To the Editor
Allergies to hen’s egg and cow’s milk are the most common allergies in infants and young children. Wilson et al.(1) reported egg white sensitization in 8% of children with a median age of 7.7 years, but prevalence varies among studies. The majority of children with hen’s egg allergy become tolerant as they grow older (“natural tolerance”), with resolution of egg allergy in ~50% by 27–72 months of age(2,3). A recent review summarizes research on food allergy resolution(4).
For patients with persistent food allergies, different therapies, including oral immunotherapy (OIT), are studied. OIT has been shown, in subjects with allergies to egg and several other foods, to successfully desensitize to the culprit food(s) while increasing levels of antigen-specific IgG4 and slowly decreasing or maintaining levels of antigen-specific IgE(5–8). Studies suggest that most participants have to continue to ingest the allergen regularly to maintain desensitization. Only a small subset reached sustained unresponsiveness for the studied timeframe when discontinuing egg consumption(5–7). In contrast, individuals who are naturally tolerant to egg don’t need to ingest egg regularly to stay desensitized.
In this exploratory study, we interrogated differences in plasma immune responses among participants who outgrew an egg allergy, those who were egg-allergic and successfully desensitized by egg-OIT, and non-egg-allergic controls. Understanding the differences among these populations may help to identify why most participants after OIT have to maintain eating the food to stay desensitized while this is not necessary in naturally developed tolerance. As a long-term goal, this may aid in the development of improved treatments for egg allergy.
We identified 24 egg-allergic participants who were successfully desensitized by egg-OIT and therefore were analyzed as participants in the pre- and post-egg OIT group, 28 non-egg-allergic participants, and 30 who outgrew their egg allergy, defined by questionnaires. All participants had other atopic diseases, including other food allergies (Table 1). We compared specific IgE (sIgE) and IgG4 (sIgG4) levels to hen’s egg and its major components (Gal d 1, Gal d 2 and Gal d 3). See the Supporting Information for details. Table S1 lists results of the pairwise analyses.
Table 1:
Study demographics.
| Non-egg-allergic | Pre-/post-egg OIT$ | Egg-allergy outgrown | P value* | |
|---|---|---|---|---|
| Participants (n) | 28 | 24 | 30 | |
| Sex female, n (%) | 19 (68%) | 8 (33%) | 8 (27%) | 0.004 |
| Age in years, median (range, IQR) | 8.5 (4–18, 3.25) | 8 (3–24, 5.25) | 10 (2–24, 5) | 0.24 |
| History of asthma | 12 (43%) | 14 (61%) [1 NA] | 19 (73%) [4 NA] | 0.08 |
| History of atopic dermatitis | 22 (79%) | 20 (87%) [1 NA] | 21 (81%) [4 NA] | 0.76 |
| History of allergic rhinitis | 21 (75%) | 15 (65%) [1 NA] | 18 (69%) [4 NA] | 0.60 |
| History of allergy to food(s) different from egg, n (%) | 27 (96%) | 23 (96%) | 30 (100%) | 0.53 |
These 24 participants were part of the pre- as well as post-egg OIT group. The pre-egg OIT demographics information is provided.
P values by Fisher’s exact test or Kruskal-Wallis test.
[NA] numbers of participants with unavailable data.
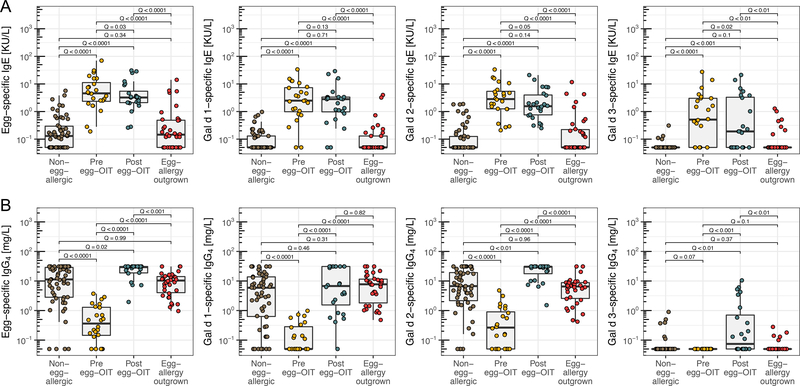
sIgE levels to egg and its components were each significantly greater (Q (FDR-adjusted P)<0.01, Figure 1A) among pre- and post-OIT participants than they were in the naturally tolerant and non-egg-allergic participants. Egg-OIT resulted in a weak reduction (model estimates between −0.1 and −0.27 (log10 scale); standard error (SE) between 0.05–0.1) of sIgE levels from pre- to post-egg-OIT for egg and Gal d 1–3 (Q between 0.02–0.13). sIgE levels between participants who were never allergic to egg vs. those who outgrew their egg allergy showed similar, negligible differences (estimates between 0.05–0.25; SE between 0.12–0.17, Q between 0.1–0.71). The percentage of participants who were sensitized to egg and Gal d 1–3, judged by sIgE>0.35KU/L, was comparable between the naturally tolerant and non-egg-allergic participants (Figure S2).
Figure 1:
sIgE (A) and sIgG4 (B) levels to egg and 3 components (Gal d 1–3) in participants who were never egg-allergic, allergic to egg and subsequently successfully desensitized by OIT to egg, or outgrew their egg allergy. Q values (FDR adjusted P) by likelihood ratio test in liner mixed effects model. Each plot had a Q < 0.0001 by Kruskal–Wzallis test.
sIgG4 to egg and Gal d 1–2 were significantly (Q<0.0001, Figure 1B) lower in egg-allergic participants than in those treated by egg-OIT or in the egg naturally tolerant or non-egg-allergic individuals. No sIgG4 to Gal d 3 was found in any of the egg-allergic participants (detection limit 0.1mg/L) but was detected in the other groups (50% of post-OIT, 15% of naturally tolerant, 11% of non-egg-allergic samples). For sIgG4 to egg and Gal d 2, post-OIT levels were greater than those in naturally tolerant and non-egg-allergic individuals (Table S1).
This study has several limitations (see also Supplement). The status of natural tolerance was determined based on questionnaire, but the time when natural tolerance was achieved, and whether egg was incorporated in their diet, are not known, nor did we have samples pre and post natural tolerance. Therefore, we also have no information about the sIgE or sIgG4 levels of these participants before they outgrew their egg allergy. Qamar et al.(9) reported no difference in egg-sIgE at the time of diagnosis of egg allergy between participants continuing to be allergic and those who became naturally tolerant, while sensitization to Gal d 1 was found to be associated with a 2.5-fold increased risk of persistent egg allergy(3). 22 of the 24 individuals who underwent egg-OIT received a course of Omalizumab. Several half-lives had passed after the last administration of Omalizumab before sample collection, but a residual effect of Omalizumab on the sIgE or sIgG4 levels cannot be excluded.
The strength of this study is that we could compare three different egg-allergic conditions with non-egg allergy.
In summary, we found that egg-OIT only weakly influenced sIgE levels, based on comparing pre- with post-OIT levels within the studied OIT time frame. Participants who became naturally tolerant to egg or were never egg-allergic showed lower sIgE levels than pre- and post-OIT participants. Other studies evaluating sIgE levels after 10+ months of egg-OIT found that egg sIgE levels were lower when compared to baseline levels(5,7). Conversely, egg-OIT caused sIgG4 levels to egg, Gal d 1, and Gal d 2 to increase to levels similar to those in naturally tolerant and non-egg-allergic participants. However, we cannot say how much the low sIgG4 levels in the group of egg-allergic participants might have reflected egg avoidance.
In conclusion, we show that egg-OIT results in sIgG4 levels similar to those in non-allergic or naturally tolerant individuals, while the sIgE levels remain greater than in these two groups. Data from our limited pilot study suggest that the greater sIgE levels in the participants post-OIT, compared to those who outgrew their egg allergy, represents one possible reason why naturally tolerant participants don’t need to ingest the culprit food to stay desensitized while it is necessary in most cases after OIT. However, given the limited statistical power (see also Statistical analysis in the Supplement), further analysis of data from larger OIT studies is needed to confirm whether sIgE levels post-OIT eventually resemble those of naturally tolerant or non-allergic individuals. If a subgroup of individuals shows such a reduction in sIgE levels, these individuals could be studied for their potential to remain desensitized to egg even after stopping daily maintenance of OIT.
Supplementary Material
Acknowledgments
This work was supported by the Stanford Maternal & Child Health Research Institute (Andorf), the Stanford NIH-NCATS-CTSA (grant no. UL1 TR001085), NIH 5U19AI104209–05 (Galli), NIH 1R01AI140134–01 (Nadeau), FARE, EAT, and the Sean N. Parker Center for Allergy and Asthma Research. We thank all research participants, and the study coordinators and clinicians, from the original studies. We thank Thermo Fisher for performing a part of the antibody measurements at no cost.
Footnotes
Conflict of interest
Dr. Borres is an employee of Thermo Fisher Scientific (which is also stated in the authors list). Dr. Galli reports grants from National Institutes of Health, personal fees from National Allergy and Infectious Disease Advisory Council of the NIH, patents (planned, pending or issued) from Stanford University, and participation in KdT Ventures Fund I, during the conduct of the study. Dr. Chinthrajah reports grants from NIAID Consortium for Food Allergy, grants from Regeneron, grants from DBV Therapeutics, grants from Aimmune Therapeutics, grants from Novartis, grants from Astellas Pharma, personal fees from Alladapt Immunotherapeutics, during the conduct of the study. Dr. Nadeau reports grants and other from NIAID, other from Novartis, personal fees and other from Regeneron, grants and other from FARE, grants from EAT, other from Sanofi, other from Astellas, other from Nestle, other from BeforeBrands, other from Alladapt, other from ForTra, other from Genentech, other from AImmune Therapeutics, other from DBV Technologies, personal fees from Astrazeneca, personal fees from ImmuneWorks, personal fees from Cour Pharmaceuticals, grants from Allergenis, grants from Ukko Pharma, other from AnaptysBio, other from Adare Pharmaceuticals, other from Stallergenes-Greer, other from NHLBI, from NIEHS, from EPA, other from WAO Center of Excellence, outside the submitted work. The remaining authors have nothing to disclose.
References
- 1.Wilson JM, Workman L, Schuyler AJ, Rifas-Shiman SL, McGowan EC, Oken E et al. Allergen sensitization in a birth cohort at midchildhood: Focus on food component IgE and IgG4 responses. J Allergy Clin Immunol 2018;141:419–423.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters RL, Dharmage SC, Gurrin LC, Koplin JJ, Ponsonby A-L, Lowe AJ et al. The natural history and clinical predictors of egg allergy in the first 2 years of life: a prospective, population-based cohort study. J Allergy Clin Immunol 2014;133:485–491. [DOI] [PubMed] [Google Scholar]
- 3.Dang TD, Peters RL, Koplin JJ, Dharmage SC, Gurrin LC, Ponsonby AL et al. Egg allergen specific IgE diversity predicts resolution of egg allergy in the population cohort HealthNuts. Allergy 2019;74:318–326. [DOI] [PubMed] [Google Scholar]
- 4.Berin MC. Mechanisms that define transient versus persistent food allergy. J Allergy Clin Immunol 2019;143:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW et al. Oral Immunotherapy for Treatment of Egg Allergy in Children. N Engl J Med 2012;367:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andorf S, Purington N, Kumar D, Long A, O’Laughlin KL, Sicherer S et al. A Phase 2 Randomized Controlled Multisite Study Using Omalizumab-facilitated Rapid Desensitization to Test Continued vs Discontinued Dosing in Multifood Allergic Individuals. EClinicalMedicine 2019;7:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caminiti L, Pajno GB, Crisafulli G, Chiera F, Collura M, Panasci G et al. Oral Immunotherapy for Egg Allergy: A Double-Blind Placebo-Controlled Study, with Postdesensitization Follow-Up. J allergy Clin Immunol Pract 2015;3:532–539. [DOI] [PubMed] [Google Scholar]
- 8.Chinthrajah RS, Purington N, Andorf S, Long A, O’Laughlin KL, Lyu SC et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Published Online First: 2019. doi: 10.1016/S0140-6736(19)31793-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qamar N, Fishbein AB, Erickson KA, Cai M, Szychlinski C, Bryce PJ et al. Naturally occurring tolerance acquisition to foods in previously allergic children is characterized by antigen specificity and associated with increased subsets of regulatory T cells. Clin Exp Allergy 2015;45:1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.