Abstract
Depressive symptoms may differ in severity and change over time in people living with HIV (PLWH). Describing depression trajectories and associated clinical characteristics of PLWH in an interventional study may help in developing a more personalized medicine approach. Using latent class growth analysis four, 15-month self-reported depression trajectories were identified in 416 PLWH participating in a collaborative care program. The four subgroups were characterized by improving (58.4% [of participants]), worsening (9.4%), highly responsive (19.5%) and persistently severe (12.7%) depressive symptoms. A high proportion of individuals were in trajectories marked by improvement. Further, the highly responsive group had on average, over 50% reduction of self-reported depressive symptoms. Self-reported trauma, posttraumatic stress disorder (PTSD), lower education and fewer HIV and psychiatry clinic visits were associated with worsening or persistently severe depressive symptom trajectories. Members of the persistently severe group were less likely to be virally suppressed after 12-months. Identifying subgroups of PLWH based on changes in self-reported depressive symptoms may further inform intervention approaches that can advance care.
Keywords: Depression, HIV viral suppression, longitudinal studies, collaborative care model, reported trauma, latent class growth analysis
Introduction
People living with HIV (PLWH) commonly experience depressive symptoms, with a prevalence rate between 28% and 44%(1–4). Many of these individuals have long term symptoms such as recurrent depressive episodes (2) and/or chronic major depressive disorder (3).
Depression is associated with poor HIV health outcomes. PLWH with major depression (compared with no major depression) are more likely to have poor adherence to antiretroviral therapy (4, 5), detectable viral loads (6), worse long-term survival (6, 7), and lower quality of life (8). In PLWH, depression severity is associated with increased risk behaviors and decreased immune functioning (9). Depression is also frequently under-treated in PLWH (2, 3).
The clinical manifestation of depression is often complex in PLWH (10). Depression may change over time and be a heterogenous condition (10–12). PLWH exhibit different long-term trajectories based in part on HIV clinical factors and the severity of depressive symptoms (10–12).
Due to the number of PLWH who experience depressive symptoms, many individuals utilize antidepressant treatment or psychotherapy (10). These interventions help PLWH manage the symptoms of HIV and depression (13). In order to guide clinical management and further personalize these interventions, there is a growing need to understand how depression changes over time, or the trajectories of depression, in PLWH in an intervention program.
Our objective was to describe the depression trajectories for PLWH who were participating in an intervention. We implemented the collaborative care model (CCM) in a busy urban HIV primary care clinic. The CCM is an evidence-based integrated care model for treating depression in primary care settings. The CCM includes routine screening for depression, measurement-based care, proactive disease management utilizing a registry, behavioral health coordination, and case consultation by the behavioral health care manager with a psychiatrist. The consulting psychiatrist makes treatment recommendations to the primary provider through the behavioral health care manager but does not directly evaluate the patient. The CCM is relatively new to HIV settings (14, 15).
After identifying homogeneous subgroups of PLWH based on depression trajectories, clinicians may begin to monitor potential nonresponders characterized by intractable or volatile depression trajectories. We study both individual-level clinical factors and neighborhood-level indicators of education and income that potentially associate with these depression trajectories.
Neighborhood-level indicators capture essential information regarding the physical and social environment within neighborhoods that influence health outcomes in people living with HIV (16). Disadvantaged neighborhoods have a higher prevalence of HIV, due in part to intersecting social conditions such as poverty, discrimination and inequality (17). Neighborhood-level poverty has been associated with lower CD4 cell counts and more racially segregated neighborhoods have been found to be associated with more depressive symptoms in PLWH (18). Further, both increased poverty and lower education have been associated with lower probability of survival after an HIV diagnosis (19, 20). In general, neighborhood disadvantage has been found to be associated with higher rates of major depression and substance use disorders (21). In PLWH, social-economic position and individual-level characteristics have been hypothesized to influence each other as well as moderate the relationship between environmental resources and constraints, such as social capital, minority stress and stigma, and psychological influences including depression and trauma (17).
Relationships between depression trajectories and subsequent viral suppression rates also should be evaluated in order to understand the impact of certain trajectories on HIV viral load. Those subgroups characterized by a favorable depression trajectory may provide insight into the type of subject likely to respond to the intervention. Our study will help clinicians understand better the progression of depression in PLWH to aid in patient care decisions.
Methods
Design and Measures
Beginning in June 2015, we implemented the CCM at a public health care system located in Cleveland, OH. The purpose of this program was to improve the identification and management of patients with depressive symptoms. A combination of manual data collection and electronic data extraction was used to collect clinical and sociodemographic characteristics on all patients screening positive for depression.
The Patient Health Questionnaire (PHQ)-2 asks about the presence of depression or anhedonia during the prior two weeks. Patients with an affirmative response to either question are prompted to complete the PHQ-9. On the PHQ-9, patients specify frequency in the past 2 weeks (0 = not at all to 3 = every day) of nine symptoms, yielding a total score (range: 0–27). Scores on this self-reported instrument provide information regarding severity of symptom and treatment response; thus the PHQ-9 is often used to guide treatment decisions (22). In particular, a PHQ-9 ≥ 10 has been previously established as a screening cutoff for depressive disorder given high sensitivity and specificity at this score (22, 23). The PHQ-9 has been validated using multiple modes for administration, clinical populations, and diverse racial and ethnic groups (24).
To implement the CCM, all patients presenting for HIV care were screened for depressive symptoms using the PHQ-2/9; a behavioral health care manager (BHM) met with patients scoring 10 or higher in clinic and at the time of the visit. The CCM was implemented clinic-wide regardless of insurance status. The BHMs reviewed current and prior symptoms, reviewed and encouraged self-management strategies for depression and often completed a full mental health assessment. Once weekly, the BHMs met with the consulting psychiatrist to review each case. The consulting psychiatrist makes recommendations for medications, counseling and/or additional evaluations. Measurement-based care involves remeasuring symptoms every three months until symptoms remit as well as augmenting therapy when there is insufficient reduction of symptoms. The CCM was implemented clinic-wide regardless of insurance status.
Total PHQ-9 scores, age, gender, race, health insurance, smoking status, CD4 count, viral load, and HIV and psychiatry clinic and ED visits were extracted from an electronic health records (EHR) and entered into a database.
Baseline HIV viral load and CD4 counts were the last values recorded prior to the initial PHQ-9 assessment. The 12-month measurements for HIV viral load and CD4 count were the last values recorded within one year post the initial PHQ-9 assessment. Viral loads ≤200 copies/ml were categorized as not detectable and viral loads > 200 copies/ml were categorized as detectable. We used 2011–2015 American Community Survey 5-year data (25) at the zip-code level (ZCTA) to obtain neighborhood median household income level and high school education attainment based on geocoding percentage.
Manual chart reviews were used to collect data on substance use history, prior diagnosis of posttraumatic stress disorder (PTSD), prior diagnosis of bipolar disorder and reported abuse or trauma. Prior diagnoses were collected from the patient’s past medical history and current problem list. Reports of abuse or trauma were based on detailed notes from BHMs, medical case managers and physician notes. Reports of trauma or abuse are standard in a full mental health assessment as well as medical case management assessments. We also recorded if the subject was prescribed psychiatric medications prior to their initial PHQ-9.
Ethical Considerations
All study procedures were approved by the local IRB (IRB # - 15–00252) and adhered to the principles outlined in the Declaration of Helsinki. Data reported here were collected retrospectively under a waiver of consent.
Statistical Analysis
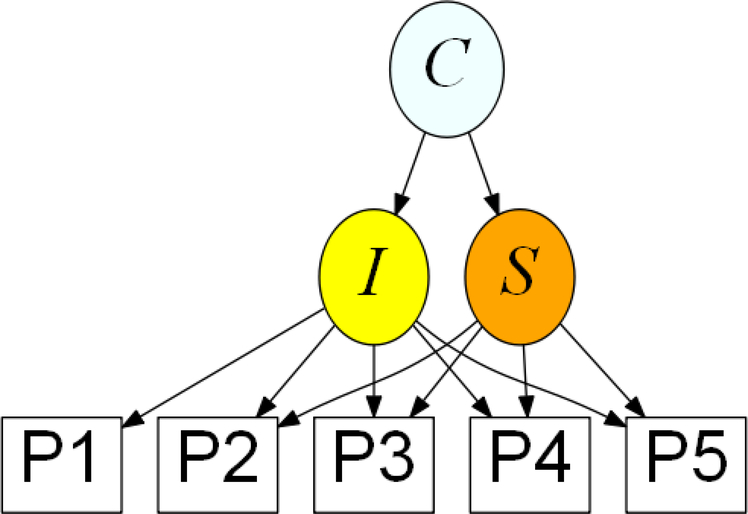
Latent Class Growth Analysis (LCGA) allows us to identify meaningful subgroups within a larger study population to examine growth trajectories over time (26). LCGA approaches are flexible modeling strategies (27). In our study, LCGA accounts for the challenges of an EHR database (i.e. individually varying follow-up appointments, irregular follow up, missingness and systematic error of patient reported scales). The latent variable mixture model for performing LCGA for our study corresponds to the path diagram in Figure 1.
Figure 1. Path diagram of latent variable mixture model used for a depression screening scale for people living with HIV in a collaborative care program over 15 months (N = 416).
I = level of PHQ-9 at baseline; S = linear rate at which PHQ-9 changes; C = categorical variable for latent class. P1-P5 are the individually time varying PHQ-9 total scores for each individual at each time point from baseline up to 5 possible time points over a 15-month period. We used the mixture model corresponding to this figure to (1) determine the optimal solution for the number of classes and (2) classify individuals into most likely latent class membership. Description of the within-class trajectories subgroups was subsequently done under no assumption of linearity.
We (1) performed LCGA using the mixture model in Figure 1 to determine the optimal solution for the number of subgroups and classify individuals into subgroups of PLWH in the collaborative care program based on longitudinal depression screening trajectories (2) described these subgroups (within-class trajectories) using local regression techniques under no assumption of linearity (3) evaluated potential predictors of subgroup membership (4) evaluated if subgroup membership associated with viral suppression rates at 12-months.
The subgroups (also termed latent classes) identified by LCGA are not known a priori, but rather are determined empirically. A trajectory shape for each class is estimated from the data, and individuals are assigned to latent classes based on their posterior probabilities (28). To identify the optimal number of classes for our data structure, statistical indices and parameter estimates are evaluated, including entropy, Akaike information criterion (AIC), Bayesian information criterion (BIC), sample-size adjusted BIC (aBIC), corrected AIC for small sample sizes (AICC) and assessment of class cell sizes (29). The one-class solution is first specified, which is then used as a comparison for solutions of increasing class size until the best solution is identified. A graphical display of the mean trajectory for the PHQ-9 scale within each subgroup helped us assign meaning to each latent class.
We used the MLR option in MPlus to perform model estimation (30, 31). The MLR approach uses maximum likelihood to estimate the parameters, but uses a robust sandwich type estimator (Huber-White sandwich estimator) to calculate standard errors (31). Robust approaches are resistant to errors under small violations of parametric assumptions.
Our model effectively handled ignorable missing data dependent on the data in hand (i.e., following a “missing at random” assumption) via full information maximum likelihood (FIML). As a result, respondents with missing data could still be included in the trajectory analysis for unbiased inference.
Multinomial regression is used to regress a nominal outcome on an explanatory variable. Subgroup membership across individuals in the study population can be summarized in a latent class nominal variable. Therefore, multinomial regression was used to determine if age, sex, race, insurance status, neighborhood income, neighborhood rates of high school graduation, HIV viral load at baseline, CD4 count at baseline, HIV clinic, psychiatry clinic and Emergency Department visits in the 12 months prior to initial visit, smoking status, reported trauma, PTSD, history of substance abuse, bipolar disorder and mental health medication use influenced subgroup membership. Due to our sample size, we first analyzed one explanatory variable at a time in a series of models. We then included all statistically significant explanatory variables in a multiple regression model. We found only a small point biserial correlation (rpb) between PTSD (rpb = 0.15) and reported trauma (rpb = 0.17) and PHQ-9 at baseline, confirming that these items are not collinear.
We used logistic regression to evaluate if subgroup membership associated with a binary indicator of HIV viral load (not detected vs. detected) at 12-months. Visits over 15-months post-baseline occurred irregularly and 12-month HIV viral load was collected with variation in timeframe. However, due to the variation in timing, we evaluated the association between subgroup trajectories and HIV viral load; we did not assume the results would provide information regarding the extent to which these trajectories influenced viral suppression.
Additionally, we used bias-corrected three step procedures (32, 33) in performing multinomial and logistic regression. These procedures account for measurement error in the assignment of latent classes (since assignment is not exact and is based on posterior probabilities).
We defined α = 0.05 for our level of significance in all statistical tests. All statistical tests were two-tailed. R program 3.5.2 and R Studio were used for data cleaning and graphics (34). LCGA was carried out using Mplus Version 8.2 (30).
Results
Study Population
The HIV clinic is part of a public health care system in Ohio. Between June 29, 2015 and December 31, 2017, the HIV clinic served 1824 total patients. Overall, 1384 (76%) were male. In self-reported racial-ethnic categories, 985 (54%) identified as Black/African American, 736 (40%) identified as White, and 258 (14%) identified as Hispanic. Patients ranged from 16–81 years of age, with 65% between 40–70 years old.
CCM subjects (N = 416) included in this study had screened positive for depressive symptoms during a first visit between June 29, 2015 and June 30, 2017 (at least six months prior to the end of the study period). All subjects had at least one follow up PHQ-9 measure after the first visit to be considered in the data. The second visit in the study was scheduled to occur three months after the first visit. Given variation in timing, these second visits occurred anywhere between 1.2 and 4.8 months with a mean of 3.3 months (standard deviation = 0.85 months). Thus, subjects had a sufficient amount of follow-up time as well as available data to evaluate depression screening changes. Baseline was the first visit in our database during our study period.
We used data on these subjects up to 15-months post baseline. We describe this population in Table I. Of note, out of the 61 of participants who were prescribed a psychiatric medication prior to initial PHQ-9 ≥10, the majority (83.5%) reported the medication was either a selective serotonin reuptake inhibitor (SSRI) or a serotonin norepinephrine reuptake inhibitor (SNRI).
Table I:
Characteristics of people living with HIV with self-reported depression
| Missing | ||||
|---|---|---|---|---|
| Factor | N = 416 | % | # | % |
| Age (Years) (mean±SD) | 42.7±11.93 | 0 | 0.0 | |
| Male | 301 | 72.4 | 0 | 0.0 |
| Race/ethnicity | 2 | 0.5 | ||
| African American/Black | 199 | 48.1 | ||
| Hispanic/Latino | 36 | 8.7 | ||
| Other | 7 | 1.7 | ||
| White (non-Hispanic) | 172 | 41.5 | ||
| Neighborhood median household incomea | 16 | 3.8 | ||
| $0–$30,000 | 140 | 35.0 | ||
| $30,000–$50,000 | 202 | 50.5 | ||
| >$50,000 | 58 | 14.5 | ||
| Neighborhood high school graduation rates ≥ 80% | 212 | 53.0 | 16 | 3.8 |
| Primary insurance Class | 2 | 0.5 | ||
| Commercial or private | 55 | 13.3 | ||
| Medicaid | 195 | 47.1 | ||
| Medicare | 107 | 25.8 | ||
| Uninsured or self-Pay | 32 | 7.7 | ||
| Other Class | 25 | 6.0 | ||
| Smoking status | 2 | 0.5 | ||
| Never | 76 | 18.4 | ||
| Former smoker | 103 | 24.9 | ||
| Current | 235 | 56.8 | ||
| Documented history of substance use | 254 | 62.4 | 9 | 2.2 |
| PHQ-9 severity | 0 | 0.0 | ||
| Moderate (10–14) | 197 | 47.4 | ||
| Moderately severe (15–20) | 119 | 28.6 | ||
| Severe (21–27) | 100 | 24.0 | ||
| HIV viral load (copies/mL) | 0 | 0.0 | ||
| Not documented | 11 | 2.6 | ||
| Undetectable (< 200) | 320 | 76.9 | ||
| Detected (≥ 200) | 85 | 20.4 | ||
| CD4+ Count (cells/μL) | 0 | 0.0 | ||
| Not documented | 31 | 7.5 | ||
| 0 – 200 | 31 | 7.5 | ||
| 201 – 350 | 55 | 13.2 | ||
| 351 – 500 | 71 | 17.1 | ||
| ≥ 501 | 228 | 54.8 | ||
| Attended at least 1 HIV clinic visit in 1 year period prior to baseline | 364 | 87.5 | 0 | 0.0 |
| Attended at least 1 psychiatry clinic visit in 1 year period prior to baseline | 80 | 19.2 | 0 | 0.0 |
| Visited emergency department once or more in 1 year period prior to baseline | 142 | 34.1 | 0 | 0.0 |
| PTSD | 74 | 17.8 | 0 | 0.0 |
| Bipolar | 80 | 19.2 | 0 | 0.0 |
| Self-report taking mental health medications prior to PHQ-9 screening | 254 | 61.1 | 0 | 0.0 |
| Self-report of trauma | 141 | 46.4 | 112 | 26.9 |
$0–$30,000 = very low income $30,000–$50,000 = low income, >$50,000 medium income
CCM participants had a depression screening assessment an average of 2.78 times (SD = 0.80) over the 15-month period under study. By inclusion criteria, all patients had at least two depression screening assessments. Most (57%) of those patients made a third, and 18% had at least four depression screening assessments over the study timeframe. Only 3% had five depression screening assessments over the study timeframe (with none more than five).
Number of Subgroups
Using LCGA, we identified four latent classes. The minimized BIC value (Table II) and interpretability of the classes support the four class solution. None of the other potential solutions had a meaningful improvement across model fit indices or interpretability as compared to the four class solution. We could also rule out the six class solution since one of the classes had zero members.
Table II.
Model fit indices for one through eight class solutions for latent class growth analysis in people living with HIV with self-reported depression.
| Number of Classes | Log-likelihood | DF | Scaling Correction for MLR | AIC | AICC | BIC | aBIC | Entropy |
|---|---|---|---|---|---|---|---|---|
| 1 | −3670.94 | 7 | 0.85 | 7354.39 | 7354.66 | 7382.60 | 7360.39 | |
| 2 | −3580.96 | 10 | 1.15 | 7181.92 | 7182.46 | 7222.22 | 7190.49 | 0.720 |
| 3 | −3567.71 | 13 | 1.33 | 7161.42 | 7162.33 | 7213.82 | 7172.57 | 0.770 |
| 4 | −3549.17 | 16 | 1.14 | 7130.33 | 7131.69 | 7194.82 | 7144.05 | 0.707 |
| 5 | −3545.66 | 19 | 1.11 | 7129.31 | 7131.23 | 7205.90 | 7145.61 | 0.608 |
| 6 | −3542.55 | 22 | 0.98 | 7129.10 | 7131.68 | 7217.78 | 7147.97 | 0.754 |
| 7 | −3528.58 | 25 | 1.07 | 7107.16 | 7110.49 | 7207.93 | 7128.60 | 0.677 |
| 8 | −3528.58 | 28 | 0.95 | 7113.16 | 7117.36 | 7226.02 | 7137.17 | 0.697 |
DF = Degrees of Freedom; MLR = maximum likelihood with robust standard errors via Hubert-White sandwich estimator; AIC = Akaike Information Criteria; AICC = corrected AIC for small sample sizes; BIC = Bayesian Information Criteria; aBIC = sample-size adjusted BIC
In terms of classification of individuals based on their most likely latent class, the four class solution did not have any low cell sizes; the smallest subgroup still had 9.4% of the cases in the sample.
Description of Subgroups
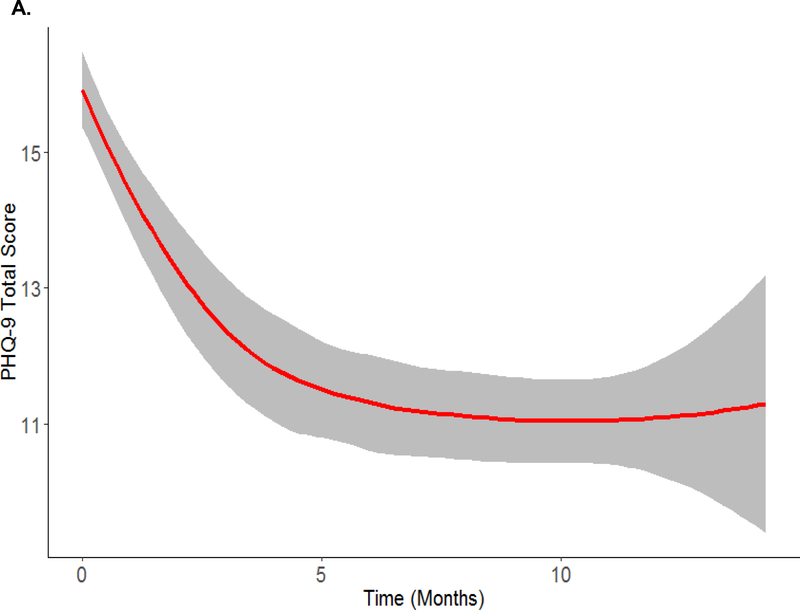
Overall, the average trajectory of self-reported depression in this sample started at moderate and decreased over the 15 months, while still above the threshold for major depression (see Figure 2A). We performed mixed-effects cubic regression analysis to estimate this overall trend [Estimate (95% bias-corrected bootstrapped confidence interval); Intercept = 15.886 (15.332,16.440); Linear Slope = −1.646 (−2.114,−1.168); Quadratic Slope = 0.178 (0.072,0.280); Cubic Slope = −0.006 (−0.012,−0.001)].
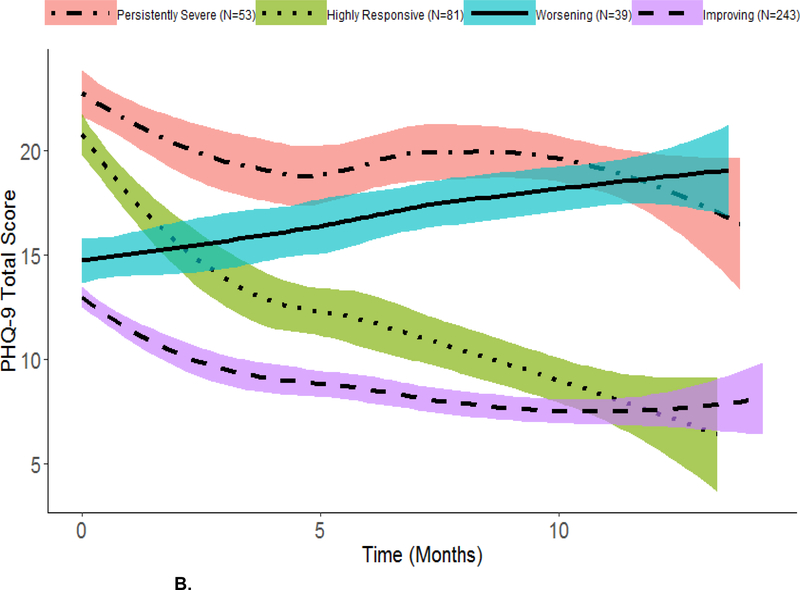
Figure 2. Overall sample (panel A) and average within-class trajectories for the four-class growth model based on most likely latent class membership clustering (panel B) using local regression smoothing.
A. Overall Trajectory (N = 416)
B. Within-Class Trajectories
Shaded region in each plot represents a 95% Confidence Interval. Individuals were classified into most likely class membership using the mixture model in Figure 1 under the assumption of linearity. Description of the within-class trajectories subgroups was done under no assumption of linearity.
Displayed in Figure 2B are the average self-reported depression trajectories for each subgroup in the four class solution.
We labeled the four subgroups as improving (58.4% [of participants]), worsening (9.4%), highly responsive (19.5%) and persistent severe (12.7%) according to self-reported depression score trajectories (see Figure 2B).
We calculated, within each subgroup, the mean (standard deviation) baseline PHQ-9 total score: persistently severe = 23 (2.55), highly responsive = 21 (2.43), worsening = 15 (2.36), improving = 13 (2.25). Similarly, we calculated the within-group mean (standard deviation) of the final recorded PHQ-9 total scores for each subject within the timeframe under study: persistently severe = 20 (4.30), highly responsive = 10 (5.56), worsening = 19 (3.43), improving = 8 (4.72).
Members of the improving group had moderate symptoms at baseline that decreased over time. Members of the worsening group also had moderate symptoms at baseline, but these symptoms increased over time. Members of the highly responsive group had severe symptoms at baseline prior to an over 50 percent reduction in the mean PHQ-9 score over the timeframe under study. Members of persistently severe group had severe depression at baseline that appeared to decrease over time with some fluctuations. However, overall, their depressive symptoms remained high.
Factors Associated with Subgroup Membership
Subgroup membership was significantly associated with neighborhood rate of high school graduation, HIV and psychiatry clinic visits, PTSD, substance abuse and reported trauma (see Table III).
Table III.
Odds Ratios and 95% Confidence Intervals for Multinomial and Logistic Regression Analysis that Do Not Contain One
| Subgroup | Reference | Covariate | Univariate Analysis | ||
|---|---|---|---|---|---|
| Odds Ratio | Lower | Upper | |||
| Persistently Severe | Improving | Education | 0.44 | 0.21 | 0.95 |
| Persistently Severe | Improving | Reported Trauma | 2.41 | 1.14 | 5.07 |
| Persistently Severe | Improving | HIV Clinic Visits | 0.27 | 0.12 | 0.62 |
| Worsening | Improving | Reported Trauma | 3.20 | 1.13 | 9.04 |
| Highly Responsive | Improving | Psychiatry Clinic Visits | 2.57 | 1.11 | 5.98 |
| Persistently Severe | Improving | PTSD | 3.38 | 1.43 | 7.98 |
| Worsening | Improving | PTSD | 3.78 | 1.17 | 12.21 |
| Highly Responsive | Improving | Substance Abuse | 3.69 | 1.21 | 11.21 |
| Persistently Severe | Highly Responsive | Education | 0.32 | 0.10 | 1.00 |
| Worsening | Highly Responsive | Substance Abuse | 0.19 | 0.05 | 0.76 |
| Outcome | |||||
| Persistently Severe | Improving | Not Detected HIV Viral Load at 12-months | 0.15 | 0.37 | 0.93 |
Education = Neighborhood High School Education Attainment Based on Geocoding (≥80 % or <80%); HIV clinic visits = attended ≥ 1 HIV clinic visit in 1-year period prior to baseline; psychiatry clinic visits = attended ≥ 1 psychiatry clinic visit in 1-year period prior to baseline; reported trauma = self-reported history of trauma under a patient chart review as described by sexual, physical or emotional abuse
We found that members of the persistently severe group were more likely to live in a neighborhood with low high school graduation rates than members of the improving and highly responsive subgroups. Members of the persistently severe and worsening symptom groups were more likely to have reported trauma and PTSD compared to members of the improving group. Members of the persistently severe group were less likely to have HIV clinic visits compared to members of the improving group. Members of the highly responsive group were more likely to have psychiatry clinic visits and a history of substance abuse compared to members of the improving group. Members of the highly responsive group were also more likely to have a history of substance abuse compared to members of the worsening group.
In multiple regression analysis, we found that members of the persistently severe group were less likely to have HIV clinic visits (OR = 0.26, 95% CI = 0.09, 0.79) compared to the improving group. Members of the highly responsive group were more likely to have visits in the psychiatry clinic (OR=2.55, 95% CI = 1.04, 6.21) and history of substance abuse (OR = 3.02, 95% CI = 1.06, 8.58) compared to members of the improving group. All other pairwise subgroup comparisons had 95% confidence intervals that contained one.
We also performed analysis to determine if subgroup membership was associated with HIV viral load at 12-months. We found that members of the persistently severe group were less likely to be virally suppressed compared to members of the improving group (see Table III). All other pairwise subgroup comparisons in analyzing viral suppression had 95% confidence intervals that contained one.
Discussion
Depression has been found to be a heterogeneous condition in PLWH (10, 11). Interventional studies analyzing data in aggregate in PLWH fail to observe intrinsic variation in subgroup response for depressive symptoms. Thus, analysis of subgroup depression trajectories in PLWH is essential for understanding (i) the progression of depression and (ii) for whom the intervention may be effective or ineffective.
We evaluated self-reported depression trajectories of participants in a collaborative care program using LCGA (35). Four subgroupings were a better fit for the data than one homogenous group based on LCGA model fit and interpretation.
Interestingly, previous LCGA non-interventional studies of PLWH also found four subgroups (10–12). These studies included PLWH both screening positive and negative for depressive symptoms.
Bengtson et al. (2018) studied 1493 HIV-infected men and 292 HIV-infected women of which 26% of men and 29% of women had depressive symptoms at baseline (10). In our urban clinic, overall 30.6% of men and 33.9% of women had depressive symptoms upon first visit. They found, over an average of 30 months of follow-up for both men and women, four similar trajectories (low, mild to moderate, improving and severe). A small additional trajectory (5% of cases) was found in men characterized by “rebounding” from moderate symptoms to improvement back to moderate symptoms.
Owora (2018) studied a baseline sample of 2260 patient records abstracted from EMR database of PLWH (11). In his study 79% of subjects were male; depressive symptoms were found in 23% of men and 29% of women at baseline. He performed LCGA on 1494 PLWH with at least one clinic visit each year during their first four years of follow-up. He also found four trajectories (low-chronic, moderate-ascending, high-episodic and high-chronic).
Kelso-Chichetto et al. (2018) (12) studied patterns of depressive symptoms across 10-years by HIV status using the Center for Epidemiologic Studies Depression (CES-D) Scale (36) from the Multicenter AIDS Cohort Study (MACS; N = 980) and Women’s Interagency HIV Study (WIHS; N = 1744). The MACS sample is a cohort of men who have sex with men and included 594 HIV positive subjects while the WIHS sample is a cohort of women, living with or at risk for HIV-infection and included 1192 HIV positive subjects. Four trajectories were found in HIV positive males (low, moderate, high and severe). A small additional trajectory (7% of cases) was found in HIV positive females characterized by severe depressive symptoms “decreasing” over time.
These previous studies all found a persistently mild group (10–12). Since, we only included subjects with self-reported depressive symptoms, this group is absent from our study. We thus focus our attention on the trajectories in PLWH from these studies that included moderate to severely depressed individuals. We also found persistently severe and worsening groups. Owora did not find steady improvement in any group with depressive symptoms(11). Bengtson et al. found a group similar to our improving group (10). Kelso-Chichetto et al. found a moderate group showing some improvement in females, but was persistently moderate in males (12). Our study differed from Bengtson et al.(10) in that the majority of our subjects were members of the improving symptom group. Our study also identified a highly responsive group, characterized by severely depressed individuals who steadily improved with, on average, over a 50% reduction in self-reported depression over 15 months. A similar group (described as “decreasing”) was found in females, but not males, in Kelso-Chichetto et al. (12). However, reduction in depression in the “decreasing” group in Kelso-Chichetto et al. (12) happened at a slower rate per year and included only 7% of HIV positive female subjects.
Symptoms of severe depression (i.e. anhedonia, fatigue, concentration problems and low self-efficacy) can lead to poor engagement with the HIV team. The collaborative care program was designed to help patients with depressive symptoms and increase engagement with the HIV team. Highly responsive and improving group members showed a positive trajectory over the course of the study. Further, the majority of subjects were in these groups with positive trajectories.
We found predictors that influenced subgroup membership. Higher neighborhood education attainment and prior HIV clinic visits made an individual more likely for membership in the improving group compared to the persistently severe group. In lieu of these findings, future studies may evaluate if the supportive care of HIV primary care services (i.e. therapeutic relationships with provider, nursing, and social work support during HIV clinical visits) have a beneficial effect for depressive symptoms.
No reported trauma or PTSD made an individual more likely for membership in the improving group compared to both the persistently severe and worsening groups. This finding is consistent with prior studies that have indicated that trauma, especially childhood trauma, is associated with treatment resistant depression (37, 38). Increased patient monitoring and intervention for such traumatic experiences could also have an impact on subsequent depressive symptoms. For example, in treating women with reported trauma in this study population, clinicians might follow practical guidelines as described by the national strategy group to develop a model of trauma-informed primary care for women living with HIV (39).
The highly responsive group was more likely to have history of substance abuse than the improving and the worsening groups. This finding may suggest perhaps substance induced depression may be a transient mood state that may improve with cessation/”washout” of the substance. Further, we found that the highly responsive group were more likely to have prior psychiatry clinic visits compared to the improving group. CCM facilitates and coordinates care with psychiatric clinic providers rather than replacing care with a psychiatric provider.
Subjects living in a neighborhood with higher education attainment were more likely to be members of the highly responsive group compared to the persistently severe group. Neighborhood-level education could be a very important factor that determines trajectories for depressive symptoms among those with severe self-reported depressive symptoms.
Research is necessary to understand adherence to the intervention program as well as associations between the intervention program and depressive symptoms before being able to draw more formal conclusions regarding these relationships described above. Our results regarding the influence of neighborhood-level education, PTSD, history of substance abuse, reported trauma and prior HIV and psychiatry clinic visits on depression trajectories are novel and promising.
We did not find that HIV viral load at baseline influenced subgroup membership. However, we found an association between HIV viral load at 12 months and subgroup membership; the persistently severe group was less likely to be virally suppressed at 12-months compared to the improving group. Thus, having a depression trajectory that remains severely high over 15-months may negatively correlate with effective management of HIV infection.
The study may have limited external validity outside the study population. In future work, our models will require further validation using other populations and perhaps data from alternate measures and scales.
Potential sources of measurement error for measures (e.g. prior diagnosis of PTSD, bipolar disorder, reported trauma) abstracted from patient’s past medical history and problem list and/or chart review include (1) individuals in care for a shorter period of time have less opportunity for conditions to be detected and (2) the presence of these measures in the chart will depend heavily on care practices. However, we aimed to limit these sources of measurement error in the data collection and modeling strategy. LCGA uses latent variables to account for measurement error in the growth in depression and assignment of latent class membership. All members of our sample had to have at least two recorded visits and at least six months of follow-up time. Thus, all subjects had at least some reasonable opportunity for these conditions to be detected. There was also an effort for systematic screening and assessment for these conditions in the collaborative care program.
This study assumes that all LCGA model assumptions are met in this study population for valid inference (i.e. within-class multivariate normality) (40). We assumed linearity in the within-class trajectories over time in performing LCGA in identifying subgroup membership, which may not be appropriate (10, 11). Our series of models did not all converge to a global solution when including a nonlinear term (i.e. quadratic term). However, while we classified members to each of the subgroups using linear assumptions, we did not assume linearity when describing the within-class trajectories. A future study is necessary in a larger sample of PLWH in a collaborative care program to verify the four class solution while modeling potential nonlinear trends in the data structure.
Further assessment for future time points (follow-up data to be collected in the future) would be valid and necessary to understand these trajectories over a longer time frame. Future steps include identifying patients with clinical characteristics that could potentially lead to depression trajectories characteristic of the persistently severe or worsening groups and target them for additional intervention. On the surface, clinicians might act readily to the initial high PHQ-9 of the highly responsive group. At the start of the study period these patients are flagged in EHR compared to the worsening group and may be more observably depressed. However, the results of this study indicate that the progression of depressive symptoms in these subjects may have helped alleviate mental health symptoms. Future studies are necessary to evaluate whether the trends found in this analysis (i.e. persistently severe group remains steadily high over time) is due to treatment resistant depression, poor quality of care, patient engagement, a combination of factors, or some other reasoning.
Conclusions
In our study, we described four depression trajectories for PLWH who are participating in an intervention at a public health system. A high proportion (77.9%) of individuals were in trajectories marked by improvement. Of special note was the highly responsive group; members of this group, on average, had over 50 percent reduction in self-reported depression over the study timeframe.
We found particular clinical factors, self-reported trauma, PTSD, lower neighborhood-level education and fewer HIV and psychiatry clinic visits, that were associated with worsening or persistently severe depressive symptom trajectories. Individuals with persistently severe depressive symptoms were also less likely to be virally suppressed. Consideration of all of these factors can personalize care for specific challenges and strengths among PLWH with depressive symptoms and may further inform intervention approaches that can advance care.
Acknowledgments
Study data were collected and managed using REDCap electronic data capture tools hosted at MetroHealth Medical Center.1,2 REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
Funding
This study was possible because of research funding by the Health Resources and Services Administration (H97HA27429–01-00) and NCATS/NIH (UL1 TR002548).
Disclosures
Dr. Mallika Lavakumar received royalties for educational material prepared on psychotic disorders for primary care physicians from Oakstone Medical Publishing and reports a book royalty agreement with Nova Science.
Dr. Doug Gunzler reports a book royalty agreement with Taylor Francis Publishing
Dr Allison Webel has received research funding from Gilead Sciences
Dr. Avery received honoraria from Gilead for participation on the PrEP speakers’ bureau and PrEP steering committee.
Footnotes
PA Harris, R Taylor, R Thielke, J Payne, N Gonzalez, JG. Conde, Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support, J Biomed Inform. 2009 Apr;42(2):377–81.
PA Harris, R Taylor, BL Minor, V Elliott, M Fernandez, L O’Neal, L McLeod, G Delacqua, F Delacqua, J Kirby, SN Duda, REDCap Consortium, The REDCap consortium: Building an international community of software partners, J Biomed Inform. 2019 May 9 [doi: 10.1016/j.jbi.2019.103208]
References
- 1.Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, et al. Psychiatric disorders and drug use among human immunodeficiency virus–infected adults in the United States. Archives of general psychiatry. 2001;58(8):721–8. [DOI] [PubMed] [Google Scholar]
- 2.Choi SK, Boyle E, Cairney J, Collins EJ, Gardner S, Bacon J, et al. Prevalence, recurrence, and incidence of current depressive symptoms among people living with HIV in Ontario, Canada: results from the Ontario HIV Treatment Network Cohort Study. PloS one. 2016;11(11):e0165816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cholera R, Pence BW, Bengtson AM, Crane HM, Christopoulos K, Cole SR, et al. Mind the Gap: Gaps in antidepressant treatment, treatment adjustments, and outcomes among patients in routine HIV care in a multisite US Clinical Cohort. PLoS One. 2017;12(1):e0166435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uthman OA, Magidson JF, Safren SA, Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle-and high-income countries: a systematic review and meta-analysis. Current HIV/AIDS Reports. 2014;11(3):291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. Journal of acquired immune deficiency syndromes (1999). 2011;58(2):181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pence BW, Mills JC, Bengtson AM, Gaynes BN, Breger TL, Cook RL, et al. Association of increased chronicity of depression with HIV appointment attendance, treatment failure, and mortality among HIV-infected adults in the United States. JAMA psychiatry. 2018;75(4):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ironson G, Fitch C, Stuetzle R. Depression and Survival in a 17-Year Longitudinal Study of People With HIV: Moderating Effects of Race and Education. Psychosomatic medicine. 2017;79(7):749–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millar BM, Starks TJ, Gurung S, Parsons JT. The impact of comorbidities, depression, and substance use problems on quality of life among older adults living with HIV. AIDS and Behavior. 2017;21(6):1684–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi T, Shacham E, Onen NF, Grubb JR, Overton ET. Depression severity is associated with increased risk behaviors and decreased CD4 cell counts. AIDS care. 2014;26(8):1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bengtson AM, Pence BW, Powers KA, Weaver MA, Mimiaga MJ, Gaynes BN, et al. Trajectories of Depressive Symptoms Among a Population of HIV-Infected Men and Women in Routine HIV Care in the United States. AIDS and Behavior. 2018:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owora AH. Major depression disorder trajectories and HIV disease progression: results from a 6-year outpatient clinic cohort. Medicine. 2018;97(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelso-Chichetto NE, Okafor CN, Cook RL, Abraham AG, Bolan R, Plankey M. Association Between Depressive Symptom Patterns and Clinical Profiles Among Persons Living with HIV. AIDS Behav. 2018;22(5):1411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuah FLH, Haldane VE, Cervero-Liceras F, Ong SE, Sigfrid LA, Murphy G, et al. Interventions and approaches to integrating HIV and mental health services: a systematic review. Health policy and planning. 2017;32(suppl_4):iv27–iv47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pence BW, Gaynes BN, Williams Q, Modi R, Adams J, Quinlivan EB, et al. Assessing the effect of Measurement-Based Care depression treatment on HIV medication adherence and health outcomes: rationale and design of the SLAM DUNC Study. Contemporary clinical trials. 2012;33(4):828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curran GM, Pyne J, Fortney JC, Gifford A, Asch SM, Rimland D, et al. Development and implementation of collaborative care for depression in HIV clinics. AIDS care. 2011;23(12):1626–36. [DOI] [PubMed] [Google Scholar]
- 16.Latkin CA, German D, Vlahov D, Galea S. Neighborhoods and HIV: a social ecological approach to prevention and care. Am Psychol. 2013;68(4):210–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pellowski JA, Kalichman SC, Matthews KA, Adler N. A pandemic of the poor: social disadvantage and the U.S. HIV epidemic. Am Psychol. 2013;68(4):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shacham E, Lian M, Önen N, Donovan M, Overton E. Are neighborhood conditions associated with HIV management? HIV Medicine. 2013;14(10):624–32. [DOI] [PubMed] [Google Scholar]
- 19.McDavid Harrison K, Ling Q, Song R, Hall HI. County-level socioeconomic status and survival after HIV diagnosis, United States. Annals of epidemiology. 2008;18(12):919–27. [DOI] [PubMed] [Google Scholar]
- 20.McMahon J, Wanke C, Terrin N, Skinner S, Knox T. Poverty, hunger, education, and residential status impact survival in HIV. AIDS Behav.2011;15(7):1503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silver E, Mulvey EP, Swanson JW. Neighborhood structural characteristics and mental disorder: Faris and Dunham revisited. Social science & medicine. 2002;55(8):1457–70. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The Phq‐9. Journal of general internal medicine. 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrando SJ, Samton J, Mor N, Nicora S, Findler M, Apatoff B. Patient health questionnaire-9 to screen for depression in outpatients with multiple sclerosis. International Journal of MS Care. 2007;9(3):99–103. [Google Scholar]
- 24.Pinto‐Meza A, Serrano‐Blanco A, Peñarrubia MT, Blanco E, Haro JM. Assessing Depression in Primary Care with the PHQ‐9: Can It Be Carried Out over the Telephone? Journal of General Internal Medicine. 2005;20(8):738–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Census Bureau. ACS Summary File Technical Documentation. 2016.
- 26.Gunzler DD, Morris N, Perzynski A, Ontaneda D, Briggs F, Miller D, et al. Heterogeneous depression trajectories in multiple sclerosis patients. Multiple Sclerosis and Related Disorders. 2016;9:163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2008;2(1):302–17. [Google Scholar]
- 28.Muthén B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55(2):463–9. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz G Estimating the dimension of a model. The annals of statistics. 1978;6(2):461–4. [Google Scholar]
- 30.Muthén LK, Muthén BO. Mplus. The comprehensive modelling program for applied researchers: user’s guide. 2012;5. [Google Scholar]
- 31.Huber PJ, editor The behavior of maximum likelihood estimates under nonstandard conditions. Proceedings of the fifth Berkeley symposium on mathematical statistics and probability; 1967. [Google Scholar]
- 32.Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Using the BCH method in Mplus to estimate a distal outcome model and an arbitrary secondary model. Mplus Web Notes. 2014;21(2):1–22. [Google Scholar]
- 33.Asparouhov T, Muthén B. Auxiliary variables in mixture modeling: Three-step approaches using M plus. Structural Equation Modeling: A Multidisciplinary Journal. 2014;21(3):329–41. [Google Scholar]
- 34.Venables WN, Smith DM, Team RDC. An introduction to R. Network Theory Ltd; 2002. [Google Scholar]
- 35.Alemayehu D, Cappelleri JC. Conceptual and analytical considerations toward the use of patient-reported outcomes in personalized medicine. American health & drug benefits. 2012;5(5):310. [PMC free article] [PubMed] [Google Scholar]
- 36.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 37.Tunnard C, Rane LJ, Wooderson SC, Markopoulou K, Poon L, Fekadu A, et al. The impact of childhood adversity on suicidality and clinical course in treatment-resistant depression. Journal of affective disorders. 2014;152:122–30. [DOI] [PubMed] [Google Scholar]
- 38.Williams LM, Debattista C, Duchemin A, Schatzberg A, Nemeroff C. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Translational psychiatry. 2016;6(5):e799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Machtinger EL, Cuca YP, Khanna N, Rose CD, Kimberg LS. From treatment to healing: the promise of trauma-informed primary care. Women’s Health Issues. 2015;25(3):193–7. [DOI] [PubMed] [Google Scholar]
- 40.Bauer DJ. Observations on the use of growth mixture models in psychological research. Multivariate Behavioral Research. 2007;42(4):757–86. [Google Scholar]