Abstract
Estimates of body composition have been derived using 3-dimensional optical imaging (3DO), but no equations to date have been calibrated using a 4-component (4C) model criterion. This investigation reports the development of a novel body fat prediction formula using anthropometric data from 3DO imaging and a 4C model. Anthropometric characteristics and body composition of 179 participants were measured via 3DO (Size Stream® SS20) and a 4C model. Machine learning was used to identify significant anthropometric predictors of body fat (BF%), and stepwise/lasso regression analyses were employed to develop new 3DO-derived BF% prediction equations. The combined equation was externally cross-validated using paired 3DO and DXA assessments (n=158), producing a R2 value of 0.78 and a constant error of (X±SD) 0.8±4.5%. 3DO BF% estimates demonstrated equivalence with DXA based on equivalence testing with no proportional bias in the Bland-Altman analysis. Machine learning methods may hold potential for enhancing 3DO-derived BF% estimates.
In recent years, the use of commercially available 3-dimensional optical imaging (3DO) devices has garnered considerable interest as a potentially useful, non-invasive method to evaluate automated anthropometry and body composition for adults1, 2 and children.3 These devices utilize infrared and visible light to create a 3-dimensional representation of a subject’s body, allowing for rapid, automatic assessment of anthropometric characteristics.4 Highly reliable circumference measurements have been produced by 3DO devices and can be employed in simple anthropometry-based body fat prediction equations.5 However, the comprehensive data collected by 3DO allows for the development of more advanced prediction models that utilize additional circumferences and ratios between body segments, which may reduce the magnitude of errors caused by increasing adiposity, different body shapes, and divergent body composition phenotypes.6 Thus, the purpose of the present investigation was to develop and cross-validate a novel body fat prediction method using anthropometric data from 3DO imaging and a reference 4-component (4C) model.
One hundred and seventy-nine participants (103F, 76M; age: [X±SD] 33.6±15.3 y; BMI: 25.1±4.2 kg/m2; body fat: 26.3±8.8; 30% racial/ethnic minorities) completed duplicate assessments via 3DO, with repositioning between assessments. Each individual assessment produced five full-body scans, which were all utilized in the present analysis. The 3DO scanner employed in the present study (Size Stream® SS20; scanner version 6.0.0.32) utilizes structured light scanning with a static configuration and was calibrated daily using a manufacturer-supplied checkered calibration board. For 4C body composition estimates, body volume was obtained from air displacement plethysmography with measured lung volume (Cosmed BOD POD®), bone mineral content (BMC) from dual-energy X-ray absorptiometry (DXA) (General Electric Lunar Prodigy with enCORE software v. 16.2), total body water from bioimpedance spectroscopy (ImpediMed SFB7), and body mass (BM) from a calibrated electronic scale. All equipment was calibrated as recommended by the device manufacturers each day prior to use, and all testing occurred after an overnight food and fluid fast. Participants provided informed consent, and the aforementioned protocol was approved by the Texas Tech University Institutional Review Board.
A rearrangement of the 4C equation of Wang et al.7 was utilized to estimate body fat percentage (BF%). For 3DO and ADP assessments, participants wore minimal form fitting clothing and a swim cap. Light athletic clothing with no metal was worn for DXA and bioimpedance spectroscopy assessments. For cross-validation purposes, paired DXA (Hologic® Discovery A) and 3DO (Size Stream® SS20) assessments (n=158) were completed at two additional testing sites. Although differences between GE® and Hologic® DXA output have been reported,8 cross-calibration was not performed for the present analysis due to the minimal use of GE® DXA variables (i.e., only BMC from the GE® scanner was used as part of the 4C model).
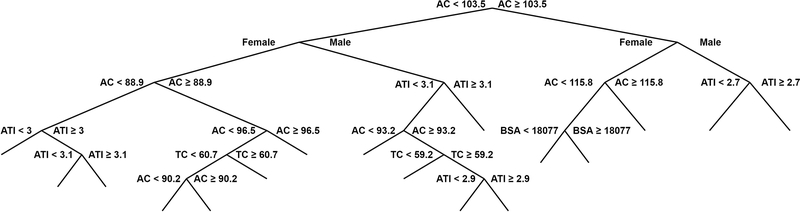
Machine learning (i.e., decision tree analysis) was utilized to determine the significant 3DO-derived factor(s) separating higher and lower BF%, based on the 4C model data. Stepwise and lasso regression analyses were performed to develop a BF% estimation formula based on approximately 200 anthropometric measurement sites identified by the 3DO scanning software. Decision tree analysis using the fitrtree function (MATLAB, Natick, MA, USA) identified lower abdomen circumference (ACLOWER) as the primary anthropometric factor that distinguished between higher and lower BF%, with a criterion separation point of 103.5 cm (40.75 inches) (Figure 1). ACLOWER was defined as the horizontal circumference at the level of the forwardmost projection point occurring inferior to the front waist point and superior to the maximum rear seat projection. A novel appendage-to-trunk circumference index (ATI) was developed for use in the BF% prediction equations, where UA is upper arm, R and L correspond to right and left, ACUPPER corresponds to the maximal circumference superior to the waist and inferior to the chest, and all values represent circumference estimated in cm.
Figure 1. Decision Tree Analysis.
Machine learning (i.e., decision tree analysis) identified lower abdomen circumference (AC) as the primary anthropometric factor that distinguished between higher and lower 4-component model body fat percentage, with a criterion separation point of 103.5 cm (40.75 inches). The decision tree procedures further produced the two body fat percentage equations displayed in the manuscript.
Abbreviations. AC: lower abdominal circumference in cm, ATI: appendage-to-trunk index, BSA: body surface area in cm2, TC: thigh circumference (right) in cm
Novel BF% prediction equations for individuals with ACLOWER less than 103.5 cm (3DO BF%Eq.1) and greater than 103.5 cm (3DO BF%Eq.2) were developed, where sex is coded as Male=1 and Female=0, BSA is body surface area measured in cm2, and all remaining variables are circumferences measured in cm. Thigh circumference was defined as the leg girth measured 5.1 cm (2.0 inches) below the crotch point.
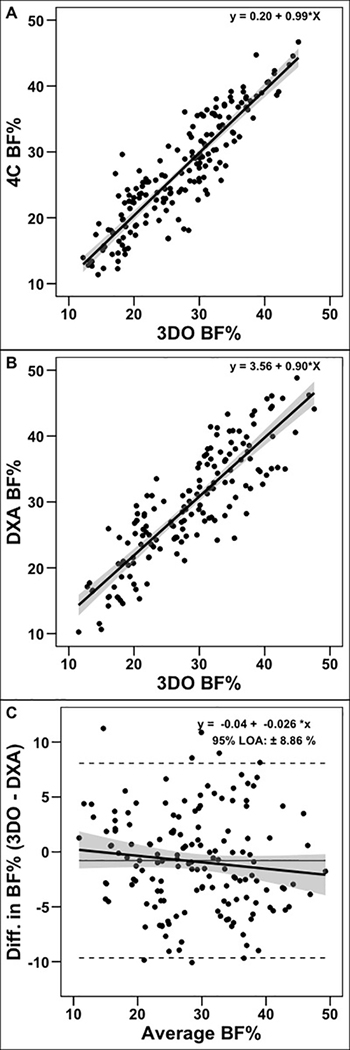
The developed method produced a R2 of 0.82 and a constant error (CE) of −0.15 ± 3.67% relative to 4C BF% data (Figure 2A). Cross-validation against DXA BF% produced an R2 value of 0.78, CE of −0.79 ± 4.52%, and root mean square error of 4.57% (Figure 2B). Additionally, 3DO and DXA BF% exhibited equivalence9 based on a ±2% body fat equivalence interval and displayed no proportional bias in the Bland-Altman analysis (Figure 2C).
Figure 2. Validation of 3DO Body Fat Estimates.
New 3DO body fat estimates were developed using paired 3DO and 4C assessments (panel A). The 3DO estimates were externally cross validated against DXA. Cross-validation indicated a small deviation from the slope of the line of identity (panel B; slope: 0.90 [95% confidence interval: 0.83, 0.98]), but no proportional bias (panel C; slope: 0.03 [95% confidence interval: −0.05, 0.10]).
Abbreviations. 3DO: 3-dimensional optical imaging, 4C: 4-component model, DXA: dual-energy x-ray absorptiometry, LOA: limits of agreement
This proof-of-concept investigation identified and validated a novel 3DO body fat prediction equation that accounts for divergent anthropometric characteristics. The combined equation was found to have acceptable validity and minimal group-level error compared to DXA, mirroring the results of Ng and colleagues,1 who reported comparable validity of a 3DO-derived prediction equation relative to DXA fat mass estimates in a multi-ethnic, mixed-sex cohort. Taken together, these findings suggest that device-specific 3DO BF% prediction equations could serve as a suitable alternative to DXA to estimate body composition in groups of healthy individuals. While the individual-level error (i.e., 95% limits of agreement) was relatively larger, further research regarding the potential utility of 3DO for tracking individual changes in body composition is warranted.
A unique feature of the present investigation is the use of the 4C model to develop the 3DO BF% prediction equation, as previous studies have not developed or validated 3DO-derived estimates using a multi-compartment model.1, 6, 10, 11 Furthermore, the novel application of machine learning techniques to determine key anthropometric factors associated with body fat variation revealed that the optimal 3DO-derived variables to predict percent body fat differ substantially between lean and obese individuals. However, limitations of the present investigation are the small sample size for machine learning techniques, which likely did not capture the full variety of body shapes and compositions, and the necessity of utilizing few regressors to avoid overfitting. Additionally, anthropometric estimates from differing 3DO systems can vary, even for similar anatomical locations, indicating that the presented equations are likely specific to the evaluated scanner.5 In the future, investigators and device manufacturers could use a similar approach to identify key anthropometric predictors in specific populations or in larger samples, potentially increasing the specificity, accuracy, and utility of 3DO-derived body composition estimation equations.
Funding
No funding was received for the data collection leading to equation development, although the project was supported in-kind by Size Stream, LLC through provision of the 3-dimensional optical scanner. The data utilized for external validation were obtained from research supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK109008, R01DK111698) and registered at Clinical Trial Registration: NCT03637855.
Footnotes
Conflict of Interest
Size Stream, LLC, the manufacturer of the 3D scanner utilized in the present study, supported the project through equipment loan/donation to the study sites. However, monetary funding was not provided to the study sites or investigators as part of this project. Size Stream, LLC was also involved in the study design, execution, analysis, and interpretation of the study results. D.B. is employed by Size Stream, LLC, and B.S. is a paid analysis consultant for Size Stream, LLC. S.B.H. is on the medical advisory board for Tanita Medical. J.A.S. has received in-kind support from Size Stream, LLC, Styku, and FIT3D and has received research funding from Hologic and General Electric (GE) Healthcare. G.M.T. has received in-kind support from Size Stream, LLC, Naked Labs Inc., RJL Systems, MuscleSound, and Biospace, Inc. (DBA InBody). The remaining authors declare no potential conflicts of interest.
REFERENCES
- 1.Ng BK, Sommer MJ, Wong MC, Pagano I, Nie Y, Fan B et al. Detailed 3-dimensional body shape features predict body composition, blood metabolites, and functional strength: the Shape Up! studies. The American Journal of Clinical Nutrition 2019. doi: 10.1093/ajcn/nqz218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tinsley GM, Moore ML, Benavides ML, Dellinger JR, Adamson BT. 3-Dimensional optical scanning for body composition assessment: A 4-component model comparison of four commercially available scanners. Clin Nutr 2020. e-pub ahead of print 2020/03/03; doi: 10.1016/j.clnu.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 3.Wong MC, Ng BK, Kennedy SF, Hwaung P, Liu EY, Kelly NN et al. Children and Adolescents’ Anthropometrics Body Composition from 3-D Optical Surface Scans. Obesity (Silver Spring, Md.) 2019; 27(11): 1738–1749. e-pub ahead of print 2019/11/07; doi: 10.1002/oby.22637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymsfield SB, Bourgeois B, Ng BK, Sommer MJ, Li X, Shepherd JA. Digital anthropometry: a critical review. European Journal of Clinical Nutrition 2018; 72(5): 680–687. doi: 10.1038/s41430-018-0145-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tinsley GM, Moore ML, Dellinger JR, Adamson BT, Benavides ML. Digital anthropometry via three-dimensional optical scanning: evaluation of four commercially available systems. European Journal of Clinical Nutrition 2019. doi: 10.1038/s41430-019-0526-6 [DOI] [PubMed] [Google Scholar]
- 6.Harbin MM, Kasak A, Ostrem JD, Dengel DR. Validation of a three-dimensional body scanner for body composition measures. Eur J Clin Nutr 2018; 72(8): 1191–1194. e-pub ahead of print 2017/12/31; doi: 10.1038/s41430-017-0046-1 [DOI] [PubMed] [Google Scholar]
- 7.Wang ZM, Xavier P-S, Kotler DP, Wielopolski L, Withers RT, Pierson J et al. Multicomponent methods: Evaluation of new and traditional soft tissue mineral models by in vivo neutron activation analysis. American Journal of Clinical Nutrition 2002; 76: 968–974. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL et al. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res 2012; 27(10): 2208–2216. e-pub ahead of print 2012/05/25; doi: 10.1002/jbmr.1654 [DOI] [PubMed] [Google Scholar]
- 9.Dixon PM, Saint-Maurice PF, Kim Y, Hibbing P, Bai Y, Welk GJ. A Primer on the Use of Equivalence Testing for Evaluating Measurement Agreement. Medicine and science in sports and exercise 2018; 50(4): 837–845. e-pub ahead of print 2017/11/15; doi: 10.1249/mss.0000000000001481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryder JR, Ball SD. Three-dimensional body scanning as a novel technique for body composition assessment: a preliminary investigation. Journal of Exercise Physiology Online 2012; 15: 1+. [Google Scholar]
- 11.Wang J, Gallagher D, Thornton JC, Yu W, Horlick M, Pi-Sunyer FX. Validation of a 3-dimensional photonic scanner for the measurement of body volumes, dimensions, and percentage body fat. The American journal of clinical nutrition 2006; 83(4): 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]