Abstract
Pain and tobacco cigarette smoking frequently co-occur, and smokers report using cigarettes to self-medicate pain. Despite the growing popularity of e-cigarettes and alternative nicotine products, no research has examined their use as a function of pain status. The goal of this study was to test cross-sectional relations between the presence of pain and current use of e-cigarettes, lifetime poly-nicotine use, and lifetime use of individual nicotine products. The sample was comprised of current daily smokers (N = 301) who were recruited to participate in a web-based longitudinal study examining predictors of cessation milestones. Results indicated that smokers who endorsed past two-week significant pain (vs. no past two-week pain) were 3 times more likely to endorse current e-cigarette use, reported having used a greater number of nicotine products in their lifetime, and were nearly 3 times more likely to endorse lifetime poly-nicotine use. In terms of individual products, smokers with pain were approximately 4 times as likely to have tried e-cigarettes, and 7 times more likely to have tried cigars. This is the first study to demonstrate that smokers who endorse significant pain are also more likely to endorse use of e-cigarettes and other combustible nicotine products. Future research is needed to examine poly-nicotine use in relation to pain reporting among more varied samples of smokers and nonsmokers.
Keywords: e-cigarettes, nicotine, tobacco, pain
Pain and tobacco cigarette smoking are highly prevalent and frequently comorbid conditions that engender a combined economic burden of more than $800 billion in healthcare costs and lost productivity in the United States each year (Gaskin & Richard, 2012; USDHHS, 2014; Xu, Bishop, Kennedy, Simpson, & Pechacek, 2015). Prevalence data indicate that rates of smoking among individuals with co-occurring pain are approximately two-to-three times greater than those observed in the general population (Michna et al., 2004; Orhurhu, Pittelkow, & Hooten, 2015; Zvolensky, McMillan, Gonzalez, & Asmundson, 2009). There is also evidence that smokers experience greater frequency and intensity of pain, and greater pain-related functional impairment (Palmer, Syddall, Cooper, & Coggon, 2003; Weingarten et al., 2008).
A reciprocal model of pain and smoking posits that these conditions interact in a bidirectional manner, resulting in greater pain and the maintenance of cigarette dependence (Ditre, Brandon, Zale, & Meagher, 2011; Ditre, Zale, & LaRowe, 2019; Zale, Maisto, & Ditre, 2016). Consistent with this framework, regular smoking has been identified as a risk factor in the onset and progression of painful conditions (Shiri, Karppinen, Leino-Arjas, Solovieva, & Viikari-Juntura, 2010; Sugiyama et al., 2010; Weingarten et al., 2009), and there is evidence that pain is a potent motivator of smoking urge and behavior (Dhingra et al., 2014; Ditre & Brandon, 2008; Ditre, Heckman, Butts, & Brandon, 2010; Kosiba, Zale, & Ditre, 2018). Nicotine has also been shown to confer acute analgesia (Ditre, Heckman, Zale, Kosiba, & Maisto, 2016), and smokers report using cigarettes to cope with pain (e.g., Jamison, Stetson, & Parris, 1991; Patterson et al., 2012).
Despite growing empirical focus on interrelations between pain and cigarette use, surprisingly limited work has examined covariation between pain and the use of other nicotine/tobacco products. The use of electronic cigarettes (e-cigarettes), cigars, smokeless tobacco, and hookah have risen sharply over the past decade (Corey et al., 2014; Delnevo et al., 2014; King, Patel, Nguyen, & Dube, 2014; Lee, Hebert, Nonnemaker, & Kim, 2014), and almost half of nicotine users report concurrent use of multiple nicotine products (Kasza et al., 2017). Poly-nicotine use (vs. single product use) is also associated with greater nicotine exposure (Bombard, Pederson, Nelson, & Malarcher, 2007; Bombard, Rock, Pederson, & Asman, 2008), and more severe nicotine dependence (Soule, Pomeranz, Moorhouse, & Barnett, 2015).
The increased prevalence of poly-nicotine use have spurred interest in identifying individual difference factors that may promote such use (Cummins, Zhu, Tedeschi, Gamst, & Myers, 2014; Hartwell, Thomas, Egan, Gilmore, & Petticrew, 2017; Jones, Popova, Weaver, Pechacek, & Eriksen, 2018). One recently published study showed that e-cigarette users with co-occurring pain (vs. no pain) were more dependent on e-cigarettes and perceived a greater number of barriers to quitting e-cigarettes (Zvolensky et al., 2018). Acute nicotine deprivation among cigarette smokers has been shown to increase pain intensity and sensitivity to experimental pain (Ditre, Zale, LaRowe, Kosiba, & De Vita, 2018), and e-cigarette users with co-occurring pain may endorse greater perceived difficulty quitting due to the exacerbation of pain during a quit attempt. Additionally, the acute pain-reducing effects of nicotine (Ditre et al., 2016) may negatively reinforce e-cigarette use and strengthen beliefs regarding the utility of e-cigarettes and other nicotine products for pain reduction. Greater lifetime nicotine exposure has further been shown to increase risk for the worsening of pain over time (De Vita, Maisto, Ansell, Zale, & Ditre, 2019). Although these findings provide evidence of a positive relation between pain and e-cigarette use/dependence, the degree to which such associations extend to concurrent traditional cigarette smokers or individuals who use other types of nicotine products remains unclear.
The goal of the current study was to examine the use of e-cigarettes and other nicotine products as a function of pain status, among current smokers. We hypothesized that smokers who endorsed past two-week significant pain (vs. no past two-week pain) would be more likely to report current use of e-cigarettes, lifetime poly-nicotine use, and lifetime use of individual nicotine products (i.e., e-cigarettes, cigars, cigarillos, pipes, hookah, chewing tobacco, dip, snus, and snuff).
Method
All study procedures were approved by the Institutional Review Board at the Medical University of South Carolina. The current sample was comprised of cigarette smokers (N = 301) recruited via ads posted on Craigslist and ResearchMatch for a longitudinal study examining cessation fatigue as a predictor of cessation milestones (Heckman et al., 2018). Participants were required to smoke at least 10 cigarettes per day on at least 25 of the last 30 days, and endorse strong intention to quit smoking in the next month (score of at least 7 out of 10) using a single item Contemplation Ladder (Biener & Abrams, 1991). These data were collected at baseline, prior to initiation of a quit attempt, using REDCap (Harris et al., 2009).
Measures
Cigarette dependence.
Cigarette dependence was assessed using the Heaviness of Smoking Index (HSI; Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989), which is comprised of two items (i.e., “How soon after you wake up do you smoke your first cigarette?” and “How many cigarettes per day do you smoke?). Previous work supports the total score as a valid and reliable measure of cigarette dependence (Borland, Yong, O’connor, Hyland, & Thompson, 2010).
E-cigarette and other nicotine product use.
Participants were asked “Over the past week (including today), on how many days have you used an e-cigarette (even a puff)?” Those who endorsed using an e-cigarette over the past 7 days were coded as a current e-cigarette users. The use of a single item for past 7-day use has shown high concordance with more detailed assessments of use (e.g., timeline follow-back; Bernstein, Rosner, & Toll, 2016). Participants were also queried regarding lifetime use (“Have you EVER tried any of the following products?”) of e-cigarettes, cigars, cigarillos, pipes, hookah, chewing tobacco, dip, snus, and snuff. Lifetime poly-nicotine use was determined based on whether current cigarette smokers endorsed having tried at least one additional nicotine product. Total number of nicotine products used and lifetime use of individual products were also tabulated.
Pain status.
Past two-week pain status was determined using a single yes/no item (i.e., “Have you had any significant pain in the past two weeks?”). The presence of past two-week pain has predicted greater scores on more comprehensive measures of pain severity (Von Korff, Ormel, Keefe, & Dworkin, 1992), and past one- and two-week pain status has been associated with smoking rates and nicotine dependence (Aigner et al., 2015; Ditre et al., 2011; Hahn, Rayens, Kirsh, & Passik, 2006).
Data Analytic Plan
All analyses were conducted using SPSS Version 24 (IBM Corp., 2016). First, t-tests and χ2 tests were used to examine differences in participant characteristics as a function of pain. Variables that differed by pain status were included as covariates in adjusted models (Pocock, Assmann, Enos, & Kasten, 2002). Adjusted models also covaried for participant sex and age, as prior research has shown these factors to be strong predictors of e-cigarette and poly-nicotine use (Jones et al., 2018; Stanton & Halenar, 2018). Second, unadjusted and adjusted logistic regression models were used to examine likelihood of current e-cigarette use and lifetime poly-nicotine use as a function of pain. Third, analysis of covariance (ANCOVA) assessed differences in total number of lifetime nicotine products as a function of pain. Finally, logistic regressions models tested differences in lifetime use of individual nicotine products (i.e., e-cigarettes, chewing tobacco, snuff, dip, snus, cigars, cigarillos, pipes, and hookah) as a function of pain. We examined unadjusted and adjusted results to determine if associations changed after the inclusion of relevant covariates.
Results
Participant Characteristics
Participant characteristics as a function of pain status are presented in Table 1. Participants were approximately 34 years old (M = 34.39, SD = 8.67), smoked nearly a pack of cigarettes per day (M = 19.44, SD = 6.74), and reported moderate levels (M = 3.18, SD = 1.09) of cigarette dependence (Heatherton et al., 1989). Over half of the total sample endorsed lifetime poly-nicotine use (65.1%), with an average of 1.58 products overall (SD = 1.67). Approximately 35% of participants reported lifetime use of e-cigarettes and 24% endorsed past week e-cigarette use.
Table 1.
Participant Characteristics as a Function of Past Two-Week Pain
| No Pain (n = 231) | Pain (n = 70) | Total Sample (n = 301) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Sociodemographic Variables | |||
| Sex | |||
| Male | 158 (68.4%) | 49 (70.0%) | 207 (68.7%) |
| Female | 73 (31.6%) | 21 (30.0%) | 94 (31.3%) |
| Race* | |||
| White | 159 (68.8%) | 57 (81.4%) | 216 (71.8%) |
| Non-white | 72 (31.2%) | 13 (18.6%) | 85 (28.2%) |
| Employment* | |||
| Employed | 206 (89.2%) | 54 (77.1%) | 260 (86.4%) |
| Unemployed | 25 (10.8%) | 16 (22.9%) | 41 (13.6%) |
| Education | |||
| High school or less | 58 (25.1%) | 10 (14.3%) | 68 (22.6%) |
| Some college or more | 173 (74.9%) | 60 (85.7%) | 233 (77.4%) |
| Relationship Status | |||
| Single | 78 (33.8%) | 19 (27.1%) | 97 (32.2%) |
| In a relationship | 153 (66.2%) | 51 (72.9%) | 204 (67.8%) |
| M (SD) | M (SD) | M (SD) | |
| Age | 34.65 (8.56) | 36.64 (11.93) | 34.39 (8.67) |
| # Drinks Past 2 Weeks | 20.36 (19.24) | 24.55 (15.17) | 22.70 (17.06) |
| Depression* | 7.94 (4.49) | 13.38 (5.33) | 9.15 (1.86) |
| Cigarettes per Day* | 17.30 (4.87) | 22.90 (6.66) | 19.44 (6.74) |
| HSI* | 2.65 (1.06) | 3.70 (0.82) | 3.18 (1.09) |
Notes:
p < .05. HSI (Heaviness of Smoking Index); Depression assessed via PHQ-9 (Patient Health Questionnaire - 9).
One quarter of participants (n = 70) endorsed past two-week significant pain. Consistent with the extant literature, participants with pain smoked a greater number of cigarettes per day (M = 22.90, SD = 6.66 vs. M = 17.30, SD = 4.87, p < .001) and endorsed greater cigarette dependence (M = 3.70, SD = .82 vs. M = 2.65, SD = 1.06, p < .001). Smokers with pain were more likely to be white, unemployed, and endorse higher levels of depression (ps < .05), thus race, employment status, and depression were included as covariates in adjusted models.
Current E-Cigarette Use
Unadjusted logistic regression analyses revealed that participants with co-occuring pain were almost 4 times more likely to report current use of e-cigarettes (OR = 4.78, 95% CI: 2.67 − 8.52, p < .001), which remained the same after adjusting for covariates (OR = 4.39, 95% CI: 2.18–8.83, p < .001). The percentage of participants who endosed current e-cigarette use as a function of pain status is presented in Figure 1.
Figure 1. Percentage of participants as a function of past two-week pain (yes/no) who reported current e-cigarette use and lifetime poly-nicotine use.
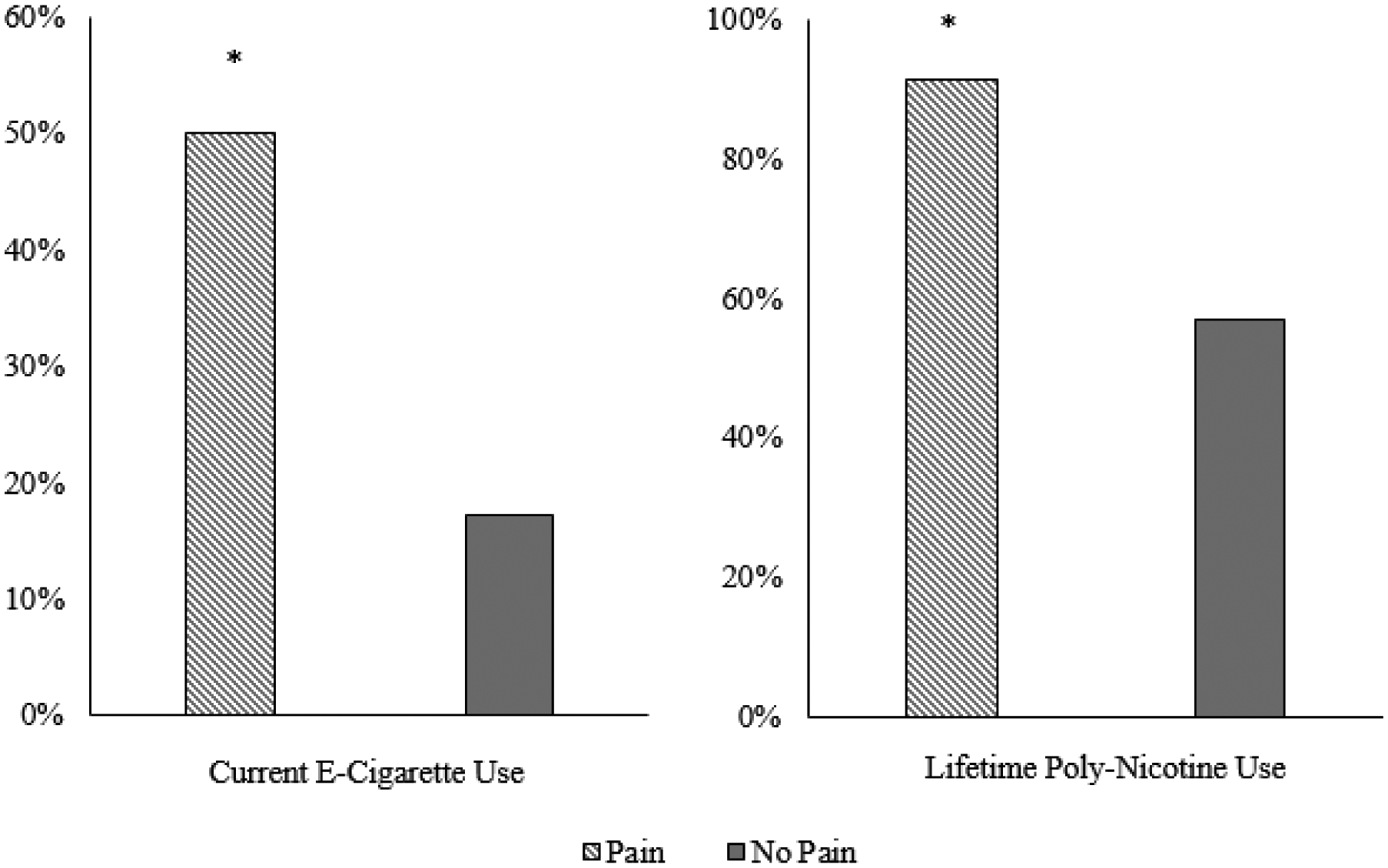
Note: * p < .05
Lifetime Poly-Nicotine Use
Approximately 90% of smokers with pain were lifetime poly-nicotine users compared to 57% without pain (see Figure 1). Unadjusted models revealed that participants with significant pain were 7 times more likely to report lifetime poly-nicotine use (OR = 8.00, 95% CI: 3.33 − 19.22 ; p < .001), which decreased after adjusting for covariates (OR = 3.83, 95% CI: 1.47 − 9.95, p < .01). With regard to number of lifetime nicotine products, smokers with pain used a greater number of products (M = 2.01, SE = .22) compared to smokers without pain (M = 1.44, SE = .21; F [1, 300] = 5.90, p =.016).
Lifetime Use of Individual Nicotine Products
Unadjusted models revealed that smokers with pain were over 3 times more likely to have tried e-cigarettes (OR = 4.61, 95% CI: 2.61 − 8.12, p < .001), almost 9 more likely to have tried cigars (OR = 9.44, 95% CI: 5.17 − 17.22, p < .001), and over twice as likely to have tried pipes (OR = 3.26, 95% CI: 1.27–8.38, p = .014), hookah (OR = 2.79, 95% CI: 1.17–6.68, p = .021), and cigarillos (OR = 2.12, 95% CI: 1.16 − 3.90, p = .015). There was no significant association between pain and use of chewing tobacco, snuff, snus, and dip (all ps > .05). In the adjusted models, smokers with significant pain were almost 3 times more likely to have tried e-cigarettes (OR = 3.78, 95% CI: 1.95– 7.35, p < .001), almost 7 times more likely to have tried cigars (OR = 7.82, 95% CI: 3.80 − 16.09, p < .001), over 2 times more likely to have tried pipes (OR = 3.66, 95% CI: 1.07 − 12.55, p = .039), and over 4 times as likely to have tried hookah (OR = 4.48, 95% CI: 1.35 − 14.82, p = .014). Conversely, smokers with pain were less likely to have tried snuff (OR = .15, 95% CI: .03 − .68, p = .015), and all adjusted logistic regression models examining ever use of cigarillos, chew, snus, and dip were not statistically significant (all ps > .05; Table 2). Because running multiple models increases the likelihood of a Type I error (Chen, Feng, & Yi, 2017), a Bonferroni correction was employed to reduce the false discovery rate and increase statistical power (Benjamini & Hochberg, 1995; Bland & Altman, 1995). After correcting for muliple models, both adjusted models examining lifetime e-cigarette and cigar use remained significant (ps < .005).
Table 2.
Adjusted Logistic Regression Models Examining Pain as a Predictor of Lifetime Use of Individual Nicotine Products
| E-Cigarettes | Cigars | Cigarillos | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| .99 | .96–1.02 | .506 | .97 | .93–1.00 | .055 | .98 | .95–1.01 | .253 | |
| Racea | .94 | .52–1.72 | .851 | .97 | .49–1.94 | .936 | 1.43 | .70–2.91 | .325 |
| Sexb | 2.07 | 1.13–3.82 | .019 | .75 | .36–1.55 | .433 | 2.86 | 1.44–5.70 | .003* |
| Employment Statusc | .38 | .17–.83 | .136 | .79 | .31–2.01 | .623 | .79 | .33–1.88 | .595 |
| Depressiond | 1.05 | .99–1.11 | .136 | 1.07 | 1.00–1.14 | .046* | 1.07 | 1.00–1.14 | .030* |
| Pain Statuse | 3.78 | 1.95–7.35 | <.001** | 7.83 | 3.80–16.10 | <.001** | 1.47 | .71–3.03 | .301 |
| Pipes | Hookah | Chewing Tobacco | |||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| .90 | .835–.98 | .013* | .88 | .82–.95 | .001* | 1.01 | .97–1.04 | .682 | |
| Racea | .86 | .24–3.00 | .807 | .52 | .18–1.54 | .238 | .33 | .18–.64 | .001* |
| Sexb | .79 | .29–2.85 | .715 | 3.81 | 1.26–11.44 | .017* | .57 | .28–1.16 | .122 |
| Employment Statusc | .20 | .06–.73 | .015* | .30 | .09–.94 | .039* | 3.24 | 1.00–10.48 | .049* |
| Depressiond | .98 | .88–1.09 | .681 | .98 | .89–1.08 | .660 | 1.09 | 1.02–1.17 | .011* |
| Pain Statuse | 3.66 | 1.07–12.55 | .039* | 4.48 | 1.36–14.82 | .014* | .56 | .25–1.24 | .154 |
| Dip | Snus | Snuff | |||||||
| OR | 95% CI | p | OR | 95% CI | p | OR | 95% CI | p | |
| 1.02 | .99–1.06 | .232 | 1.04 | .99–1.08 | .077 | 1.07 | 1.02–1.12 | .005* | |
| Racea | .65 | .32–1.32 | .234 | 1.37 | .62–3.04 | .441 | 1.23 | .49–3.08 | .661 |
| Sexb | .74 | .34–1.62 | .451 | .83 | .38–1.90 | .659 | .88 | .34–2.28 | .785 |
| Employment Statusc | 1.26 | .42–3.79 | .676 | 1.78 | .53–6.02 | .355 | 2.9 | .57–15.44 | .194 |
| Depressiond | 1.03 | .96–1.11 | .370 | 1.06 | .98–1.14 | .169 | 1.08 | .98–1.19 | .118 |
| Pain Statuse | .50 | .19–1.30 | .154 | .54 | .21–1.36 | .191 | .14 | .03–.69 | .015* |
Notes:
Race (0 – Non-White, 1 – White);
Sex (0 – Male, 1 – Female);
(Employment Status (0 – Unemployed, 1 – Employed);
Depression – Patient Health Questionnaire (PHQ – 9);
Pain Status (0 – No Pain, 1 - Pain);
p < .05;
p < .001 and significant after Bonferroni Correction.
Discussion
This is the first study to examine current e-cigarette use and lifetime poly-nicotine product use among cigarette smokers as a function of co-occurring pain. Results indicated that smokers with past two-week significant pain (vs. no past two-week pain) were over 3 times more likely to endorse concurrent use of e-cigarettes. In addition, smokers with co-occurring pain reported having used a greater number of nicotine products in their lifetime, and were almost 3 times more likely to endorse lifetime poly-nicotine use (i.e., use of any nicotine product in addition to traditional combustible cigarettes). Specifically, smokers with pain were more likely to have tried e-cigarettes, cigars, hookah, and pipes. Smokers with pain were less likely to have tried snuff. After correcting for multiple models (Benjamini & Hochberg, 1995; Bland & Altman, 1995), smokers with pain were still more likely to endorse lifetime use of e-cigarettes and cigars. No differences were observed regarding lifetime use of cigarillos, chew, snus, or dip.
Given the acute analgesic effects of nicotine (Ditre et al., 2016), one possible explanation for these findings is that smokers with co-occurring pain may also use e-cigarettes and other nicotine products in an effort to extend/supplement exposure to nicotine (e.g., between traditional cigarettes or during times when smoking is not permitted). Indeed, smokers report using cigarettes for pain-coping (Hooten, Shi, Gazelka, & Warner, 2011), and thus may be more likely to also use e-cigarettes and other nicotine products to self-medicate pain symptoms. An alternative explanation is that smoking more cigarettes per day (smokers with pain in this study consumed approximately 32% more cigarettes per day than those without pain) while concurrently using a greater number of other nicotine products could increase lifetime nicotine exposure, which in turn may facilitate pain and elevate risk for developing painful conditions (De Vita et al., 2019). Collectively, these initial findings support the utility of assessing e-cigarette and other nicotine product use among smokers with pain. Additional research is needed to elucidate potentially bidirectional effects between poly-nicotine use and the experience of pain (e.g., Ditre et al., 2011; Ditre et al., 2019).
E-cigarettes use has increased seven-fold over the past decade (McMillen, Gottlieb, Shaefer, Winickoff, & Klein, 2014), and empirical interest in the dual use of e-cigarettes and traditional cigarettes has increased in commensurate fashion (e.g., Jones et al., 2018; Stanton & Halenar, 2018). Despite previous work showing pain to be associated with greater e-cigarette dependence (Zvolensky et al., 2018), we are not aware of any work examining pain and dual use of e-cigarettes and cigarettes. There has been some question in this line of research as to whether dual users may decrease their consumption of combustible cigarettes in the context of e-cigarette uptake (Rahman, Hann, Wilson, Mnatzaganian, & Worrall-Carter, 2015). There is also growing interest in identifying individual factors that contribute to e-cigarette use (e.g., Hartwell et al., 2017). The present findings suggest that daily cigarette smokers with pain may be more likely to initiate and continue use of e-cigarettes. However, patterns of e-cigarette use among smokers with co-occurring pain remain unclear, including whether smokers titrate their cigarette consumption following initiation of e-cigarette use. Future research is needed to examine interplay between pain and dual use of e-cigarettes and traditional cigarettes.
Although smokers with co-occurring pain were more likely to use e-cigarettes and other combustible nicotine products (e.g., cigars), there was no association between significant pain and lifetime use of cigarillos, chewing tobacco, dip, and snus. One potential explanation for these findings is that smokers with pain may prefer inhaled/combustible nicotine delivery systems. In e-cigarettes and combustible nicotine products, nicotine smoke or vapor is taken in through the mouth, throat, and lungs, and can reach the brain in as little as seven seconds (Henningfield & Keenan, 1993; Russell & Feyerabend, 1978). In comparison, smokeless tobacco products vary in nicotine content (Henningfield, Radzius, & Cone, 1995), take longer to reach peak blood levels (Henningfield & Keenan, 1993), and smokers report feeling less confident in their ability to control nicotine intake when using these products (Sami et al., 2012). Additionally, the route of administration in e-cigarettes and combustible nicotine products more closely resembles that of traditional tobacco cigarettes. Indeed, current smokers report that they are more likely to consider switching to an inhaled product, like e-cigarettes, relative to a smokeless tobacco product (Anic et al., 2018).
Several important limitations should be noted. First, cross-sectional assessment of poly-nicotine use and pain status prohibits inferences regarding temporal or causal effects. Second, although previous studies observed interplay between cigarette use/dependence and the presence of recent pain (e.g., Hahn et al., 2006), this study was limited to a single item assessing past two-week significant pain. In addition, whereas past two-week pain could indicate the presence of a persistently painful condition, chronic pain status was not assessed, and it is not possible to determine from these data whether past use of nicotine products covaried with pain experience. Future research should examine e-cigarette and poly-nicotine use in relation to clinical pain characteristics (e.g., persistence, intensity, impairment) over the same period of time (e.g., Turk & Melzack, 2011). Third, although binary variables are useful for estimating odds ratios, future work would benefit from incorporating more granular assessment of nicotine product use (e.g., frequency/duration, current poly-nicotine use, time since last use), along with biological assays of nicotine exposure (e.g., Marsot & Simon, 2016; SRNT, 2002). Fourth, comorbid psychopathology and several candidate transdiagnostic factors (e.g., pain-related anxiety, anxiety sensitivity, distress intolerance) have been proposed as potential mechanisms in bidirectional pain-smoking associations (Ditre et al., 2019). Consistent with prior research (Holmes et al., 2010), we covaried for relevant factors (e.g., depression) in the present analysis; however, future prospective research should examine these variables as mediators of pain-smoking relations. Finally, the current sample was comprised of daily smokers who were motivated to quit in the next 30 days, and the extent to which these findings may generalize to lighter smokers or those not intending to quit remains unclear.
In summary, these data suggest that use of e-cigarettes and other nicotine products is highly prevalent among tobacco cigarette smokers with co-occurring pain. Despite preliminary findings showing associations between pain and e-cigarette dependence (Zvolensky et al., 2018) and literature documenting interrelations between pain and smoking (Ditre et al., 2011; Ditre et al., 2019), this is the first study that examined e-cigarette and poly-nicotine use as a function of significant pain. Future research is needed to elucidate prevalence and patterns of alternative nicotine product use among smokers and non-smokers with varying levels of pain.
Public Significance Statement:
This is the first study to demonstrate that tobacco cigarette smokers with co-occurring pain are more likely to also use e-cigarettes and other nicotine products (relative to smokers without co-occurring pain). Smokers with pain may use additional nicotine products to help manage painful symptoms. Concurrent use of multiple nicotine products among smokers with pain may impede smoking cessation and contribute to the maintenance of addiction.
Acknowledgments
This research was supported by the Chairman’s Research Development Fund Pilot Grant Program, Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina. BWH was supported by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health (grant numbers K12 DA031794 and K23 DA041616).
Footnotes
We have no conflicts of interest to declare.
There has been no prior dissemination of the ideas and data appearing in this manuscript.
These findings were presented at the 2019 annual meeting for the Society for Research on Nicotine and Tobacco. There has been no other prior dissemination of the ideas and data appearing in the manuscript.
References
- Aigner CJ, Cinciripini PM, Anderson KO, Baum GP, Gritz ER, & Lam CY (2015). The association of pain with smoking and quit attempts in an electronic diary study of cancer patients trying to quit. Nicotine & Tobacco Research, 18(6), 1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anic GM, Holder-Hayes E, Ambrose BK, Rostron BL, Coleman B, Jamal A, & Apelberg BJ (2018). E-cigarette and smokeless tobacco use and switching among smokers: Findings from the National Adult Tobacco Survey. American Journal of Preventive Medicine, 54(4), 539–551. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57(1), 289–300. [Google Scholar]
- Bernstein SL, Rosner J, & Toll B (2016). Concordance between timeline follow-back and single-question assessment of self-reported smoking in a clinical trial. Substance Abuse, 37(3), 398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, & Abrams DB (1991). The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology, 10(5), 360. [DOI] [PubMed] [Google Scholar]
- Bland JM, & Altman DG (1995). Multiple significance tests: The Bonferroni method. The BMJ, 310(6973), 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombard JM, Rock VJ, Pederson LL, & Asman KJ (2008). Monitoring polytobacco use among adolescents: Do cigarette smokers use other forms of tobacco? Nicotine & Tobacco Research, 10(11), 1581–1589. [DOI] [PubMed] [Google Scholar]
- Borland R, Yong H-H, O’connor R, Hyland A, & Thompson M (2010). The reliability and predictive validity of the Heaviness of Smoking Index and its two components: Findings from the International Tobacco Control Four Country study. Nicotine & Tobacco Research, 12(suppl_1), S45–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-Y, Feng Z, & Yi X (2017). A general introduction to adjustment for multiple comparisons. Journal of Thoracic Disease, 9(6), 1725–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey CG, King BA, Coleman BN, Delnevo CD, Husten CG, Ambrose BK, & Apelberg BJ (2014). Little filtered cigar, cigarillo, and premium cigar smoking among adults--United States, 2012–2013. Morbidity and Mortality Weekly Report, 63(30), 650–654. [PMC free article] [PubMed] [Google Scholar]
- Cummins SE, Zhu S-H, Tedeschi GJ, Gamst AC, & Myers MG (2014). Use of e-cigarettes by individuals with mental health conditions. Tobacco Control, 23(suppl 3), iii48–iii53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita MJ, Maisto SA, Ansell EB, Zale EL, & Ditre JW (2019). Pack-Years of Tobacco Cigarette Smoking as a Predictor of Spontaneous Pain Reporting and Experimental Pain Reactivity. Experimental and Clinical Psychopharmacology, 8(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnevo CD, Wackowski OA, Giovenco DP, Manderski MTB, Hrywna M, & Ling PM (2014). Examining market trends in the United States smokeless tobacco use: 2005–2011. Tobacco Control, 23(2), 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhingra LK, Homel P, Grossman B, Chen J, Scharaga E, Calamita S, … Portenoy R (2014). Ecological Momentary Assessment of Smoking Behavior in Persistent Pain Patients. Clinical Journal of Pain, 30(3), 205–213. [DOI] [PubMed] [Google Scholar]
- Ditre JW, & Brandon TH (2008). Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. Journal of Abnormal Psychology, 117(2), 467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, & Meagher MM (2011). Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychological Bulletin, 137(6), 1065–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Gonzalez BD, Simmons VN, Faul LA, Brandon TH, & Jacobsen PB (2011). Associations between pain and current smoking status among cancer patients. Pain, 152(1), 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, & Brandon TH (2010). Effects of expectancies and coping on pain-induced motivation to smoke. Journal of Abnormal Psychology, 119(3), 524–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Zale EL, Kosiba JD, & Maisto SA (2016). Acute analgesic effects of nicotine and tobacco in humans: a meta-analysis. Pain, 157(7), 1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, & LaRowe LR (2019). A reciprocal model of pain and substance use: Transdiagnostic considerations, clinical implications, and future directions. Annual Review of Clinical Psychology, 15, 503–528. [DOI] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, LaRowe LR, Kosiba JD, & De Vita MJ (2018). Nicotine deprivation increases pain intensity, neurogenic inflammation, and mechanical hyperalgesia among daily tobacco smokers. Journal of Abnormal Psychology, 127(6), 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin DJ, & Richard P (2012). The economic costs of pain in the United States. The Journal of Pain, 13(8), 715–724. [DOI] [PubMed] [Google Scholar]
- Hahn EJ, Rayens MK, Kirsh KL, & Passik SD (2006). Brief report: Pain and readiness to quit smoking cigarettes. Nicotine & Tobacco Research, 8(3), 473–480. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell G, Thomas S, Egan M, Gilmore A, & Petticrew M (2017). E-cigarettes and equity: A systematic review of differences in awareness and use between sociodemographic groups. Tobacco Control, 26(e2), e85–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, & Robinson J (1989). Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction, 84(7), 791–799. [DOI] [PubMed] [Google Scholar]
- Heckman BW, Dahne J, Germeroth LJ, Mathew AR, Santa Ana EJ, Saladin ME, & Carpenter MJ (2018). Does cessation fatigue predict smoking-cessation milestones? A longitudinal study of current and former smokers. Journal of Consulting and Clinical Psychology, 86(11), 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield JE, & Keenan RM (1993). Nicotine delivery kinetics and abuse liability. Journal of Consulting and Clinical Psychology, 61(5), 743. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Radzius A, & Cone EJ (1995). Estimation of available nicotine content of six smokeless tobacco products. Tobacco Control, 4(1), 57. [Google Scholar]
- Holmes A, Williamson O, Hogg M, Arnold C, Prosser A, Clements J, … O’Donnell M (2010). Predictors of pain severity 3 months after serious injury. Pain Medicine, 11(7), 990–1000. [DOI] [PubMed] [Google Scholar]
- Hooten WM, Shi Y, Gazelka HM, & Warner DO (2011). The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain, 152(1), 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp. (2016). IBM SPSS statistics for windows (Version 24). Armonk, NY: IBM Corp. [Google Scholar]
- Jamison RN, Stetson BA, & Parris WC (1991). The relationship between cigarette smoking and chronic low back pain. Addictive Behaviors, 16(3–4), 103–110. [DOI] [PubMed] [Google Scholar]
- Jones DM, Popova L, Weaver SR, Pechacek TF, & Eriksen MP (2018). A national comparison of dual users of smokeless tobacco and cigarettes and exclusive cigarette smokers, 2015–2016. Nicotine & Tobacco Research, 20(suppl_1), S62–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KA, Ambrose BK, Conway KP, Borek N, Taylor K, Goniewicz ML, … Hyland AJ (2017). Tobacco-product use by adults and youths in the United States in 2013 and 2014. New England Journal of Medicine, 376, 342–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BA, Patel R, Nguyen KH, & Dube SR (2014). Trends in awareness and use of electronic cigarettes among US adults, 2010–2013. Nicotine & Tobacco Research, 17(2), 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosiba JD, Zale EL, & Ditre JW (2018). Associations between pain intensity and urge to smoke: Testing the role of negative affect and pain catastrophizing. Drug and Alcohol Dependence, 187, 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YO, Hebert CJ, Nonnemaker JM, & Kim AE (2014). Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Preventive Medicine, 62, 14–19. [DOI] [PubMed] [Google Scholar]
- Marsot A, & Simon N (2016). Nicotine and cotinine levels with electronic cigarette: A review. International Journal of Toxicology, 35(2), 179–185. [DOI] [PubMed] [Google Scholar]
- McMillen RC, Gottlieb MA, Shaefer RMW, Winickoff JP, & Klein JD (2014). Trends in electronic cigarette use among US adults: Use is increasing in both smokers and nonsmokers. Nicotine & Tobacco Research, 17(10), 1195–1202. [DOI] [PubMed] [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, … Jamison RN (2004). Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. Journal of Pain Symptom Management, 28(3), 250–258. [DOI] [PubMed] [Google Scholar]
- Orhurhu VJ, Pittelkow TP, & Hooten WM (2015). Prevalence of smoking in adults with chronic pain. Tobacco Induced Diseases, 13(1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer KT, Syddall H, Cooper C, & Coggon D (2003). Smoking and musculoskeletal disorders: Findings from a British national survey. Annals of the Rheumatic Diseases, 62(1), 33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AL, Gritzner S, Resnick MP, Dobscha SK, Turk DC, & Morasco BJ (2012). Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. The Journal of Pain, 13(3), 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Assmann SE, Enos LE, & Kasten LE (2002). Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: current practice and problems. Statistics in Medicine, 21(19), 2917–2930. [DOI] [PubMed] [Google Scholar]
- Rahman MA, Hann N, Wilson A, Mnatzaganian G, & Worrall-Carter L (2015). E-cigarettes and smoking cessation: Evidence from a systematic review and meta-analysis. PloS One, 10(3), e0122544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M, & Feyerabend C (1978). Cigarette smoking: A dependence on high-nicotine boli. Drug Metabolism Reviews, 8(1), 29–57. [DOI] [PubMed] [Google Scholar]
- Sami M, Timberlake DS, Nelson R, Goettsch B, Ataian N, Libao P, & Vassile E (2012). Smokers’ perceptions of smokeless tobacco and harm reduction. Journal of Public Health Policy, 33(2), 188–201. [DOI] [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, & Viikari-Juntura E (2010). The association between smoking and low back pain: A meta-analysis. American Journal of Medicine, 123(1), 87 e87–35. [DOI] [PubMed] [Google Scholar]
- Soule EK, Pomeranz JL, Moorhouse MD, & Barnett TE (2015). Multiple tobacco use and increased nicotine dependence among people with disabilities. Disability and Health Journal, 8(2), 258–263. [DOI] [PubMed] [Google Scholar]
- Stanton CA, & Halenar MJ (2018). Patterns and Correlates of multiple tobacco product use in the United States. Nicotine & Tobacco Research, 20(suppl_1), S1–S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, & Kumagai S (2010). Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Annals of the Rheumatic Diseases, 69(01), 70–81. [DOI] [PubMed] [Google Scholar]
- Turk DC, & Melzack R (2011). Assessment of chronic pain in epidemiological and health services research In Turk DC & Melzack R (Eds.), Handbook of pain assessment (Vol. 3rd ed , pp. 455–473). New York, NY: The Guilford Press [Google Scholar]
- USDHHS. (2014). The health consequences of smoking—50 years of progress: a report of the Surgeon General. Retrieved from Atlanta, GA. [Google Scholar]
- SRNT Subcommittee on Biochemical Verification (2002). Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research, 4(2), 149–159. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Ormel J, Keefe FJ, & Dworkin SF (1992). Grading the severity of chronic pain. Pain, 50(2), 133–149. [DOI] [PubMed] [Google Scholar]
- Weingarten TN, Moeschler SM, Ptaszynski AE, Hooten WM, Beebe TJ, & Warner DO (2008). An assessment of the association between smoking status, pain intensity, and functional interference in patients with chronic pain. Pain Physician, 11(5), 643–653. [PubMed] [Google Scholar]
- Weingarten TN, Podduturu VR, Hooten WM, Thompson JM, Luedtke CA, & Oh TH (2009). Impact of tobacco use in patients presenting to a multidisciplinary outpatient treatment program for fibromyalgia. The Clinical Journal of Pain, 25(1), 39–43. [DOI] [PubMed] [Google Scholar]
- Xu X, Bishop EE, Kennedy SM, Simpson SA, & Pechacek TF (2015). Annual healthcare spending attributable to cigarette smoking: an update. American Journal of Preventive Medicine, 48(3), 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, & Ditre JW (2016). Anxiety and Depression in Bidirectional Relations Between Pain and Smoking: Implications for Smoking Cessation. Behavior Modification, 40(1–2), 7–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Garey L, Mayorga NA, Rogers AH, Orr MF, Ditre JW, & Peraza N (2018). Current pain severity and electronic cigarettes: an initial empirical investigation. Journal of Behavioral Medicine, 42(3), 461–468. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, McMillan K, Gonzalez A, & Asmundson GJ (2009). Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine & Tobacco Research, 11(12), 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]


