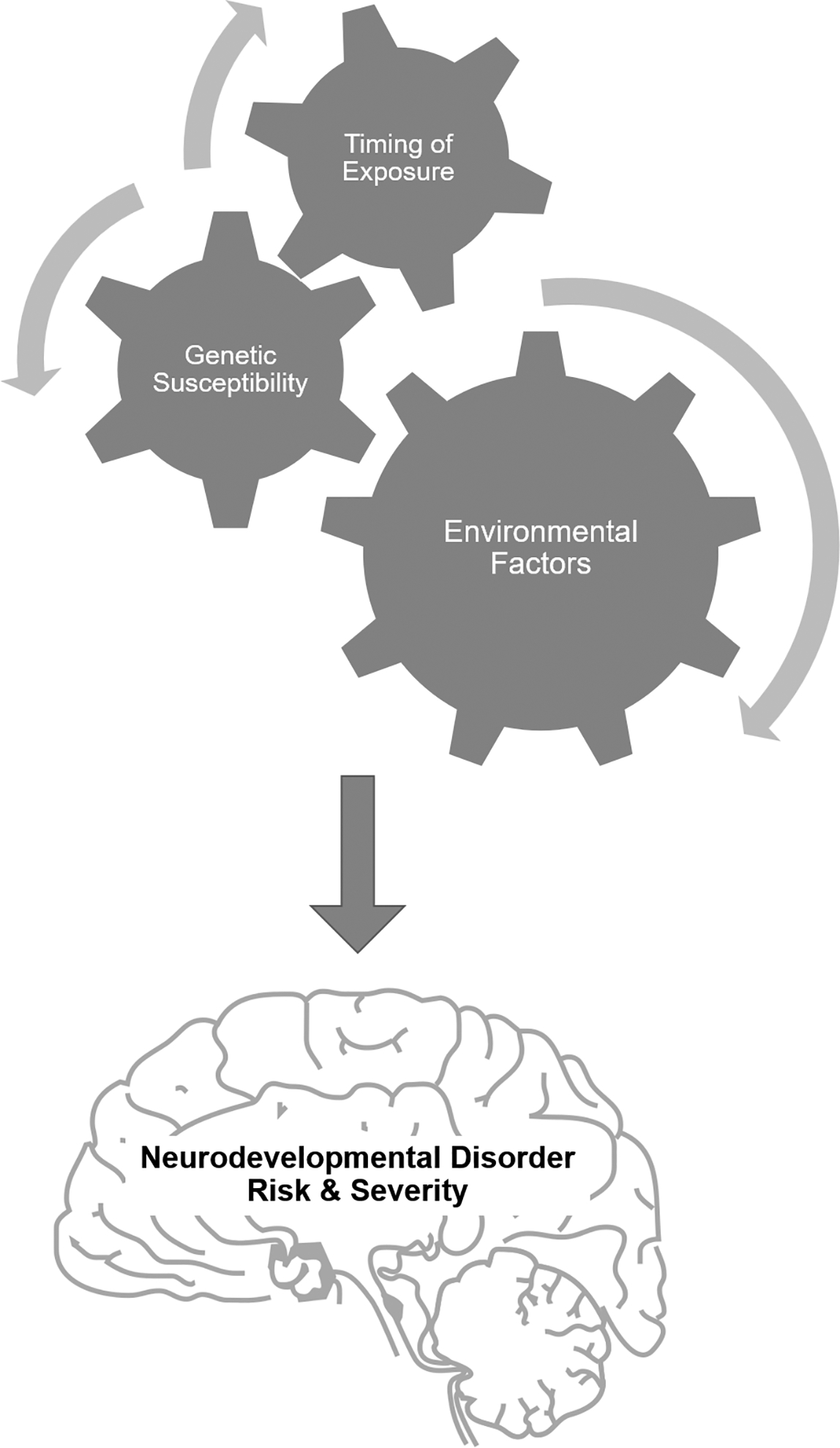
Accumulating evidence indicates that individual risk for NDDS is determined by a complex interplay of genetic risk factors that confer susceptibility, exposure to environmental stressors, including neurotoxic chemicals, and the timing of environmental exposures. Exposures that occur during critical neurodevelopmental windows pose a greater risk to the developing brain.
The convergence of legacy and contemporary NDL-PCBs on CREB signaling has important implications in the context of PCB developmental neurotoxicity (Figure 2). CREB is a key transcriptional regulator of dendritic growth in response to diverse stimuli, including activity (Redmond et al. 2002; Wayman et al. 2006). Therefore, the inappropriate activation of this transcriptional pathway as a result of PCB exposure may have significant functional consequences in the developing brain, including cortical overgrowth and hyperconnectivity, which are phenotypes observed in ASD and other neurodevelopmental disorders [reviewed in (Stamou et al. 2013)]. Consistent with this suggestion, mutations in CREB and/or CREB signaling are implicated in the pathogenesis of numerous NDDs (Bu et al. 2017; D’Andrea et al. 2015; Ngounou Wetie et al. 2015; Todd and Mack 2001). These observations together with clinical evidence indicating that altered dendritic complexity is as a common pathologic feature of diverse NDDs (Alaerts et al. 2016; Copf 2016; Supekar et al. 2013), suggest the human relevance of experimental evidence that developmental PCB exposure alters dendritic complexity of developing neurons.
7. Data gaps and directions for future study
A critical data gap is the paucity of data regarding the developmental neurotoxicity of the contemporary non-Aroclor PCB congeners. Evidence of increasing human exposure to these congeners (Hornbuckle and Robertson 2010; Hu and Hornbuckle 2010; Koh et al. 2015; Sethi et al. 2019) underscores the need to address this data gap. There is also a need to determine which of the persistent legacy NDL PCBs to which humans are exposed constitute the greatest risk to the developing human brain, and to identify genetic polymorphisms that modify individual susceptibility to PCB developmental neurotoxicity. Given recent data indicating that hydroxylated and sulfated PCB metabolites are also potent drivers of neurotoxic effects, further research into PCB metabolic fate and the actions of PCB metabolites is also warranted. In light of data documenting significantly elevated levels of airborne PCBs, including PCBs 11 and 95, in schools (Thomas et al. 2012) and outdoor environments (Hu et al. 2008), another critical data gap is the lack of information regarding the relative neurotoxic impact of diet vs. inhalation as routes of PCB exposure (Ampleman et al. 2015; Lehmann et al. 2015). Addressing these data gaps will be critical for rigorously assessing the risks that PCBs pose to the developing human brain.
To address these data gaps, it will be important to implement a number of experimental approaches. First is an urgent need for comprehensive analyses of the PCB congener profile that comprises current human exposures. Epidemiological studies need to move away from the typical practice of measuring a single or small subset of PCB congeners as indicators of cumulative PCB exposure (Longnecker et al. 2003). Further, many human studies have focused on DL congeners, despite evidence from animal studies suggesting that DL PCBs are likely not responsible for many of the cognitive and behavioral abnormalities observed in humans (Bernhoft et al. 1994; Bushnell and Rice 1999; Schantz et al. 1996). Future human studies should endeavor to quantify all 209 PCB congeners or at least include an increased number and wider variety of PCB congeners in exposure assessments to gain a more comprehensive understanding of contemporary human exposures. Human studies should also leverage emerging mechanistic data from experimental studies to stratify cohorts by relevant genetic factors that may modify risk of PCB developmental neurotoxicity (Granillo et al. 2019).
Nonclinical studies need to move away from using industrial Aroclor PCB mixtures because these do not model congener profiles relevant to current human exposures (Frame et al. 1996; Koh et al. 2015; Longnecker et al. 2003; Sethi et al. 2018; Sethi et al. 2019). Rather, researchers should use PCB congeners and mixtures that mimic the contemporary congener compositions and levels observed in human sera, placenta, and/or cord blood to better model PCB exposures in the gestational environment. There is also need for nonclinical studies that establish causal relationships between molecular, cellular and behavioral endpoints. Behavioral assessments should focus on specific domains previously identified as targets of PCB exposure in epidemiological studies, which are primarily executive function and cognitive flexibility. In vitro and alternative models should be developed to more rapidly screen legacy and contemporary PCB congeners, both individually and as mixtures, to establish relative potencies and mechanistic convergence and divergence. Results from such screens will be useful for prioritizing PCB congeners to test in animal models and will inform interpretation of results generated using PCB mixtures in vivo.
8. Conclusions
PCBs remain a continuing environmental health concern, although recent data suggest that the profile of PCB congeners of concern may have shifted over the past several decades. Contemporary human exposures are increasingly predominated by legacy NDL PCBs and lightly chlorinated non-Aroclor PCBs. Mechanistic data indicate that NDL PCBs alter normal trajectories of neurodevelopment by modulating dendritic arborization, while recent in vitro data suggest lightly chlorinated contemporary PCBs influence neurodevelopment by modulating both dendritic and axonal growth. Interestingly, while the proximal signaling events mediating the dendritic effects of these two groups of PCBs are divergent, with NDL PCBs enhancing dendritic growth via RyR-dependent mechanisms, and PCB 11 influencing dendritic growth via RyR-independent mechanisms, both converge on CREB signaling. These observations suggest that CREB signaling may be a relevant target for stratifying epidemiological studies of PCB developmental neurotoxicity, and for setting up high throughput screens using CREB signaling as the relevant outcome to identify those PCBs that pose the greatest risk to the developing human brain.
Footnotes
9. Compliance with Ethical Standards
The authors have no conflicts of interest to disclose. This work was supported by the United States National Institute of Environmental Health (grant numbers R01 ES014901, R01 ES014901–09S1 (ViCTER supplement), P30 ES023513, T32 ES007059). The sponsors were not involved in the writing of the paper or in the decision to submit the work for publication.
10. References
- Alaerts K, Swinnen SP, Wenderoth N (2016) Sex differences in autism: a resting-state fMRI investigation of functional brain connectivity in males and females Soc Cogn Affect Neurosci 11:1002–1016 doi: 10.1093/scan/nsw027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC, Thorne PS (2015) Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements Environmental science & technology 49:1156–1164 doi: 10.1021/es5048039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes Fernandes EC, Hendriks HS, van Kleef RG, Reniers A, Andersson PL, van den Berg M, Westerink RH (2010a) Activation and potentiation of human GABAA receptors by non-dioxin-like PCBs depends on chlorination pattern Toxicol Sci 118:183–190 doi: 10.1093/toxsci/kfq257 [DOI] [PubMed] [Google Scholar]
- Antunes Fernandes EC, Hendriks HS, van Kleef RG, van den Berg M, Westerink RH (2010b) Potentiation of the human GABA(A) receptor as a novel mode of action of lower-chlorinated non-dioxin-like PCBs Environ Sci Technol 44:2864–2869 doi: 10.1021/es902321a [DOI] [PubMed] [Google Scholar]
- Bansal R, You SH, Herzig CT, Zoeller RT (2005) Maternal thyroid hormone increases HES expression in the fetal rat brain: an effect mimicked by exposure to a mixture of polychlorinated biphenyls (PCBs) Brain Res Dev Brain Res 156:13–22 doi: 10.1016/j.devbrainres.2005.01.007 [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Hohmann CF (1997) Behavioral consequences of abnormal cortical development: insights into developmental disabilities Behav Brain Res 86:121–142 [DOI] [PubMed] [Google Scholar]
- Berghuis SA, Bos AF, Sauer PJ, Roze E (2015) Developmental neurotoxicity of persistent organic pollutants: an update on childhood outcome Archives of toxicology 89:687–709 doi: 10.1007/s00204-015-1463-3 [DOI] [PubMed] [Google Scholar]
- Bernhoft A, Nafstad I, Engen P, Skaare JU (1994) Effects of Pre- and Postnatal Exposure to 3,3′,4,4′,5-Pentachlorobiphenyl on Physical Development, Neurobehavior and Xenobiotic Metabolizing Enzymes in Rats Environ Toxicol Chem 13:1589–1597 doi: 10.1002/etc.5620131007 [DOI] [Google Scholar]
- Berridge MJ (2006) Calcium microdomains: organization and function Cell Calcium 40:405–412 doi: 10.1016/j.ceca.2006.09.002 [DOI] [PubMed] [Google Scholar]
- Boix J, Cauli O, Felipo V (2010) Developmental exposure to polychlorinated biphenyls 52, 138 or 180 affects differentially learning or motor coordination in adult rats. Mechanisms involved Neuroscience 167:994–1003 doi: 10.1016/j.neuroscience.2010.02.068 [DOI] [PubMed] [Google Scholar]
- Brini M, Cali T, Ottolini D, Carafoli E (2014) Neuronal calcium signaling: function and dysfunction Cell Mol Life Sci 71:2787–2814 doi: 10.1007/s00018-013-1550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q et al. (2017) CREB Signaling Is Involved in Rett Syndrome Pathogenesis J Neurosci 37:3671–3685 doi: 10.1523/JNEUROSCI.3735-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ, Rice DC (1999) Behavioral assessments of learning and attention in rats exposed perinatally to 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) Neurotoxicology and teratology 21:381–392 [DOI] [PubMed] [Google Scholar]
- Carpenter DO (2006) Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health Rev Environ Health 21:1–23 [DOI] [PubMed] [Google Scholar]
- Chandrasekaran V, Lea C, Sosa JC, Higgins D, Lein PJ (2015) Reactive oxygen species are involved in BMP-induced dendritic growth in cultured rat sympathetic neurons Mol Cell Neurosci 67:116–125 doi: 10.1016/j.mcn.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lin Y, Dang K, Puschner B (2017) Quantification of Polychlorinated Biphenyls and Polybrominated Diphenyl Ethers in Commercial Cows’ Milk from California by Gas Chromatography-Triple Quadruple Mass Spectrometry PLoS One 12:e0170129doi: 10.1371/journal.pone.0170129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheslack-Postava K et al. (2013) Maternal serum persistent organic pollutants in the Finnish Prenatal Study of Autism: A pilot study Neurotoxicology and teratology 38:1–5 doi: 10.1016/j.ntt.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni D, Sindaco R, Bertazzi PA (2012) Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: a review, 1989–2010 Environ Int 44:151–162 doi: 10.1016/j.envint.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Copf T (2016) Impairments in dendrite morphogenesis as etiology for neurodevelopmental disorders and implications for therapeutic treatments Neuroscience and biobehavioral reviews 68:946–978 doi: 10.1016/j.neubiorev.2016.04.008 [DOI] [PubMed] [Google Scholar]
- Curran CP et al. (2012) Ahrd Cyp1a2(−/−) mice show increased susceptibility to PCB-induced developmental neurotoxicity Neurotoxicology 33:1436–1442 doi: 10.1016/j.neuro.2012.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea I et al. (2015) Lack of kinase-independent activity of PI3Kgamma in locus coeruleus induces ADHD symptoms through increased CREB signaling EMBO Mol Med 7:904–917 doi: 10.15252/emmm.201404697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewailly E, Mulvad G, Pedersen HS, Ayotte P, Demers A, Weber JP, Hansen JC (1999) Concentration of organochlorines in human brain, liver, and adipose tissue autopsy samples from Greenland Environ Health Perspect 107:823–828 doi: 10.1289/ehp.99107823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Y, Lee DK (2012) Effects of polychlorinated biphenyls on the development of neuronal cells in growth period; structure-activity relationship Exp Neurobiol 21:30–36 doi: 10.5607/en.2012.21.1.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle EC (2010) Human genetic disorders of axon guidance Cold Spring Harb Perspect Biol 2:a001784doi: 10.1101/cshperspect.a001784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubig PA, Aguiar A, Schantz SL (2010) Lead and PCBs as risk factors for attention deficit/hyperactivity disorder Environ Health Perspect 118:1654–1667 doi: 10.1289/ehp.0901852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W et al. (2017) Enantioselectivity of 2,2’,3,5’,6-Pentachlorobiphenyl (PCB 95) Atropisomers toward Ryanodine Receptors (RyRs) and Their Influences on Hippocampal Neuronal Networks Environmental science & technology 51:14406–14416 doi: 10.1021/acs.est.7b04446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame GM, Cochran JW, Bowadt SS (1996) Complete PCB congener distributions for 17 aroclor mixtures determined by 3 HRGC systems optimized for comprehensive, quantitative, congener-specific analysis Hrc- Journal of High Resolution Chromatography 19:657–668 [Google Scholar]
- Fritsch EB, Pessah IN (2013) Structure-activity relationship of non-coplanar polychlorinated biphenyls toward skeletal muscle ryanodine receptors in rainbow trout (Oncorhynchus mykiss) Aquat Toxicol 140–141:204–212 doi: 10.1016/j.aquatox.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauger KJ, Kato Y, Haraguchi K, Lehmler HJ, Robertson LW, Bansal R, Zoeller RT (2004) Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors Environ Health Perspect 112:516–523 doi: 10.1289/ehp.6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giera S, Bansal R, Ortiz-Toro TM, Taub DG, Zoeller RT (2011) Individual polychlorinated biphenyl (PCB) congeners produce tissue- and gene-specific effects on thyroid hormone signaling during development Endocrinology 152:2909–2919 doi: 10.1210/en.2010-1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldey ES, Crofton KM (1998) Thyroxine replacement attenuates hypothyroxinemia, hearing loss, and motor deficits following developmental exposure to Aroclor 1254 in rats Toxicol Sci 45:94–105 doi: 10.1006/toxs.1998.2495 [DOI] [PubMed] [Google Scholar]
- Granillo L et al. (2019) Polychlorinated biphenyls influence on autism spectrum disorder risk in the MARBLES cohort Environ Res 171:177–184 doi: 10.1016/j.envres.2018.12.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory P (2000) Industrial applications of phthalocyanines Journal of Porphyrins and Phthalocyanines (JPP) 04:432–437 doi: [DOI] [Google Scholar]
- Grimm FA, He X, Teesch LM, Lehmler HJ, Robertson LW, Duffel MW (2015) Tissue Distribution, Metabolism, and Excretion of 3,3’-Dichloro-4’-sulfooxy-biphenyl in the Rat Environmental science & technology 49:8087–8095 doi: 10.1021/acs.est.5b01499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA et al. (2017) Identification of a sulfate metabolite of PCB 11 in human serum Environ Int 98:120–128 doi: 10.1016/j.envint.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Capozzi SL, Kraeutler TM, Rodenburg LA (2014) Global distribution and local impacts of inadvertently generated polychlorinated biphenyls in pigments Environmental science & technology 48:8573–8580 doi: 10.1021/es502291b [DOI] [PubMed] [Google Scholar]
- Hagmar L (2003) Polychlorinated biphenyls and thyroid status in humans: a review Thyroid 13:1021–1028 doi: 10.1089/105072503770867192 [DOI] [PubMed] [Google Scholar]
- Hendriks HS, Antunes Fernandes EC, Bergman A, van den Berg M, Westerink RH (2010) PCB-47, PBDE-47, and 6-OH-PBDE-47 differentially modulate human GABAA and alpha4beta2 nicotinic acetylcholine receptors Toxicol Sci 118:635–642 doi: 10.1093/toxsci/kfq284 [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I et al. (2018) A Prospective Study of Environmental Exposures and Early Biomarkers in Autism Spectrum Disorder: Design, Protocols, and Preliminary Data from the MARBLES Study Environ Health Perspect 126:117004doi: 10.1289/EHP535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EB, Feng W, Zheng J, Dong Y, Li X, Lehmler HJ, Pessah IN (2017) An Extended Structure-Activity Relationship of Nondioxin-Like PCBs Evaluates and Supports Modeling Predictions and Identifies Picomolar Potency of PCB 202 Towards Ryanodine Receptors Toxicol Sci 155:170–181 doi: 10.1093/toxsci/kfw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf NB, Ruder AM, Succop P (2009) Background levels of polychlorinated biphenyls in the U.S. population The Science of the total environment 407:6109–6119 doi: 10.1016/j.scitotenv.2009.08.035 [DOI] [PubMed] [Google Scholar]
- Hornbuckle K, Robertson L (2010) Polychlorinated biphenyls (PCBs): sources, exposures, toxicities Environmental science & technology 44:2749–2751 doi: 10.1021/es100801f [DOI] [PubMed] [Google Scholar]
- Hsu ST, Ma CI, Hsu SK, Wu SS, Hsu NH, Yeh CC, Wu SB (1985) Discovery and epidemiology of PCB poisoning in Taiwan: a four-year followup Environ Health Perspect 59:5–10 doi: 10.1289/ehp.59-1568088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC (2010) Inadvertent polychlorinated biphenyls in commercial paint pigments Environmental science & technology 44:2822–2827 doi: 10.1021/es902413k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC (2008) Discovery of non-aroclor PCB (3,3’-dichlorobiphenyl) in Chicago air Environmental science & technology 42:7873–7877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglefield JR, Mundy WR, Shafer TJ (2001) Inositol 1,4,5-triphosphate receptor-sensitive Ca(2+) release, store-operated Ca(2+) entry, and cAMP responsive element binding protein phosphorylation in developing cortical cells following exposure to polychlorinated biphenyls J Pharmacol Exp Ther 297:762–773 [PubMed] [Google Scholar]
- Inglefield JR, Shafer TJ (2000) Polychlorinated biphenyl-stimulation of Ca(2+) oscillations in developing neocortical cells: a role for excitatory transmitters and L-type voltage-sensitive Ca(2+) channels J Pharmacol Exp Ther 295:105–113 [PubMed] [Google Scholar]
- Itoh S et al. (2018) Association of maternal serum concentration of hydroxylated polychlorinated biphenyls with maternal and neonatal thyroid hormones: The Hokkaido birth cohort study Environ Res 167:583–590 doi: 10.1016/j.envres.2018.08.027 [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Miyazaki W, Takeshita A, Kuroda Y, Koibuchi N (2002) Polychlorinated biphenyls suppress thyroid hormone-induced transactivation Biochem Biophys Res Commun 299:384–388 doi: 10.1016/s0006-291x(02)02659-1 [DOI] [PubMed] [Google Scholar]
- Jensen S (1972) The PCB Story Ambio 1:123–131 [Google Scholar]
- Keil KP, Sethi S, Lein PJ (2019) Sex-Dependent Effects of 2,2’,3,5’,6-Pentachlorobiphenyl on Dendritic Arborization of Primary Mouse Neurons Toxicological sciences : an official journal of the Society of Toxicology 168:95–109 doi: 10.1093/toxsci/kfy277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinefelter K et al. (2018) Genetic differences in the aryl hydrocarbon receptor and CYP1A2 affect sensitivity to developmental polychlorinated biphenyl exposure in mice: relevance to studies of human neurological disorders Mamm Genome 29:112–127 doi: 10.1007/s00335-017-9728-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PR, Tilson HA (2000) Neurochemical effects of environmental chemicals: in vitro and in vivo correlations on second messenger pathways Ann N Y Acad Sci 919:97–105 [DOI] [PubMed] [Google Scholar]
- Koh WX, Hornbuckle KC, Marek RF, Wang K, Thorne PS (2016) Hydroxylated polychlorinated biphenyls in human sera from adolescents and their mothers living in two U.S. Midwestern communities Chemosphere 147:389–395 doi: 10.1016/j.chemosphere.2015.12.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh WX, Hornbuckle KC, Thorne PS (2015) Human Serum from Urban and Rural Adolescents and Their Mothers Shows Exposure to Polychlorinated Biphenyls Not Found in Commercial Mixtures Environmental science & technology 49:8105–8112 doi: 10.1021/acs.est.5b01854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouidhi S, Clerget-Froidevaux MS (2018) Integrating Thyroid Hormone Signaling in Hypothalamic Control of Metabolism: Crosstalk Between Nuclear Receptors Int J Mol Sci 19 doi: 10.3390/ijms19072017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann GM, Christensen K, Maddaloni M, Phillips LJ (2015) Evaluating health risks from inhaled polychlorinated biphenyls: research needs for addressing uncertainty Environ Health Perspect 123:109–113 doi: 10.1289/ehp.1408564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesiak A, Zhu M, Chen H, Appleyard SM, Impey S, Lein PJ, Wayman GA (2014) The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation J Neurosci 34:717–725 doi: 10.1523/JNEUROSCI.2884-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZM et al. (2018) Association of In Utero Persistent Organic Pollutant Exposure With Placental Thyroid Hormones Endocrinology 159:3473–3481 doi: 10.1210/en.2018-00542 [DOI] [PubMed] [Google Scholar]
- Libersat F, Duch C (2004) Mechanisms of dendritic maturation Mol Neurobiol 29:303–320 doi: 10.1385/MN:29:3:303 [DOI] [PubMed] [Google Scholar]
- Llansola M, Montoliu C, Boix J, Felipo V (2010) Polychlorinated biphenyls PCB 52, PCB 180, and PCB 138 impair the glutamate-nitric oxide-cGMP pathway in cerebellar neurons in culture by different mechanisms Chem Res Toxicol 23:813–820 doi: 10.1021/tx900440q [DOI] [PubMed] [Google Scholar]
- Llansola M, Piedrafita B, Rodrigo R, Montoliu C, Felipo V (2009) Polychlorinated biphenyls PCB 153 and PCB 126 impair the glutamate-nitric oxide-cGMP pathway in cerebellar neurons in culture by different mechanisms Neurotox Res 16:97–105 doi: 10.1007/s12640-009-9055-8 [DOI] [PubMed] [Google Scholar]
- Londono M, Shimokawa N, Miyazaki W, Iwasaki T, Koibuchi N (2010) Hydroxylated PCB induces Ca2+ oscillations and alterations of membrane potential in cultured cortical cells J Appl Toxicol 30:334–342 doi: 10.1002/jat.1501 [DOI] [PubMed] [Google Scholar]
- Longnecker MP et al. (2003) Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment Environ Health Perspect 111:65–70 doi: 10.1289/ehp.5463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Croen LA, Sjodin A, Yoshida CK, Zerbo O, Kharrazi M, Windham GC (2017) Polychlorinated Biphenyl and Organochlorine Pesticide Concentrations in Maternal Mid-Pregnancy Serum Samples: Association with Autism Spectrum Disorder and Intellectual Disability Environ Health Perspect 125:474–480 doi: 10.1289/EHP277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek RF, Thorne PS, Wang K, Dewall J, Hornbuckle KC (2013) PCBs and OH-PCBs in serum from children and mothers in urban and rural U.S. communities Environmental science & technology 47:3353–3361 doi: 10.1021/es304455k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Klaassen CD (2010) Differential effects of polychlorinated biphenyl congeners on serum thyroid hormone levels in rats Toxicological sciences : an official journal of the Society of Toxicology 117:36–44 doi: 10.1093/toxsci/kfq187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre JK, Beauchamp DA (2007) Age and trophic position dominate bioaccumulation of mercury and organochlorines in the food web of Lake Washington The Science of the total environment 372:571–584 doi: 10.1016/j.scitotenv.2006.10.035 [DOI] [PubMed] [Google Scholar]
- Mitchell MM, Woods R, Chi LH, Schmidt RJ, Pessah IN, Kostyniak PJ, LaSalle JM (2012) Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder Environ Mol Mutagen 53:589–598 doi: 10.1002/em.21722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitoma C et al. (2015) Yusho and its latest findings-A review in studies conducted by the Yusho Group Environ Int 82:41–48 doi: 10.1016/j.envint.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Miyazaki W, Iwasaki T, Takeshita A, Tohyama C, Koibuchi N (2008) Identification of the functional domain of thyroid hormone receptor responsible for polychlorinated biphenyl-mediated suppression of its action in vitro Environ Health Perspect 116:1231–1236 doi: 10.1289/ehp.11176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy WR, Shafer TJ, Tilson HA, Kodavanti PR (1999) Extracellular calcium is required for the polychlorinated biphenyl-induced increase of intracellular free calcium levels in cerebellar granule cell culture Toxicology 136:27–39 [DOI] [PubMed] [Google Scholar]
- Nave KA, Werner HB (2014) Myelination of the nervous system: mechanisms and functions Annu Rev Cell Dev Biol 30:503–533 doi: 10.1146/annurev-cellbio-100913-013101 [DOI] [PubMed] [Google Scholar]
- Naveau E et al. (2014) Alteration of rat fetal cerebral cortex development after prenatal exposure to polychlorinated biphenyls PLoS One 9:e91903doi: 10.1371/journal.pone.0091903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngounou Wetie AG, Wormwood KL, Charette L, Ryan JP, Woods AG, Darie CC (2015) Comparative two-dimensional polyacrylamide gel electrophoresis of the salivary proteome of children with autism spectrum disorder J Cell Mol Med 19:2664–2678 doi: 10.1111/jcmm.12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent AS et al. (2016) Early exposure to Aroclor 1254 in vivo disrupts the functional synaptic development of newborn hippocampal granule cells Eur J Neurosci 44:3001–3010 doi: 10.1111/ejn.13437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencikova K et al. (2018) In vitro profiling of toxic effects of prominent environmental lower-chlorinated PCB congeners linked with endocrine disruption and tumor promotion Environ Pollut 237:473–486 doi: 10.1016/j.envpol.2018.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders Nat Neurosci 14:285–293 doi: 10.1038/nn.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, Lein PJ (2010) Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity Pharmacol Ther 125:260–285 doi: 10.1016/j.pharmthera.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Lehmler HJ, Robertson LW, Perez CF, Cabrales E, Bose DD, Feng W (2009) Enantiomeric specificity of (−)-2,2’,3,3’,6,6’-hexachlorobiphenyl toward ryanodine receptor types 1 and 2 Chem Res Toxicol 22:201–207 doi: 10.1021/tx800328u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Lein PJ, Seegal RF, Sagiv SK (2019) Neurotoxicity of polychlorinated biphenyls and related organohalogens Acta Neuropathol doi: 10.1007/s00401-019-01978-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson A, Bourguignon JP, Parent AS (2016) Exposure to endocrine disrupting chemicals and neurodevelopmental alterations Andrology 4:706–722 doi: 10.1111/andr.12211 [DOI] [PubMed] [Google Scholar]
- Pittenger C, Kandel ER (2003) In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus Philos Trans R Soc Lond B Biol Sci 358:757–763 doi: 10.1098/rstb.2002.1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond L, Kashani AH, Ghosh A (2002) Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription Neuron 34:999–1010 [DOI] [PubMed] [Google Scholar]
- Rosenquist AH et al. (2017) Prenatal and Postnatal PCB-153 and p,p’-DDE Exposures and Behavior Scores at 5–9 Years of Age among Children in Greenland and Ukraine Environ Health Perspect 125:107002doi: 10.1289/EHP553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovet JF (2014) The role of thyroid hormones for brain development and cognitive function Endocr Dev 26:26–43 doi: 10.1159/000363153 [DOI] [PubMed] [Google Scholar]
- Royland JE, Kodavanti PR (2008) Gene expression profiles following exposure to a developmental neurotoxicant, Aroclor 1254: pathway analysis for possible mode(s) of action Toxicol Appl Pharmacol 231:179–196 doi: 10.1016/j.taap.2008.04.023 [DOI] [PubMed] [Google Scholar]
- Sable HJK, Schantz SL (2006) Executive Function following Developmental Exposure to Polychlorinated Biphenyls (PCBs): What Animal Models Have Told Us In: Levin ED, Buccafusco JJ (eds) Animal Models of Cognitive Impairment. Frontiers in Neuroscience; Boca Raton (FL), [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA (2010) Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children Am J Epidemiol 171:593–601 doi: 10.1093/aje/kwp427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samso M, Feng W, Pessah IN, Allen PD (2009) Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating PLoS Biol 7:e85doi: 10.1371/journal.pbio.1000085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Seo BW, Moshtaghian J, Peterson RE, Moore RW (1996) Effects of gestational and lactational exposure to TCDD or coplanar PCBs on spatial learning Neurotoxicology and teratology 18:305–313 [DOI] [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC (2003) Effects of PCB exposure on neuropsychological function in children Environ Health Perspect 111:357–576 doi: 10.1289/ehp.5461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EK, Luo L (2001) How do dendrites take their shape? Nat Neurosci 4:359–365 doi: 10.1038/86006 [DOI] [PubMed] [Google Scholar]
- Sethi S et al. (2017a) Detection of 3,3’-Dichlorobiphenyl in Human Maternal Plasma and Its Effects on Axonal and Dendritic Growth in Primary Rat Neurons Toxicological sciences : an official journal of the Society of Toxicology 158:401–411 doi: 10.1093/toxsci/kfx100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Keil KP, Lein PJ (2017b) Species and Sex Differences in the Morphogenic Response of Primary Rodent Neurons to 3,3’-Dichlorobiphenyl (PCB 11) Toxics 6 doi: 10.3390/toxics6010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Keil KP, Lein PJ (2018) 3,3’-Dichlorobiphenyl (PCB 11) promotes dendritic arborization in primary rat cortical neurons via a CREB-dependent mechanism Archives of toxicology 92:3337–3345 doi: 10.1007/s00204-018-2307-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S et al. (2019) Comparative Analyses of the 12 Most Abundant PCB Congeners Detected in Human Maternal Serum for Activity at the Thyroid Hormone Receptor and Ryanodine Receptor Environmental science & technology 53:3948–3958 doi: 10.1021/acs.est.9b00535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang H, Li Y, Wang T, Wang P, Zhang H, Zhang Q, Jiang G (2014) The presence of polychlorinated biphenyls in yellow pigment products in China with emphasis on 3,3’-dichlorobiphenyl (PCB 11) Chemosphere 98:44–50 doi: 10.1016/j.chemosphere.2013.09.075 [DOI] [PubMed] [Google Scholar]
- Stamou M, Streifel KM, Goines PE, Lein PJ (2013) Neuronal connectivity as a convergent target of gene x environment interactions that confer risk for Autism Spectrum Disorders Neurotoxicology and teratology 36:3–16 doi: 10.1016/j.ntt.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A (2001) Basic and applied aspects in the microbial degradation of azo dyes Appl Microbiol Biotechnol 56:69–80 doi: 10.1007/s002530100686 [DOI] [PubMed] [Google Scholar]
- Supekar K et al. (2013) Brain hyperconnectivity in children with autism and its links to social deficits Cell reports 5:738–747 doi: 10.1016/j.celrep.2013.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Anezaki K, Kojima H (2017) Effects of unintentional PCBs in pigments and chemical products on transcriptional activity via aryl hydrocarbon and nuclear hormone receptors Environ Pollut 227:306–313 doi: 10.1016/j.envpol.2017.04.059 [DOI] [PubMed] [Google Scholar]
- Thomas K, Xue J, Williams R, Jones P, Whitaker D (2012) Polychlorinated Biphenyls (PCBs) in School Buildings: Sources, Environmental Levels, and Exposures.. United States Environmental Protection Agency, [Google Scholar]
- Todd PK, Mack KJ (2001) Phosphorylation, CREB, and Mental Retardation Pediatr Res 50:672doi: 10.1203/00006450-200112000-00002 [DOI] [PubMed] [Google Scholar]
- Wadzinski TL et al. (2014) Endocrine disruption in human placenta: expression of the dioxin-inducible enzyme, CYP1A1, is correlated with that of thyroid hormone-regulated genes. J Clin Endocrinol Metab 99:E2735–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA et al. (2012a) PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth Environ Health Perspect 120:1003–1009 doi: 10.1289/ehp.1104833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA et al. (2008) An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP Proc Natl Acad Sci U S A 105:9093–9098 doi: 10.1073/pnas.0803072105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR (2006) Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2 Neuron 50:897–909 doi: 10.1016/j.neuron.2006.05.008 [DOI] [PubMed] [Google Scholar]
- Wayman GA et al. (2012b) PCB-95 promotes dendritic growth via ryanodine receptor-dependent mechanisms Environ Health Perspect 120:997–1002 doi: 10.1289/ehp.1104832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SS, Birnbaum LS (2009) An overview of the effects of dioxins and dioxin-like compounds on vertebrates, as documented in human and ecological epidemiology J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27:197–211 doi: 10.1080/10590500903310047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G (2011) Developmental aspects of environmental neurotoxicology: lessons from lead and polychlorinated biphenyls Journal of the neurological sciences 308:9–15 doi: 10.1016/j.jns.2011.05.020 [DOI] [PubMed] [Google Scholar]
- Wong PW, Joy RM, Albertson TE, Schantz SL, Pessah IN (1997) Ortho-substituted 2,2’,3,5’,6-pentachlorobiphenyl (PCB 95) alters rat hippocampal ryanodine receptors and neuroplasticity in vitro: evidence for altered hippocampal function Neurotoxicology 18:443–456 [PubMed] [Google Scholar]
- Yang D et al. (2014) PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms Toxicological sciences : an official journal of the Society of Toxicology 138:379–392 doi: 10.1093/toxsci/kft334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D et al. (2009) Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats Environ Health Perspect 117:426–435 doi: 10.1289/ehp.11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Kodavanti PR (2001) Possible molecular targets of halogenated aromatic hydrocarbons in neuronal cells Biochem Biophys Res Commun 280:1372–1377 doi: 10.1006/bbrc.2001.4283 [DOI] [PubMed] [Google Scholar]
- Zahalka EA, Ellis DH, Goldey ES, Stanton ME, Lau C (2001) Perinatal exposure to polychlorinated biphenyls Aroclor 1016 or 1254 did not alter brain catecholamines nor delayed alternation performance in Long-Evans rats Brain Res Bull 55:487–500 [DOI] [PubMed] [Google Scholar]
- Zimmer KE et al. (2009) Altered Stress-Induced Cortisol Levels in Goats Exposed to Polychlorinated Biphenyls (PCB 126 and PCB 153) During Fetal and Postnatal Development J Toxicol Environ Health, A 72:164–172 doi: 10.1080/15287390802539004 [DOI] [PubMed] [Google Scholar]
- Zoeller RT (2007) Environmental chemicals impacting the thyroid: targets and consequences Thyroid 17:811–817 doi: 10.1089/thy.2007.0107 [DOI] [PubMed] [Google Scholar]
- Zoeller TR, Dowling AL, Herzig CT, Iannacone EA, Gauger KJ, Bansal R (2002) Thyroid hormone, brain development, and the environment Environ Health Perspect 110 Suppl 3:355–361 doi: 10.1289/ehp.02110s3355 [DOI] [PMC free article] [PubMed] [Google Scholar]


