Abstract
Gonadal sex differentiation is increasingly recognized as a remarkably plastic process driven by species-specific genetic or environmental determinants. Among aquatic vertebrates, gonadal sex differentiation is a frequent endpoint in studies of endocrine disruption with little appreciation of underlying developmental mechanisms. Work in model organisms has highlighted the diversity of master sex-determining genes rather than uncovering any broad similarities prompting the highly conserved developmental decision of testes versus ovaries. Here we use molecular genetic markers of chromosomal sex combined with traditional histology to examine the transition of the bipotential gonads to ovaries or testes in threespine stickleback (Gasterosteus aculeatus). Serially-sectioned threespine stickleback fry were analyzed for qualitative and quantitative indications of sexual differentiation, including changes in gonadal morphology, number of germ cells and the incidence of gonadal apoptosis. We show that threespine stickleback sampled from anadromous and lacustrine populations are differentiated gonochorists. The earliest sex-specific event is a premeiotic increase in primordial germ cell number followed by a female-specific spike in apoptosis in the undifferentiated gonad of genetic females. The data suggest that an increase in PGC number may direct the undifferentiated gonad toward ovarian differentiation.
Keywords: stickleback, Gasterosteus, sex determination, primordial germ cell, PGC, apoptosis
The 25,000 species of teleost fish display a profound diversity of sex-determining mechanisms (Wootton, 1998; Mank et al., 2006). Teleost sex-determining pathways usually result in the developmentally irreversible decision for individuals to become male or female, but these developmental pathways are remarkably labile and can often be altered by environmental cues including the presence of pollutants (Devlin and Nagahama, 2002). Therefore, teleosts may provide crucial models for understanding the interacting genetic and environmental factors for sex determination, and provide us with a better understanding of the evolution of these mechanisms.
The lability of sex determination in teleosts is potentially influenced by morphological factors, such as the common origin of gonadal somatic tissues (Francis, 1992) and a population of totipotent primordial germ cells (PGCs) that are capable of differentiating into male or female gametes (Yoshizaki et al., 2002). The PGCs are specified early in embryonic development and likely undergo a short first wave of mitosis preceding their migration to the gonad anlagen (Hamaguchi, 1982; Yoshizaki et al., 2002; Raz, 2003). During their migration, PGCs are presumed to be mitotically inactive (Hardisty, 1967; Hamaguchi, 1982; Timmermans and Taverne, 1989). After they colonize the gonad, PGCs proliferate in a second wave of mitosis prior to meiotic division.
Research on several teleost species suggests that the timing of the second wave of germ cell proliferation is advanced in females relative to males (Gasterosteus aculeatus: Shimizu and Takahashi, 1980; Odontesthes bonariensis (pejerrey): Strüssmann et al., 1996; Onchorhynchus keta (chum salmon): Robertson, 1953; Onchorynchus mykiss (rainbow trout): Lebrun et al., 1982; Oryzias latipes (medaka): Satoh and Egami, 1972, Quirk and Hamilton, 1973, Kobayashi et al., 2004; Puntius conchonius (rosy barb): Çek, 2006; Salmo trutta (brown trout): Ashby, 1957; Xiphophorus helleri (black swordtail): Essenberg, 1923, Vallowe, 1957; Xiphophorus maculatus (southern platyfish): Wolf 1931; see also: Hardisty, 1967; Hunter and Donaldson, 1983; Strüssmann and Nakamura, 2002). Evidence of sexual dimorphism in PGC number, however, often relies on retrospective inference of gonadal sex and has not been characterized utilizing molecular markers of sex chromosomes in most species. One exception is medaka (O. latipes), where XX and YY males were employed to produce offspring of known genetic sex. In these offspring, similar numbers of PGCs migrated to the bipotential gonad in both genetic sexes, but by 2 days after hatching, gonads from genetic females had more germ cells than did gonads from genetic males (Quirk and Hamilton, 1973). Recent studies have confirmed these findings using PCR-based sex genotyping (i.e., Kobayashi et al., 2004). Widespread early differentiation of female gonochoristic (nonhermaphroditic) teleosts (Hunter and Donaldson, 1983) is consistent with the hypothesis that male PGC proliferation is repressed in medaka (Satoh and Egami, 1972; Kobayashi et al., 2004).
Threespine stickleback (Gasterosteus aculeatus; Teleostei, Gasterosteiformes, Bell and Foster, 1994), are well-investigated models for understanding the process of evolution and adaptive radiation (Schluter, 1994; Peichel et al., 2001; Cresko et al., 2004), and increasingly microevolution and development (Cresko et al., 2003; Shapiro et al., 2004; Colosimo et al., 2005; Peichel, 2005; Cresko et al., 2007). Throughout the northern hemisphere, genetically and morphologically distinct populations of threespine stickleback have evolved over the last 14,000 years from the colonization of multiple freshwater lakes by anadromous populations (Bell and Foster, 1994). While much of this recent work has centered on limnetic/benthic and anadromous/freshwater differences with respect to trophic morphology and armor plating reduction, respectively (Foster and Baker, 2004; Peichel, 2005) opportunity is increasing for understanding the evolution of other phenotypic responses that are correlated with these environmental shifts. Among these is plasticity in life history characteristics including reproductive traits such as gamete number production (Baker and Foster, 2002).
Simultaneously, threespine stickleback are emerging as a model for studying both the genetic controls of sex determination (Peichel et al., 2004) and the effects of pollution on sex differentiation (i.e., Katsiadaki et al., 2002; Hahlbeck et al., 2004; Bernhardt et al., 2006; see: Cresko et al., 2007). Threespine stickleback exhibit male heterogamety (Peichel et al., 2004) and are likely differentiated gonochorists. In differentiated gonochorists, the gonad develops directly into an ovary or testis. Undifferentiated gonochorists (juvenile hermaphrodites), such as Danio rerio (zebrafish), first develop ovaries and later in about 50% of individuals the ovarian structures degenerate and testes form (Yamamoto, 1969; Takahashi, 1977; Maack and Segner, 2003).
In threespine stickleback, the literature is inconclusive regarding the incidence of environmental sex determination and hermaphroditism. Infrequent reports have described oocytes in the testes of wild-caught threespine stickleback, suggesting that intersex fish occasionally exist in wild populations (Craig-Bennett, 1931; Borg and van den Hurk, 1983). Additionally, environmental factors such as temperature may influence gonadal sex in this species (Lindsey, 1962). The prevalence of hermaphroditism or environmental sex determination in stickleback is relevant to the use of this species as a biomarker for endocrine disruption (Katsiadaki et al., 2002).
Here we address the surprising lack of information on early gonadogenesis in threespine stickleback. Swarup (1958) and Shimizu and Takahashi (1980) identified differences in numbers of germ cells between putative male and female fry, but they came to opposite conclusions regarding which sex contains more germ cells in early development. Although these two studies lacked a genetic marker for sex and inferred gonadal sex retrospectively, the data suggest that mitotic proliferation of germ cells is an early event in differentiation of stickleback gonads.
This study explores the hypothesis that a premeiotic increase in female PGCs may be the earliest sexually dimorphic trait yet recorded in the differentiating threespine stickleback gonad. In addition to addressing this primary hypothesis, our data provide a definitive answer to the question of whether sticklebacks are differentiated gonochorists. It also provides an analysis of the normal course of development of the gonads that will be indispensable to future endocrine disruption studies that use morphological criteria as an endpoint, and may help elucidate the sex-determining mechanism in this species.
MATERIALS AND METHODS
Animal Husbandry
Saltwater and anadromous forms of threespine stickleback (Gasterosteus aculeatus, Linnaeus) are hypothesized to be ancestral to freshwater (lacustrine) forms (Bell and Foster, 1994; Baker et al., 1998). Fertilizations were made from two populations, a laboratory-bred lacustrine population, that originated from Bear Paw Lake (N 61.6139, W 149.7529) near Cook Inlet, Alaska (hereafter referred to as “Bear Paw”, the population from which the individual was chosen for the reference stickleback genome sequence <http://ensembl.genome.tugraz.at/Gasterosteus_aculeatus/index.html>), and a laboratory-bred anadromous population from Rabbit Slough (N 61.5595, W 149.2583), also near Cook Inlet (Cresko et al., 2004). Fry were fed with Artemia spp. (San Francisco Bay Brand, Newark, CA) and maintained in 6 ppt Instant Ocean (Aquarium Systems, Mentor, OH) in reverse osmosis water at a constant 19.5°C (S.D. = 0.57, N = 26 days).
Samples were collected at 11, 12, 15, 18, 19, 22, 25, and 26 days postfertilization (dpf). Fry were euthanized in 250 mg/l MS-222 (ethyl m-aminobenzonate, Sigma Chemical Co., St. Louis, MO, E-1626). All fry were coded as to population of origin and age. The standard length (SL; measured from the tip of snout to the posterior edge of the last vertebra) in millimeters was recorded and caudal fins were removed to 100% ethanol with sterilized instruments and stored at −20°C for DNA isolation and sex genotyping. All fry (N = 64) were fixed in Bouin’s Solution (0.9% picric acid [aqueous], 9% formaldehyde, 5% acetic acid, Sigma HT10-1-128).
Histology
The majority of fry were serially sectioned in a sagittal orientation at a thickness of 6 μm. A few individuals were serially sectioned transversely at the same thickness. Sections were stained with hematoxylin and eosin. The histological processing technique has been described elsewhere (Bernhardt et al., 2006).
Germ cells were distinguished on the basis of cellular morphology at ≥400× magnification for counting and 800–1,200× magnification for the examination of nuclear morphology using plan apochromatic lenses. Germ cells were identified according to the criteria of Nieuwkoop and Sutasurya (1979). PGCs were large, oval, and the pale spherical nucleus contained a prominent nuclear membrane and large basophilic nucleolus (Fig. 1). Germ cells in the early phases of meiosis were particularly distinct, displaying an irregular nuclear membrane, nucleolus, and either chromosomal aggregates or synapsed chromosomes. To ensure that each cell was counted only once, only germ cells displaying a prominent nuclear envelope and nucleolus were counted. The total number of germ cells in each fry was counted without prior knowledge of its sex, by examining serial sections of both gonads and manipulating the focal plane within each section. Only fry containing an entirely intact series of gonad sections were included in the final analysis. In the five female fry sampled at 25 and 26 dpf, the germ cell number was estimated by counting every third section and multiplying by three, due to the large number of germ cells. In these five fry, a few germ cells were large vitellogenic oocytes with a nuclear diameter greater than the thickness of the section, so the reported value is likely a slight overestimate.
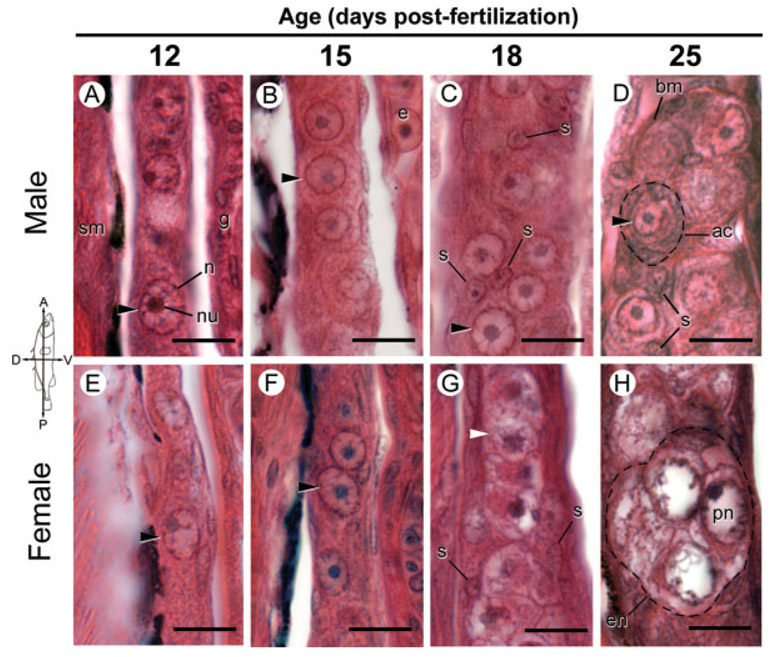
Fig. 1.
Gonadal histology of Rabbit Slough (anadromous) threespine stickleback (Gasterosteus aculeatus) fry with an emphasis on germ cell morphology 12–25 dpf. Photographs are sagittal sections of comparable regions of genetically male (A–D) and female (E–H) gonads stained with hematoxylin and eosin oriented as indicated by the compass. Fish were sampled from an anadromous population derived from Rabbit Slough and genetic sex was determined by PCR genotyping as described in the Methods. A-F: Black arrowheads point to representative primordial (premeiotic) germ cells. C, D: somatic cells (s) in testes associate with premeiotic germ cells in presumptive acini. G, H: Meiotic germ cells (white arrowheads and perinucleolar (pn) stage oocytes) are prevalent in ovaries 18 and 25 dpf, respectively. ac, acinar-like cyst; bm, basement membrane; e, erythrocyte; en, eggnest; g, gut; n, nucleus; nu, nucleolus; pn, perinucleolar oocyte; s, somatic cell nuclei; sm, skeletal muscle. Scale bars: 10 μm. See Figure 2 for Bear Paw (lacustrine) equivalent.
The incidence of apoptosis was estimated by computing the ratio of all clearly apoptotic figures to total nonapoptotic germ cells in 10 sections distributed evenly through both gonads. Apoptotic cells were identified by morphological criteria including the observation of cell shrinkage, chromatin condensation and margination, nuclear fragmentation, and the formation of apoptotic bodies (Wylie et al., 1980). Germ cell number was used in the denominator in lieu of gonad size, based on the strong correlation between SL and germ cell number and the even distribution of apoptotic cells and germ cells through the gonad. Predicted germ cell number (Predicted germ cell number = [Germ cells in 10 sections/10] × [Number of sections containing gonads]) was highly correlated with actual germ cell number computed by a complete gonad cell count, (R2 = 0.88 for all counted fry) indicating that the counted cells accurately reflected a consistent subset of the total gonad cell population.
Sections were viewed with an Olympus BX60 brightfield microscope, photographed using an Optronics Microfire digital camera controlled by PictureFrame software (Optronics, Goleto, CA). All statistical analysis was carried out using JMP, Version 6. SAS Institute, Cary, NC, 1989–2005.
Sex Genotyping
The genetic sex of each sectioned fry was determined using sex-specific primers designed to amplify a male-specific DNA fragment with a length of ~370 bp (Griffiths et al., 2000). These primers also yield a band at 600 bp present in both sexes which serves as a positive control for DNA quality and for the identification of females. DNA was isolated according to standard protocols. PCR and amplified fragment length polymorphism analysis was performed as described in Bernhardt et al. (2006).
RESULTS
Morphological Sex Differentiation of the Gonads
No qualitative differences were observed in the morphology of differentiating gonads between the anadromous (Rabbit Slough) population and the lacustrine (Bear Paw) population.
11 and 12 dpf.
In the youngest animals examined, 11 and 12 dpf male and female fry, the gonad was located within the peritoneal cavity dorsal to the intestine and ventral to the skeletal muscle of the caudal fin and trunk. Male and female gonads were indistinguishable at 11 and 12 dpf, which corresponded to ~3 and 4 days after hatching. The bilateral undifferentiated gonads were lateral to and beneath the archinephric ducts and they extended ventrally from their mesenteric connection between the peritoneum and the gut. No gonadal cavities were apparent in any sections, including a complete set of serial transverse sections of two 11 dpf Rabbit Slough male fry.
Large distinct PGCs occurred throughout the gonads (Figs. 1A,E and 2A,E). Mean diameter of germ cell nuclei was 6.08 μm (N = 25, S.D. = 0.91). A single large, basophilic nucleolus was usually associated with filaments radiating toward the nuclear membrane, a characteristic observed in both male and female PGC nuclei (e.g., Figs. 1A,E and 2A,E). Occasional mitotic PGCs and rare apoptotic figures were observed in the gonads of both sexes. There was no apparent difference in the sizes of the left and right gonads or in male and female gonads, though gonad size was not rigorously explored. Additionally, examination of serial sections for PGCs outside of the gonads led to no obvious indication of ongoing PGC migration into the gonadal tissue.
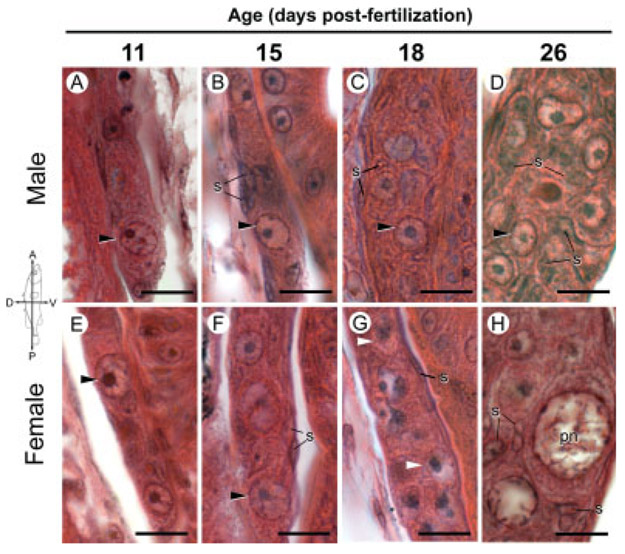
Fig. 2.
Gonadal histology of Bear Paw (lacustrine) threespine stickleback (Gasterosteus aculeatus) fry with an emphasis on germ cell morphology 11–26 dpf. Photographs are sagittal sections of comparable regions of genetically male (A–D) and female (E–H) gonads stained with hematoxylin and eosin oriented as indicated by the compass. This figure is intended for comparison with Figure 1 of the Rabbit Slough anadromous population; the essential features of both populations are the same. Genetic sex was determined by PCR genotyping as described in the Methods. A–F: Black arrowheads point to representative primordial (premeiotic) germ cells. G, H: Meiotic germ cells (white arrowheads and perinucleolar (pn) stage oocytes) are prevalent in ovaries 18 and 26 dpf, respectively. pn, perinucleolar oocyte; s, somatic cell nuclei. Scale bars indicate 10 μm.
15dpf.
By day 15, transverse sections showed the anterior ends of the gonads suspended from the mesentery at the posterior end of the air bladder (Fig. 3B,E,H). The anterior portion of the gonads appeared rounder than at earlier stages, but grossly the gonads appeared to flatten posteriorly and converge towards the midline (Fig. 3C,F,I). The gonads maintained a connection with the mesentery and were tightly interposed between the dorsal surface of the gut and the peritoneal wall. The posterior termini of the gonads were situated directly ventral to the archinephric ducts (Fig. 3D,G,J). Here the gonads contained few PGCs and they tapered into thin strands of somatic cells that appeared to form a single aggregate medially near the posterior of the gut. No ducts or cavities were visible within the gonads of either sex of either lacustrine or anadromous populations at 15 dpf. Except at the extreme posterior aspect of the gonad, the PGCs appeared to be distributed evenly over the entire length of the gonad in both sexes and populations (Fig. 4A,B).
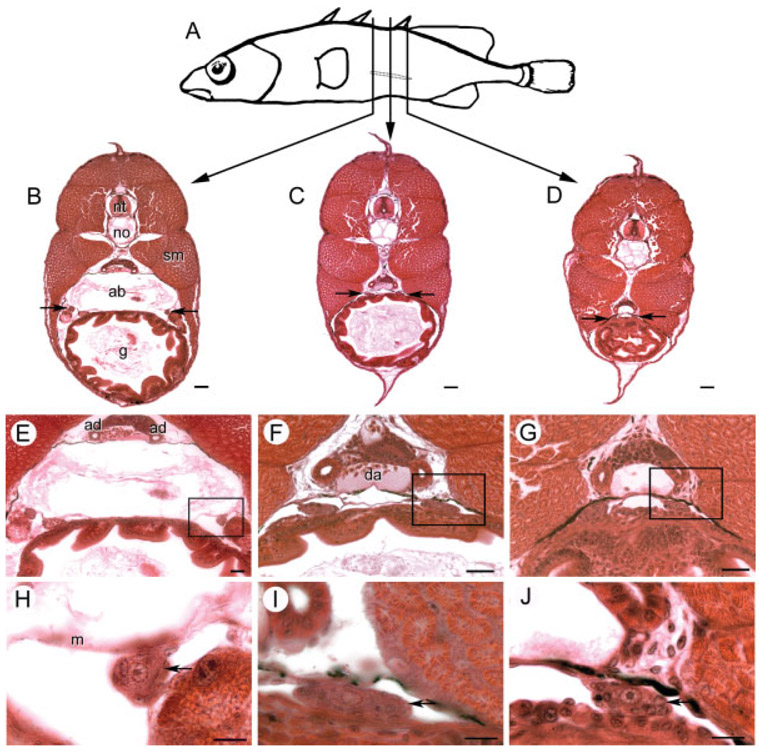
Fig. 3.
Anatomical orientation of developing threespine stickleback (Gasterosteus aculeatus) gonads at 15 dpf. A: Vertical lines, about 540 μm apart depict locations of transverse sections through the outlined left gonad of a female fry sampled from the Bear Paw population. B–D: Transverse sections with arrows showing location of the gonads. E–G: Higher magnifications show the relationship of the gonad to the mesentery (m) and archinephric duct/mesonephros. H–I: Boxed regions of above sections emphasize that gonads (arrows) flatten and approach the midline toward the posterior aspect. ab, air bladder; ad, archinephric duct; da, dorsal aorta; g, gut; m, mesentery; no, notochord; nt, neural tube; sm, skeletal muscle. Scale bars (B–D): 50 μm; (E–G): 25 μm; (I–K): 10 μm.
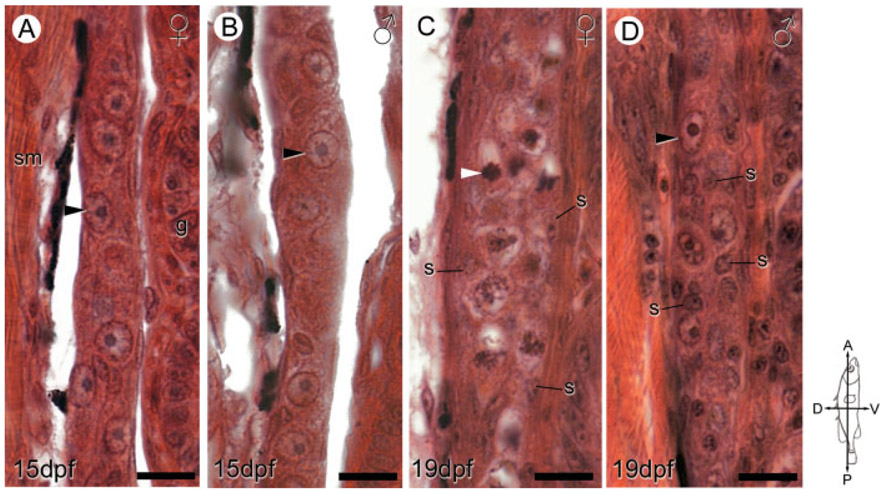
Fig. 4.
Sex-specific ovarian and testicular differentiation between 15 and 19 dpf. Representative sagittal sections of genetically female (A, C) and male (B, D) anadromous threespine stickleback (Gasterosteus aculeatus) fry at 15 dpf (A, B) and 19 dpf (C, D) highlight the dimorphic distribution of gonadal somatic (s) cells and onset of meiosis in female germ cells (C, white arrowheads). Female fry display peripheral distribution of somatic cells (C), while male fry possess many somatic cells in the gonad interior (D). The compass denotes the orientation of the fry. Black arrowheads show primordial (premeiotic) germ cells. g, gut; s, somatic cell nuclei; sm, skeletal muscle. Scale bars: 10 μm.
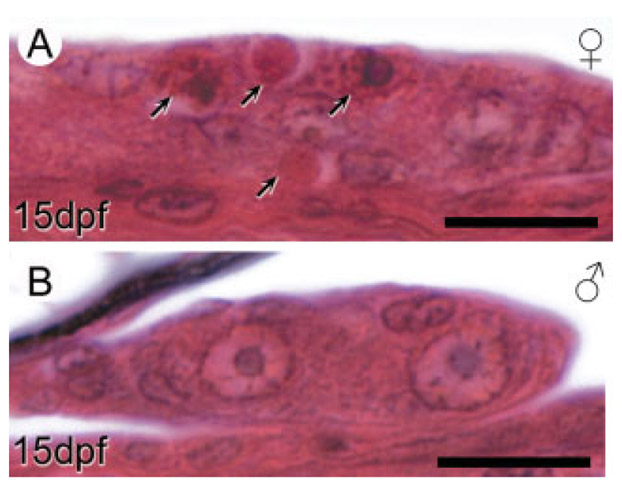
Male and female gonads did not show differences in the distribution of somatic cells within or on the gonad surface (Fig. 4A,B). In general, a single layer of somatic cells containing thin, elongated nuclei lined the surface of developing gonads. Ovaries in some female fry at this age exhibited numerous apoptotic cells and frequent mitotic germ cells (Fig. 5A) while apoptotic cells were rarely encountered in males (Fig. 5B). Also apparent was an increased number of PGCs in ovaries compared to testes (Fig. 4A,B, discussed below).
Fig. 5.
Apoptosis in male and female gonads of anadromous threespine stickleback (Gasterosteus aculeatus) fry at 15 dpf. Photographs are transverse sections of the left gonad in representative female (A) and male (B) fry. A: Arrows indicate both early apoptotic cells with highly basophilic nuclear fragments and acidophilic apoptotic bodies. Substantial numbers of apoptotic cells appeared in female but not male gonads at this stage. Cross sections are positioned similar to the location of the section in Figure 3C. Scale bars: 10 μm.
Examination of the nuclear morphology of all PGCs in complete serial sections of 18 fry failed to reveal any meiotic germ cells at 15 dpf in either sex (Figs. 1B,F and 2B,F). PGCs in 15 dpf larvae were similar in size and structure to those in younger animals.
18 and 19dpf.
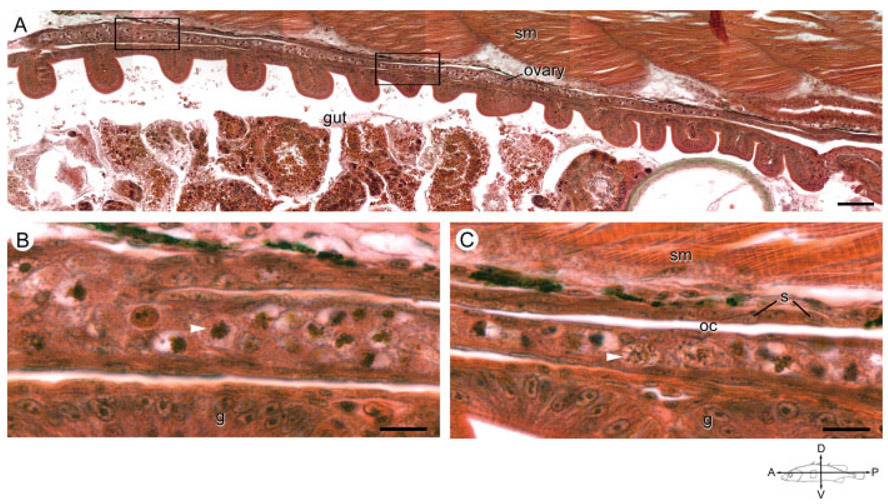
All 18 and 19dpf ovaries contained numerous meiotic germ cells (Figs. 1G, 2G, 4D, and 6A-C). In female fry, the anterior portion of the gonad appeared approximately double the width and more heavily populated with germ cells than ovaries of 15 dpf animals (Fig. 4A,C). The interior of the ovary contained only a few somatic cells distributed among groups of four or more closely associated germ cells (Figs. 1G, 2G, and 4C). The majority of the somatic cells were flattened stromal cells on the periphery of the gonad (Fig. 3C). By 18 and 19 dpf, the ovaries of a few individuals had developed a slit running the length of the long axis of the ovary. The thin space, a possible precursor to the ovarian cavity, was lined with somatic cells (Fig. 6A-C).
Fig. 6.
The ovarian cavity in an anadromous female threespine stickleback (Gasterosteus aculeatus) at 19 dpf. A: Photograph is a low magnification of the longitudinal axis of an ovary in a female fish sampled from the Rabbit Slough population at 19 dpf. Scale bar indicates 25 μm. Compass indicates the orientation of the fish. B, C: Magnification of the anterior (B) and posterior (C) boxed areas from (A) highlight an apparent fold in the anterior portion of the ovary forming a thin slit, ovarian cavity (oc), lined with somatic cells. White arrowheads indicate representative meiotic germ cells. Note that germ cells occur predominately on the ventral aspect of the slit. g, gut; oc, ovarian cavity; s, somatic cell nuclei; sm, skeletal muscle. Scale bar indicates 10 μm.
All of the examined females had initiated meiosis by 18 dpf. Germ cells in ovaries from all females sampled from anadromous and lacustrine populations at 18 and 19 dpf appeared to be in all stages of oogenesis up to the onset of vitellogenesis, though the approximate percentages of premeiotic, and meiotic stages varied among individual females. Meiotic germ cells were identified by the criteria set forth in the methods, including the presence of thick strands of condensed chromatin together with a prominent nucleolus and distinct nuclear membrane. Occasionally, basophilic nuclear aggregates localized to the periphery of the nucleus. The majority of female germ cells were distributed in nests consisting of four or more closely associated oocytes (Fig. 1G).
Male gonads at 18 and 19 dpf exhibited striking differences in the distribution of somatic cells compared to females and to males sampled a few days earlier (Figs. 1C and 4D). In contrast to the peripheral stromal cells of females, males had numerous somatic cells within the interior region of the gonad. These somatic cells with basophilic nuclei often surrounded a single PGC, forming cyst-like structures. In comparison to female fry, males appeared to possess fewer germ cells (Fig. 4C,D, discussed below). In testes, few mitotic germ cells were observed in either anadromous or lacustrine fish populations at 18 and 19 dpf and the nuclear morphology and size of germ cells was essentially identical to fish sampled a week earlier (Figs. 1C,B and 2C,B).
25 and 26 dpf.
The ovaries of 25 and 26 dpf fry were packed with groups of meiotic and premeiotic germ cells (Figs. 1H and 2H). A few flattened somatic cells surrounded centrally located nests of meiotic germ cells. Scattered primordial follicles first appeared in the interior of the ovary at this stage (Fig. 2H). The average nuclear diameter of meiotic germ cells in primordial follicles was 12.72 μm (N = 25, S.D. = 1.74), over twice that of the average nuclear diameter of PGCs (6.08 μm). Germ cells in follicles possessed nucleoli and chromatin localized in clumps along the periphery of the nucleus, similar to perinucleolar oocytes in early vitellogenesis described in zebrafish (Maack and Segner, 2013). However, these primordial follicles were relatively rare and outnumbered by germ cells in the early stages of meiotic prophase and PGCs. Day 25 and 26 ovaries had occasional apoptotic germ cells, though their numbers were greatly reduced compared to earlier stages. Ovaries at 25 and 26 dpf had mitotic somatic cells scattered throughout the gonad. In the posterior ovary, the thin ovarian cavity terminated within a loosely organized epithelium, a possible precursor to the gonoduct.
Although germ cells in 25 and 26 dpf testes displayed a nuclear morphology similar to that of germ cells in fry at earlier ages (Figs. 1D and 2D), a significant development was the encasement of groups of one or more germ cells by layers of somatic cells (Fig. 1D). A definitive, thin, acidophilic basement membrane encompassed each cyst-like structure. The cyst-like structures were distinguishable from nests of oogonia in that the germ cell plasma membranes were clearly visible. Strands of somatic cells oriented along the long axis of the gonad partitioned germ cells into blindly ending tubules.
Thus, the morphological observations presented above suggest that, threespine stickleback are differentiated gonochorists, and the earliest sign of gonadal differentiation in this species is an increase in germ cell number and apoptosis in presumptive ovaries.
Proliferation of PGCs
To test the hypothesis that females, regardless of population of origin, show accelerated PGC proliferation relative to males, the total number of germ cells in left and right gonads of 63 threespine stickleback fry of measured SL were determined in both the anadromous (Rabbit Slough) and lacustrine (Bear Paw) populations for animals between 11 and 26 dpf. This two-way design, in addition to allowing for the test of an effect of sex on PGC number allowed for a simultaneous test of significance of population, and a sex by population interaction, over time.
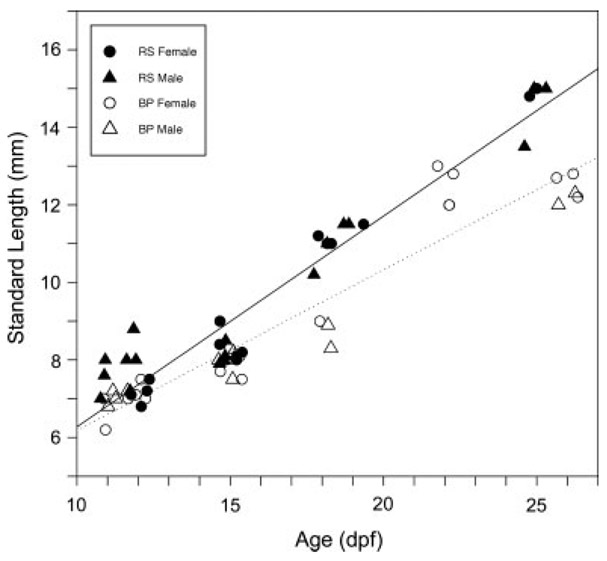
Over the course of the experiment SL increased approximately twofold between 11 and 26 dpf in both populations. An analysis of variance indicated that Rabbit Slough fry were already larger than Bear Paw fry at 11 and 12 dpf (RS = 7.56 mm, S.D. = 0.590, N = 11; BP = 7.00 mm, S.D. = 0.337, N = 10; F1,17 = 5.4, P < 0.05). This relative difference between the populations was retained through 26 dpf (Fig. 7), but there were no sex-specific differences in SL in either population throughout the study. (At 11 and 12 dpf males were 7.53 mm, S. D. = 0.61, N = 11 and females were 7.04 mm, S. D. = 0.37, N = 10).
Fig. 7.
Size of threespine stickleback (Gasterosteus aculeatus) fry differs by population but not by sex within the critical period of sex differentiation. Anadromous Rabbit Slough fry (RS, solid symbols) increase in SL (mm) more rapidly than lacustrine Bear Paw fry (BP, open symbols), but male (triangles) and female (circles) fry show no difference in growth rate. Best fit lines are shown for each population (dotted, Bear Paw; solid, Rabbit Slough). Genetic sex determined as described in the Methods. BP, Bear Paw; dpf, days postfertilization; RS, Rabbit Slough.
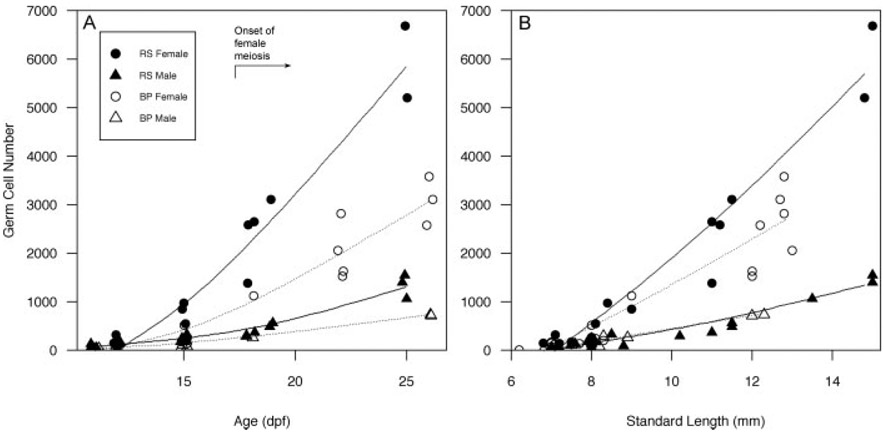
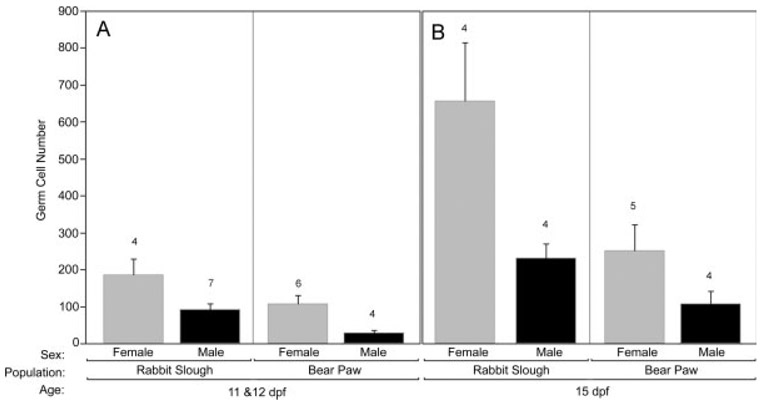
PGC number increased with age (Fig. 8A) and SL (Fig. 8B) in both males and females in both populations. An analysis of covariance at 11 and 12 dpf showed that females already possessed more germ cells than did males (Fig. 9A; F1,13 = 7.56, P < 0.02) and while population-specific effects in PGC number were evident (Fig. 9A), this difference was not significant after taking SL into consideration.
Fig. 8.
Effect of age, size, and sex on numbers of germ cells in anadromous and lacustrine threespine stickleback (Gasterosteus aculeatus) populations during the period of gonadal differentiation. A: Total germ cells increase rapidly in females (circles) relative to males (triangles) and Rabbit Slough fry (RS, solid symbols) possess more germ cells at each age than Bear Paw fry (BP, open symbols). The timing of the initiation of meiosis in genetic females is indicated. B: Population differences in SL (mm) do not account for differences in numbers of germ cells in females. Splines are shown for eased visualization (dotted = Bear Paw, solid = Rabbit Slough). Germ cells were counted based on cellular morphology in two populations of threespine stickleback as described in the Methods. Genetic sex was determined by PCR (as described).
Fig. 9.
The effects of sex and population of origin on germ cell number in threespine stickleback (Gasterosteus aculeatus) fry. A: Germ cell number in 11 and 12 dpf threespine stickleback fry from Rabbit Slough and Bear Paw populations. B: Germ cell number in 15 dpf fry from both populations. Increased germ cell number is apparent in females starting at 11 and 12 dpf (A), and Rabbit Slough fry possess more germ cells than the Bear Paw population. Germ cell number and genetic sex determined as described in the Methods. Error bars show standard error; numbers above each bar indicate sample size.
At 15 dpf, this general pattern continued. There were no sex specific differences in SL (males 8.03 mm, S.D. = 0.282, N = 8; females 8.13 mm, S.D. = 0.406, N = 10) and Rabbit Slough fry were marginally larger than Bear Paw fry (RS = 8.24 mm, S.D. = 0.343, N = 9; BP = 7.92 mm, S.D. = 0.290, N = 9; F1,14 = 3.95, P = 0.067). An analysis of covariance at this age showed that females possessed more germ cells than did males (Fig. 9B; F1,9 = 6.05, P < 0.05) and while population-specific effects in PGC number remained evident (Fig. 9B), the difference between the populations was only marginally significant after removing the effects of size (F1,9 = 3.48, P < 0.10). Thus, compared to males, females had more than twice as many PGCs beginning at 11 and 12 dpf regardless of population of origin and SL.
For samples older than 15 dpf, sample size was insufficient to test for population differences in SL. But examination of Figure 8 clearly shows that the trajectories for increasing PGC number are maintained in a sex-specific and population specific manner. A two way analysis of covariance of all fry older than 15 dpf (N = 24) substantiated higher numbers of PGCs in females relative to males even after removing the effects of length and population of origin (F1,19 = 48.0, P < 0.001).
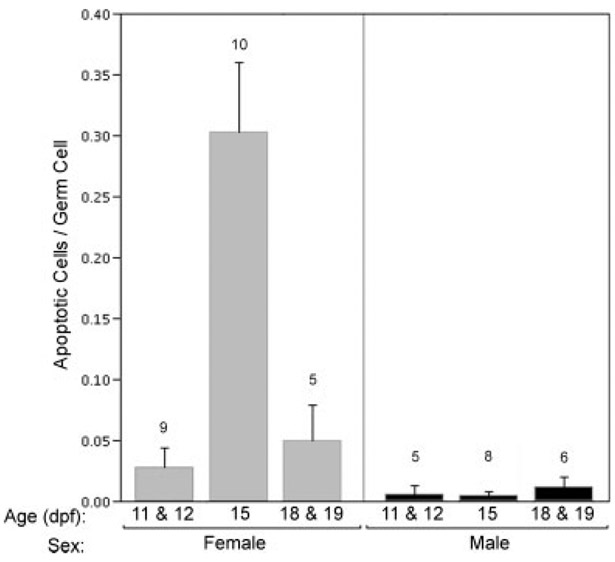
A Wave of Germ Cell Apoptosis in Females Precedes Meiosis
To address the possibility that cell death accounts for reduced PGC numbers in males, we examined the incidence of apoptosis within the developing gonad from 11 and 12 dpf through 18 and 19 dpf, expressing the results as the ratio of apoptotic cells to nonapoptotic germ cells. Morphological criteria were insufficient to categorize shrunken cells in the late stages of apoptosis as germ cells or somatic cells, but numerous cells in early apoptotic stages were clearly distinguished as germ cells. Anadromous and lacustrine populations did not show a significant difference in the mean ratio of apoptotic cells to germ cells (F1,40 = 1.07, P > 0.3), so data from both populations were pooled for subsequent analysis. Between 11/12 dpf and 15 dpf, prior to the onset of meiosis at 18 dpf, the percentage of apoptotic gonadal cells in females increased more than fivefold (Fig. 10). During the same interval there was no difference in apoptosis in male gonads. At 15 dpf, in mean ratio of apoptotic cells to germ cells in males was less than 1% (mean = 0.005, S.D. = 0.006, N = 5; Fig. 10), whereas among female gonads at 15 dpf, the mean percentage of apoptotic cells was ~30% (mean = 0.303, S.D. = 0.171, N = 9; Fig. 10). Thus, a premeiotic spike in ovarian apoptosis coupled with greater overall numbers of PGCs in presumptive ovaries lends further support to the conclusion that the rate of proliferation of PGCs in female gonads is significantly greater than in differentiating male gonads.
Fig. 10.
Effect of age and sex on rates of apoptosis in threespine stickleback (Gasterosteus aculeatus) fry. Data are expressed as the ratio of the total number of apoptotic cells to nonapoptotic germ cells in 10 sections spanning the entirety of the gonads. Cells were counted based on cellular morphology, as described in the Methods. Data collected from individual fry from anadromous and lacustrine populations were pooled for the analysis. Female fry display a peak in the ratio of apoptotic cells to germ cells. Genetic sex was determined by PCR as described. Error bars display standard error; numbers above each bar indicate sample size.
DISCUSSION
Results reported here demonstrate that threespine stickleback sampled from both an anadromous and a lacustrine population are differentiated gonochorists. Threespine stickleback directly develop testes or ovaries from undifferentiated gonadal anlagen shortly after hatching. The intermittent observation of intersex individuals in the field (Craig-Bennett, 1931; Borg and van den Hurk, 1983) does not contradict our laboratory findings when viewed in light of the apparent ease with which the administration of hormones or endocrine disrupting chemicals can alter phenotypic sex (Hahlbeck et al., 2004; Bernhardt et al., 2006). Despite the absence of cytologically distinguishable sex chromosomes (Cuñado et al., 2002), the occurrence of genetic markers for sex (Griffiths et al., 2000) and the identification of a single sex-determining region at the distal end of linkage group 19 support the conclusion that sex chromosomes exist in threespine stickleback (Peichel et al., 2004). Males were consistently heterozygous for this region, prompting the supposition that stickleback exhibit XX/XY sex determination (Peichel et al., 2004). Unfortunately, sequencing much of the sex-determining region of the heterogametic chromosome has not led to the identification of definitive sex-determining genes due to the large number of repetitive sequences (Peichel et al., 2004).
The present data show that a several fold increase in the number of PGCs in gonads of female versus male fry precedes by several days traditional morphological criteria for ovarian or testicular differentiation. In principle, any of several hypotheses could account for the observed difference in PGC number. The difference could be the result of (1) fewer PGCs colonizing male gonads, (2) delayed migration of PGCs into the male gonad, (3) increased PGC death in males, or (4) earlier or more rapid proliferation of germ cells in genetically female stickleback. No studies have addressed the migration of the PGCs in threespine stickleback. There is, however, little variation in the number of PGCs that colonize the gonads of other teleosts (Hardisty, 1967; Braat, 1999). In fact, the majority of vertebrates examined originally possess between 20 and 100 PGCs (Hardisty, 1967). The use of molecular markers to track PGCs in zebrafish, and two gobies (Gymnogobius urotaenia and Leucopsarion petersii) revealed that the number of PGCs colonizing the bilateral gonads in these teleost species has the small range of ~21–30 (Yoon et al., 1997; Saito et al., 2004; Miyake et al., 2006, respectively). Histological sampling methods in Puntius conchonius (rosy barb) also confirmed that the average number of colonizing germ cells falls within this range (Timmermans and Taverne, 1989), while Quirk and Hamilton (1973) reported 23–48 PGCs migrate to the gonads in medaka, with no significant difference between genetic males and females.
Here, we looked for, but did not observe migrating PGCs in serial sections of 11 and 12 dpf fry. Two fry, however, one male and one female, possessed only 7 PGCs in the gonads at 11 dpf. Without the benefit of cellular markers, it is impossible to exclude the possibility that some PGCs remained outside the gonad in these individuals. At the earliest ages examined, 11 and 12 dpf, there were more germ cells in females than males (Least square mean = 160.9, S.E. = 22.3 vs. 59.9, S.E. = 29.1, respectively), a result consistent with increased colonization of the gonad by PGCs or precocious PGC proliferation in females. However, to our knowledge no previous studies in any teleost have reported sex-specific differences in the number of germ cells originally colonizing the gonad, or differences in the timing of PGC migration between sexes. In addition, we found that in 15 dpf stickleback ovaries, rapid PGC proliferation was coincident with massive apoptosis just before the onset of meiosis, whereas modest cell death was consistently observed in male gonads (Fig. 10). This result further emphasizes the dramatic sexual dimorphism in PGC mitotic rate. Because male germ cell death did not account for the sexual dimorphism in germ cell number, the most parsimonious explanation is that earlier or more rapid PGC proliferation in females is the immediate consequence of genotypic sex-determination in threespine stickleback.
As in mammals, XX/XY sex-determining systems are common in other vertebrate taxa. In nonmammalian vertebrates, however, only one male sex-determining gene has been described, DMY in Oryzias latipes (medaka) and Oryzias curvinotus (Hainan medaka), (Matsuda et al., 2002, 2003; Kondo et al., 2004; Matsuda, 2005). Interestingly, DMY is not the sex-determining gene in species closely related to medaka (Schartl, 2004; Matsuda, 2005). Comparative genomics supports the general view that remarkable diversity exists among animal sex-determining genes and that Y chromosomes rapidly evolve from autosomes. The Y chromosome of threespine stickleback is estimated to be less than 10-million-years-old, and Peichel et al. (2004) suggest the sex-determining systems of closely related stickleback species may provide general insights in the evolution of teleost sex determination.
In stickleback with male heterogametic sex determination, two types of mechanisms could account for advanced ovarian differentiation: X-linked gene dosage effects or Y-linked inhibitory, male determining genes. In other teleosts evidence for a dosage effect of a master sex-determining gene is lacking. However, mutations in DMY are known to produce XY females in medaka (Shinomiya et al., 2004). Recently, introduction of a DMY transgene or overexpression of DMY produced fer-tile XX males, whereas knockdown of DMY triggered male-to-female sex reversal in medaka (Matsuda et al., 2007). Further, transplanting XY somatic cells into XX recipients redirected some chimeric female gonads to develop as testes in medaka (Shinomiya et al., 2002).
Whether an early increase in PGCs is required for gonadal sex differentiation is difficult to directly assess (but see Kurokawa et al., 2007). As mentioned above, genetically female medaka display advanced germ cell proliferation. In medaka, DMY overexpression decreased and DMY knockdown increased numbers of germ cells compared to control animals (Matsuda et al., 2002, 2007; Paul-Prasanth et al., 2006) supporting the notion that a repressor, regulated by the somatic cell DMY gene product (Kobayashi et al., 2004), acts to limit PGC mitosis in male medaka (Matsuda, 2005).
Although exogenous steroids can influence gonadal sex during a critical period of sex differentiation in teleosts, this influence may be downstream of master sex-determining genes. Recent work in medaka demonstrated that estrogen provoked the onset of meiosis in males, but numbers of PGCs were not increased (Suzuki et al., 2005; Paul-Prasanth et al., 2006), although these results contradict a previous report (Onitake, 1972). In stickleback, a detailed analysis of the developmental timing of estrogen and androgen disruption of sex determination proved that the window of hormone sensitivity was within 14 dpf, coincident with premeiotic proliferation of female PGCs in the current study (Hahlbeck et al., 2004). Unfortunately no data on numbers of PGCs in sex-reversed individuals were collected and the endpoint of male to female sex reversal was the occurrence of perinucleolar oocytes in 5–6-week-old juveniles. Thus, exogenous steroids and perhaps their environmental mimics, have the potential to interfere with a highly conserved step in the sex differentiation pathway, possibly bypassing master sex-determining genes.
The rapid proliferation of female germ cells and relative delay in the differentiation of male gonads observed in threespine stickleback fry is not necessarily at odds with the general path of sex differentiation in mammals. In mice, postmigratory PGCs, regardless of their genetic sex, proliferate and then enter meiosis whether located in embryonic ovaries, extragonadal sites in males or females, or in vitro, supporting the supposition that the events of early oogenesis are cell autonomous (McLaren, 2003). Conversely, both XX and XY PGCs in male mice arrest premeiotically in contact with developing Sertoli cells in embryonic testes (McLaren, 2003), suggesting that germ cell-Sertoli cell interactions may initially inhibit further spermatocyte development. In threespine stickleback, a similar cell–cell interaction is suggested in testicular acini that develop coincident with female meiosis at ~18 dpf (Figs. 1C,D, 2C,D, and 4D).
A suite of reproductive and life history differences exists between the hypothesized ancestral, anadromous population represented by the modern Rabbit Slough population and the descendant lacustrine Bear Paw population. Rabbit Slough individuals are larger, shorter lived and may produce only 5–6 clutches of 400 large eggs each (~2000 eggs/lifetime). In contrast, individuals from the freshwater Bear Paw population are smaller, longer lived, and may produce 10 clutches of 100 small eggs (~1000 eggs/life time; John Baker, Personal Communication). Even at the early developmental stages reported here, we detected differences in body size between these two populations. In addition, the magnitude of germ cell proliferation corresponded to body size differences between these purported ancestral and descendent populations (i.e., Rabbit Slough fry were larger and displayed increased germ cell proliferation), despite the fact that there was no sexual dimorphism in body size throughout the study. In fact, young Rabbit Slough males were marginally larger than females of the same age and population, emphasizing the finding that female increase in PGCs is not related to body size differences between sexes. Among males, Rabbit Slough fry also exhibited higher germ cell numbers than did Bear Paw males at each age, but approximately equivalent numbers at each length. This may indicate that significant differences in the process of sexual differentiation have evolved coincident with their ecological differences and associated life history differences.
In conclusion, threespine stickleback are differentiated gonochorists. Morphological sex differentiation begins in females with an increase in PGC number relative to males before 11 dpf, but more work needs to be done to pinpoint the exact timing of divergence. Comparative data suggest that a female-first expansion of the population of PGCs may be the important sex-determining factor in teleosts, enabled by chromosomal sex-determining genes in some species. These data emphasize the importance of comparative approaches in studying the process of sex differentiation. Because of its recently sequenced genome, the presence of genetic markers for sex and divergent reproductive characteristics, threespine stickleback may be a particularly useful model system for exploring the molecular genetics of sex determination and the effects of environmental pollutants on sexual development of aquatic species.
ACKNOWLEDGMENTS
Research began at the University of Oregon with support of a Research Opportunity Award to RHK and was concluded through a collaborative effort with institutional support from Reed College and the Betty C. Liu Memorial Biology Research Fellowship. We thank John Baker, Steve Black, Ruth Bremiller, Mark Currey, Joe Dunham, Poh Kheng Loi, and Patrick Phillips.
Contract grant sponsor: NSF; Grant number: 948117312; Contract grant sponsor: 2000 Undergraduate Biological Sciences Education Program Howard Hughes Medical Institute.
LITERATURE CITED
- Ashby KR. 1957. The effect of steroid hormones on the brown trout (Salmo trutta L.) during the period of gonadal differentiation. J Embryol Exp Morphol 5:225–249. [Google Scholar]
- Baker JA, Foster SA. 2002. Phenotypic plasticity for life history traits in a stream population of threespine stickleback, Gasterosteus aculeatus, L. Ecol Freshwater Fish 11:20–29. [Google Scholar]
- Baker JA, Foster SA, Heins DC, Bell MA, King RW. 1998. Variation in female life-history traits among Alaskan populations of the threespine stickleback, Gasterosteus aculeatus L. (Pisces: Gasterosteidae). Biol J Linn Soc 63:141–159. [PubMed] [Google Scholar]
- Bell MA, Foster SA. 1994. Introduction to the evolutionary biology of the threespine stickleback In: Bell MA, Foster SA, editors. The Evolutionary Biology of the Threespine Stickleback. Oxford: Oxford University Press; pp 1–27. [Google Scholar]
- Bernhardt RR, von Hipell FA, Cresko WA. 2006. Percholorate induces hermaphroditism in threespine sticklebacks. Environ Toxicol Chem 25:2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg B, van den Hurk R. 1983. Oocytes in the testes of the threespined stickleback, Gasterosteus aculeatus. Copeia 1983: 259–261. [Google Scholar]
- Braat AK, Speksnijder JE, Zivkovic D. 1999. Germ line development in fishes. Int J Dev Biol 43:745–760. [PubMed] [Google Scholar]
- Cek S 2006. Early gonadal development and sex differentiation in rosy barb (Puntius conchonius). Anim Biol 56:335–350. [Google Scholar]
- Colosimo PF, Hosemann KE, Balabhadra S, Villarreal G Jr., Dickson M, Grimwood J, Schmutz J, Myers RM, Schluter D, Kingsley DM. 2005. Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science 307:1928–1933. [DOI] [PubMed] [Google Scholar]
- Craig-Bennett A 1931. The reproductive cycle of the three-spined stickleback, Gasterosteus aculeatus, Linn. Philos Trans Royal Soc Lond, Series B (Containing Papers of a Biological Character) 219:197–279. [Google Scholar]
- Cresko WA, Yan Y-L, Baltrus DA, Amores A, Singer A, Rodrí-guez-Marí A, Postlethwait JH. 2003. Genome duplication, subfunction partitioning, and lineage divergence: Sox9 in stickleback and zebrafish. Dev Dynam 228:480–489. [DOI] [PubMed] [Google Scholar]
- Cresko WA, Amores A, Wilson C, Murphy J, Currey M, Phillips P, Bell MA, Kimmel CB, Postlethwait JH. 2004. Parallel genetic basis for repeated evolution of armor loss in Alaskan threespine stickleback populations. PNAS 101:6050–6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wa Cresko, Kl McGuigan, Phillips PC Postlethwait JH. 2007. Studies of threespine stickleback developmental evolution: progress and promise. Genetica 129:105–126. [DOI] [PubMed] [Google Scholar]
- Cufiado N, Barrios J, San Miguel E, Amaro R, Fernández C, Hermida M, Santos JL. 2002. Synamptonemal complex analysis in oocytes and spermatocytes of threespine stickleback, Gasterosteus aculeatus (Teleostei, Gasterosteidae). Genetica 114:53–56. [DOI] [PubMed] [Google Scholar]
- Devlin RH, Nagahama Y. 2002. Sex determination and sex differentiation in fish: an overview of genetic, physiological and environmental influences. Aquaculture 208:191–364. [Google Scholar]
- Essenberg JM. 1923. Sex differentiation in the viviparous teleost Xiphophorus helleri Heckel. Biol Bull 45:46–96. [Google Scholar]
- Foster SA, Baker JA. 2004. Evolution in parallel: new insights from a classic system. Trends Ecol Evol 19:546–459. [DOI] [PubMed] [Google Scholar]
- Francis RC. 1992. Sexual lability in teleosts: developmental factors. Q Rev Biol 67:1–18. [Google Scholar]
- Griffiths R, Orr KJ, Adam A, Barber I. 2000. DNA sex identification in the three-spined stickleback. J Fish Biol 57:1331–1334. [Google Scholar]
- Hahlbeck E, Griffiths R, Bengtsson B-E. 2004. The juvenile three-spined stickleback (Gasterosteus aculeatus) as a model organism for endocrine disruption. I. Sexual differentiation. Aquat Toxicol 70:287–310. [DOI] [PubMed] [Google Scholar]
- Hamaguchi S 1982. A light- and electron-microscopic study on the migration of primordial germ cells in the teleost, Oryzias latipes. Cell Tissue Res 227:139–151. [DOI] [PubMed] [Google Scholar]
- Hardisty MW. 1967. The number of vertebrate primordial germ cells. Biol Rev Cambr Philos Soc 42:265–287. [DOI] [PubMed] [Google Scholar]
- Hunter GA, Donaldson EM. 1983. Hormonal sex control and its application to fish culture In: Hoar WS, Randall JD, Donaldson EM, editors. Fish Physiology, Vol. IX Part B. Behavior and Fertility Control. New York: Academic Press; p 223–303. [Google Scholar]
- Katsiadaki I, Scott AP, Mayer I. 2002. The potential of the three-spined stickleback (Gasterosteus aculeatus L.) as a combined biomarker for oestrogens and androgens in European waters. Mar Environ Res 54:725–728. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Matsuda M, Kajiura-Kobayashi H, Suzuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y. 2004. Two DM domain genes DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Dev Dynam 231:518–526. [DOI] [PubMed] [Google Scholar]
- Kondo M, Nanda I, Hornung U, Schmid M, Schartl M. 2004. Evolutionary origin of the medaka Y chromosome. Curr Biol 14:1664–1669. [DOI] [PubMed] [Google Scholar]
- Kurokawa H, Saito D, Nakamura S, Katoh-Fukui Y, Ohta k, Baba T, Morohashi K, Tanaka M. 2007. Germ cells are essential for sexual dimorphism in the medaka gonad. PNAS 104:16958–16963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun C, Billard R, Jalabert B. 1982. Changes in the number of germ cells in the gonads of the rainbow trout (Salmo gairdneri) during the first 10 post-hatching weeks. Reprod Nutr Dev 22:405–412. [DOI] [PubMed] [Google Scholar]
- Lindsey C 1962. Experimental study of meristic variation in a population of threespine sticklebacks, Gasterosteus aculeatus. Can J Zool 40:271–312. [Google Scholar]
- Maack G, Segner H. 2003. Morphological development of the gonads in zebrafish. J Fish Biol 62:895–906. [Google Scholar]
- Mank JE, Promislow DEL, Avise JC. 2006. Evolution of alternative sex-determining mechanisms in teleost fishes. Biol J Linn Soc 87:83–93. [Google Scholar]
- Matsuda M 2005. Sex determination in the teleost medaka, Oryzias latipes. Annu Rev Genet 39:293–307. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M. 2002. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 417:559–563. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Sato T, Toyazaki Y, Nagahama Y, Hamaguchi S, Sakaizumi M. 2003. Oryzias curvinotus has DMY, a gene that is required for male development in the medaka, O. latipes. Zool Sci 20:159–161. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Shinomiya A, Kinoshita M, Suzuki A, Kobayashi T, Paul-Prasanth B, Lau E-l, Hamaguchi S, Sakaizumi M, Nagahama Y. 2007. DMY gene induces male development in genetically female (XX) medaka fish. PNAS 104:3865–3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren A 2003. Primordial germ cells in the mouse. Dev Bio 262:1–15. [DOI] [PubMed] [Google Scholar]
- Miyake A, Saito T, Kashiwagi T, Ando D, Yamamoto A, Suzuki T, Nakatsuji N, Nakatsuji T. 2006. Cloning and pattern of expression of the shirouo vasa gene during embryogenesis and its roles in PGC development. Int J Dev Biol 50:619–625. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Sutasurya LA. 1979. Primordial Germ Cells in the Chordates. Cambridge: Cambridge University Press. [Google Scholar]
- Onitake K 1972. Morphological studies of normal sex-differentiation and induced sex-reversal process of gonads in the medaka, Oryzias latipes. Annot Zool Jpn 45:159–169. [Google Scholar]
- Paul-Prasanth B, Matsuda M, Lau E-L, Suzuki A, Sakai F, Kobayashi T, Nagahama Y. 2006. Knock-down of DMY initiates female pathway in the genetic male medaka, Oryzias latipes. Biochem Biophys Res Commun 351:815–819. [DOI] [PubMed] [Google Scholar]
- Peichel CL. 2005. Fishing for the secrets of vertebrate evolution in threespine sticklebacks. Dev Dynam 234:815–823. [DOI] [PubMed] [Google Scholar]
- Peichel CL, Nereng KS, Ohgi KA, Cole BLE, Colosimo PF, Buerkle CA, Schluter D, Kingsley DM. 2001. The genetic architecture of divergence between threespine stickleback species. Nature 414:901–906. [DOI] [PubMed] [Google Scholar]
- Peichel CL, Ross JA, Matson CK, Dickson M, Grimwood J, Schmutz J, Myers RM, Mori S, Schluter D, Kingsley DM. 2004. The master sex-determination locus in threespine sticklebacks is on a nascent Y chromosome. Curr Biol 14:1416–1424. [DOI] [PubMed] [Google Scholar]
- Quirk JG, Hamilton JB. 1973. Number of germ cells in known male and known female genotypes of vertebrate embryos (Oryzias latipes). Science 180:963–964. [DOI] [PubMed] [Google Scholar]
- Raz E 2003. Primordial germ cell development: the zebrafish perspective. Nat Rev Genet 4:690–700. [DOI] [PubMed] [Google Scholar]
- Robertson JG. 1953. Sex differentiation in the Pacific salmon Onchorynchus keta (Walbaum). Can J Zool 31:73–79. [Google Scholar]
- Saito T, Otani S, Fujimoto T, Suzuki T, Nakatsuji T, Arai K, Yamaha E. 2004. The germ line lineage in ukigori, Gymnogobius species (Teleostei: Gobiidae) during embryonic development. Int J Dev Biol 48:1079–1085. [DOI] [PubMed] [Google Scholar]
- Satoh N, Egami N. 1972. Sex differentiation of germ cells in the teleost, Oryzias latipes, during normal embryonic development. J Embryol Exp Morphol 28:385–395. [PubMed] [Google Scholar]
- Schartl M 2004. Sex chromosome evolution in non-mammalian vertebrates. Curr Opin Genet Dev 14:634–641. [DOI] [PubMed] [Google Scholar]
- Schluter D 1994. Experimental evidence that competition promotes divergence in adaptive radiation. Science 266:798–801. [DOI] [PubMed] [Google Scholar]
- Shapiro MD, Marks ME, Peichel CL, Blackman BK, Nereng KS, Jonsson B, Schluter D, Kingsley DM. 2004. Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature 428:717–723. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Takahashi H. 1980. Process of sex differentiation of the gonad and gonoduct of the three-spined stickleback, Gasterosteus aculeatus L. Bull Fac Fish Hokkaido Univ 31:137–148. [Google Scholar]
- Shinomiya A, Shibata N, Sakaizumi M, Hamaguchi S. 2002. Sex reversal of genetic females (XX) induced by the transplantation of XY somatic cells in the medaka, Oryzias latipes. Int J Dev Biol 46:711–717. [PubMed] [Google Scholar]
- Shinomiya A, Otake H, Togashi K-I, Hamaguchi S, Sakaizumi M. 2004. Field survey of sex-reversals in the medaka, Oryzias latipes: genotypic sexing of wild populations. Zool Sci 21:613–619. [DOI] [PubMed] [Google Scholar]
- Strüssmann CA, Nakamura M. 2002. Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol Biochem 26:13–29. [Google Scholar]
- Strussmann CA, Takashima F, Toda K, 1996. Sex differentiation and hormonal feminization in pejerrey Odontesthes bonariensis. Aquaculture 139:31–45. [Google Scholar]
- Suzuki A, Nakamoto M, Kato Y, Shibata N. 2005. Effects of estradiol-17b on germ cell proliferation and DMY expression during early sexual differentiation of the medaka Oryzias latipes. Zool Sci 22:791–796. [DOI] [PubMed] [Google Scholar]
- Swarup H 1958. The reproductive cycle and development of the gonads in Gasterosteus aculeatus (L). Proc Zool Soc (Calcutta) 11:47–55. [Google Scholar]
- Takahashi H 1977. Juvenile hermaphroditism in the zebrafish, Brachydanio rerio. Bull Fac Fish Hokkaido Univ 28:57–65. [Google Scholar]
- Timmermans LPM, Taverne N. 1989. Segregation of primordial germ cells: Their numbers and fate during early development of Barbus conchonius (Cyprinidae, Teleostei) as indicated by 3H-thymidine incorporation. J Morphol 202:225–237. [DOI] [PubMed] [Google Scholar]
- Vallowe HH. 1957. Sexual differentiation in the teleost fish, Xiphophorus hellerii, as modified by experimental treatment. Biol Bull 112:422–429. [Google Scholar]
- Wolf LE. 1931. History of the germ cells in the viviparous teleost Platypoecilus maculatus. J Morphol 52:428–439. [Google Scholar]
- Wootton RJ. 1998. Ecology of Teleost Fishes, 2nd ed. Dordrecht: Kluwer Academic Publishers; 393p. [Google Scholar]
- Wylie AH, Kerr JFR, Currie AR. 1980. Cell death: The significance of apoptosis. Int Rev Cytol 68:251–306. [DOI] [PubMed] [Google Scholar]
- Yamamoto T 1969. Sex differentiation In: Hoar WS, Randall DJ, editors. Fish Physiology, Vol. 3, Chapter 3 New York: Academic Press; pp 117–175. [Google Scholar]
- Yoon C, Kawakami K, Hopkins N. 1997. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cellstage embryos and is expressed in the primordial germ cells. Development 124:2157–3166. [DOI] [PubMed] [Google Scholar]
- Yoshizaki G, Takeuchi Y, Kobayashi T, Ihara S, Takeuchi T. 2002. Primordial germ cells: The blueprint for a piscine life. Fish Physiol Biochem 26:3–12. [Google Scholar]