Abstract
Background
Advances in radiotherapy (RT) have led to improved oncologic outcomes for women with gynecologic cancers, however, the long-term effects and survivorship implications need further evaluation. The purpose of our study was to determine the incidence of pelvic fractures and changes in bone mineral density (BMD) following pelvic RT.
Methods
We prospectively studied 239 women who had pelvic RT for cervical, endometrial or vaginal cancer between 2008 and 2015. BMD scans and biomarkers of bone turnover were obtained at baseline and three months, one year and two years following RT. Imaging studies were assessed for pelvic fracture for up to 5 years. Patients with osteopenia, osteoporosis or pelvic fracture at any point were referred to the endocrinology service for evaluation and treatment.
Results
Median age at diagnosis was 51 years; 132 patients (56%) were menopausal. Primary diagnoses were cervical (64%), endometrial (30%) and vaginal cancer (6%). Sixteen patients (7.8% 95% CI: 4.5–12.4%) had pelvic fractures with actuarial rates of 3.6%, 12.7% and 15.7% at one, two, and three years respectively. Fractures were associated with baseline osteoporosis (p=0.002), higher baseline bone alkaline phosphatase (p=0.001) and older age (p=0.007). The proportion of patients with osteopenia/osteoporosis increased from 50% at baseline to 58%, 59%, and 70% at 3 months, 1 year and 2 years, respectively.
Conclusions
A high proportion of women had significant decreases in BMD following pelvic RT, with 7.8% diagnosed with a pelvic fracture. BMD screening and pharmacologic intervention should be strongly considered in these high-risk women.
Keywords: bone mineral density, pelvic fractures, radiotherapy, cervical cancer, endometrial cancer, vaginal cancer, survivorship
Precis:
Pelvic radiotherapy was associated with decreases in bone mineral density and the development of pelvic fractures. Screening for osteoporosis with pharmacologic intervention should be considered in these high-risk women.
Introduction
Pelvic fractures, particularly hip fractures, are a major source of morbidity and mortality.1 In the United States, there are over 250,000 hip fractures attributed to osteoporosis per year, with a 10 to 20% mortality rate within the first 6 months following a fracture.1,2 The measurement of bone mineral density (BMD) correlates with bone strength and predicts the risk of fracture.3,4,5 Pelvic radiotherapy (RT) for gynecologic malignancies has been shown to result in demineralization of bone matrix, with a pelvic fracture rate ranging from 1.7% to 89%.6,7,8
Our group previously performed a retrospective analysis of 300 women treated with curative-intent RT for cervical cancer. Pelvic fractures were noted in 29 of 300 patients (9.7%).9 Based on our retrospective findings, we determined that further study was needed to better understand the rate of pelvic fractures and associated risk factors in women undergoing RT. We therefore performed a prospective evaluation of women treated with definitive pelvic RT for cervical, endometrial or vaginal cancer. We sought to determine the incidence of pelvic fractures, associated risk factors, as well as changes in BMD and serum biomarkers of bone turnover.
Patients and Methods
We performed a prospective cohort study of women treated with definitive or adjuvant RT or concurrent chemotherapy with RT (chemoRT) for primary or recurrent cervical, endometrial or vaginal cancer at The University of Texas MD Anderson Cancer Center from September 2008 through July 2015. Institutional Review Board approval was obtained and all patients provided informed consent. Patients were excluded if they were undergoing palliative intent radiotherapy, had bone metastases, were receiving brachytherapy only, had undergone previous pelvic radiotherapy, had an existing pelvic fracture within the proposed radiation field, and/or were unwilling or unable to provide informed consent for the study.
Demographic information was collected from the patient’s electronic medical record and included: age, body mass index (BMI), race, ethnicity, smoking history and menopausal status. Pathologic characteristics including histological diagnosis, disease status and cancer stage were also obtained. The treatment characteristics were reviewed for the type, dose and duration of radiotherapy and if neoadjuvant, concurrent or adjuvant chemotherapy was received.
BMD scans were obtained at baseline, three months, one year and two years after completing radiotherapy. Dual-energy absorptiometry (DXA) (Discovery, Hologic Inc, Bedford, MA, USA) was used to assess the BMD. T-scores and Z-scores in the lumbar spine (L1–L4) and the left femoral neck and femur trochanter were assessed. BMD values were categorized into three groups, according to the World Health Organization criteria (normal, osteopenia and osteoporosis).10
Serum biomarkers of bone turnover were obtained at the same time intervals and included bone-specific alkaline phosphatase (BAP), C-terminal telopeptide of type I collagen (CTX), procollagen Type I N Propeptide (PINP). Vitamin D, calcium, phosphorus, and parathyroid hormone (PTH) levels were obtained at baseline and treated if abnormal.
Imaging studies were reviewed for pelvic fractures prior to beginning treatment, at the completion of therapy and then annually (or as performed per standard of care) for up to 5 years following the completion of treatment. These included magnetic resonance imaging (MRI), computed tomography (CT), and/or positron emission tomography/computed tomography (PET/CT) scan per standard of care and physician preference. Patients with osteopenia, osteoporosis or a pelvic fracture diagnosed at any point in the study were referred to the endocrinology service for evaluation and management.
All study data were collected and managed using the Research Electronic Data Capture (REDCap) tools hosted at MD Anderson Cancer Center.11 REDCap is a secure, web-based application designed to support data capture for research studies. Statistical analyses included the use of summary statistics to describe the clinical and demographic characteristics of the study population. Time to pelvic fracture was measured from the end of RT to the earliest date of first fracture or date of last follow up imaging study and was estimated with the product limit estimator of Kaplan and Meier. The product limit estimate at two years was used to estimate the incidence of women with pelvic fractures. Univariate Cox proportional hazards regression models were conducted to estimate the association of several potential risk factors with pelvic fracture. Descriptive statistics were used to summarize BMD and serum biomarkers. Changes in BMD and serum biomarkers were estimated along with 95% confidence intervals. Linear mixed models (LMM) were conducted to assess T-scores, Z-scores, BAP, and CTX measures over multiple time points. Scores were regressed onto time and a random intercept was included. LMMs were also conducted to assess biomarker changes over time. Biomarkers were regressed onto time and a random intercept was included. We also used Cox proportional hazards regression models to assess the baseline biomarker levels association with fractures. All statistical analyses were performed using Stata/MP v15.0 (College Station, TX).
Results
The study included 239 women with cervical, endometrial or vaginal cancer who underwent definitive radiotherapy for primary or recurrent disease during the study period. The median age at diagnosis was 51 years (range, 23 to 88). Demographic and clinical characteristics are shown in Table 1. The majority of patients (83.2%) were white. At study entry, 56.4% of patients were menopausal. The median BMI was 28.6 kg/m2 (range, 15.7–63.7). Vitamin D deficiency (<20 ng/mL) was diagnosed and treated at baseline in 42.7% of patients. The primary diagnoses were cervical (63.6%), endometrial (30.5%) and vaginal cancer (5.9%). The majority of patients (94.9%) had primary disease and 5.1% had recurrent disease.
Table 1.
Demographic and clinical characteristics (n=239)
| Characteristic | N | % |
|---|---|---|
| Age (years) | ||
| Mean (SD) | 50.5 | 13.3 |
| Median (Min-Max) | 51.0 | 23–88 |
| BMI (kg/m2) | ||
| Mean (SD) | 29.7 | 7.4 |
| Median (Min-Max) | 28.6 | 15.7–63.7 |
| Race | ||
| White | 193 | 83.2 |
| Black | 33 | 14.2 |
| Asian Pacific Islander | 6 | 2.6 |
| Unknown | 7 | |
| Ethnicity | ||
| Hispanic | 49 | 20.6 |
| Non-Hispanic | 189 | 79.4 |
| Unknown | 1 | |
| History of smoking | ||
| No | 158 | 66.4 |
| Yes | 80 | 33.6 |
| Unknown | 1 | |
| Menopausal at study entry | ||
| No | 102 | 43.6 |
| Yes | 132 | 56.4 |
| Unknown | 5 | |
| Diagnosis | ||
| Cervical | 152 | 63.6 |
| Endometrial | 73 | 30.5 |
| Vaginal | 14 | 5.9 |
| Disease Status | ||
| Primary | 221 | 94.9 |
| Recurrent | 12 | 5.1 |
| Unknown | 6 | |
| Vitamin D deficiency (< 20 ng/mL) | ||
| No | 130 | 57.3 |
| Yes | 97 | 42.7 |
| Unknown | 12 |
SD: Standard Deviation
Complete information regarding the RT treatment parameters was available for 230 patients and is shown in Table 2. The median external beam total dose was 4500 cGy (range: 3780–6600 cGy). Pelvic RT modalities included 4-Field in 131 patients (57%), Anterior to Posterior (AP/PA) in 18 patients (7.8%) and Intensity Modulated Radiation Therapy (IMRT) in 81 patients (35.2%). Extended field RT was given to 57 patients (24.8%). Brachytherapy, in addition to external beam RT, was given to 191 patients (83.4%). Concurrent chemotherapy was administered to 194 patients (84.4%), with the majority (93.8%) receiving cisplatin. Adjuvant chemotherapy following surgery and/or RT was administered to 43 patients (18.7%), with the majority (83.7%) receiving paclitaxel and carboplatin.
Table 2:
Treatment characteristics of study population (n = 230)
| Characteristic | N | % |
|---|---|---|
| Total Dose (cGy) | ||
| Mean (SD) | 4558 | 303 |
| Median (Min-Max) | 4500 | 3780–6600 |
| Radiation modality (Pelvis) | ||
| 4-Field | 131 | 57.0 |
| AP/PA | 18 | 7.8 |
| IMRT | 81 | 35.2 |
| Extended field | ||
| Yes | 57 | 24.8 |
| No | 173 | 75.2 |
| Extended field modality | ||
| 4-Field | 23 | 40.4 |
| AP/PA | 0 | 0 |
| IMRT | 34 | 59.6 |
| Brachytherapy | ||
| No | 38 | 16.6 |
| Yes | 191 | 83.4 |
| Chemotherapy | ||
| Concurrent | 194 | 84.4 |
| Adjuvant | 43 | 18.7 |
SD: Standard Deviation
There were 34 women who did not have post-treatment imaging available for review; the remaining 205 women had at least one imaging study that could be assessed for the presence of fracture. The mean number of imaging studies for each patient was 5.6 (SD 4.0; range, 1 to 22). Of these 205 patients, 16 developed a fracture (7.8%, 95% CI: 4.5 – 12.4%). The median follow-up time for all surviving patients was 13.7 months (range: 0.9 – 74.1). The median follow-up time for those with fractures was 12.9 months (range: 4.5 – 27.0). The one-year incidence of pelvic fractures was 3.6% (95% CI: 1.5 – 8.5). The two-year and three-year incidence of pelvic fractures were 12.7% (95% CI: 7.6 – 20.7%) and 15.7% (95% CI: 9.7 – 24.8), respectively (Figure 1). The locations of the fractures are shown in Table 3 and included the sacrum (n=12, 75.0%), the lumbar spine (n=2, 12.5%), both the sacrum and lumbar spine (n=1, 6.3%), and both the sacrum and pubis (n=1, 6.3%). Nine patients (56.2%) had pain associated with the fractures; and seven patients (43.8%) were asymptomatic. The patients with fractures were treated with bisphosphonates and/or pain medications. One patient required kyphoplasty due to L5 compression. None of the patients experienced major complications such as osteonecrosis or osteomyelitis.
Figure 1:
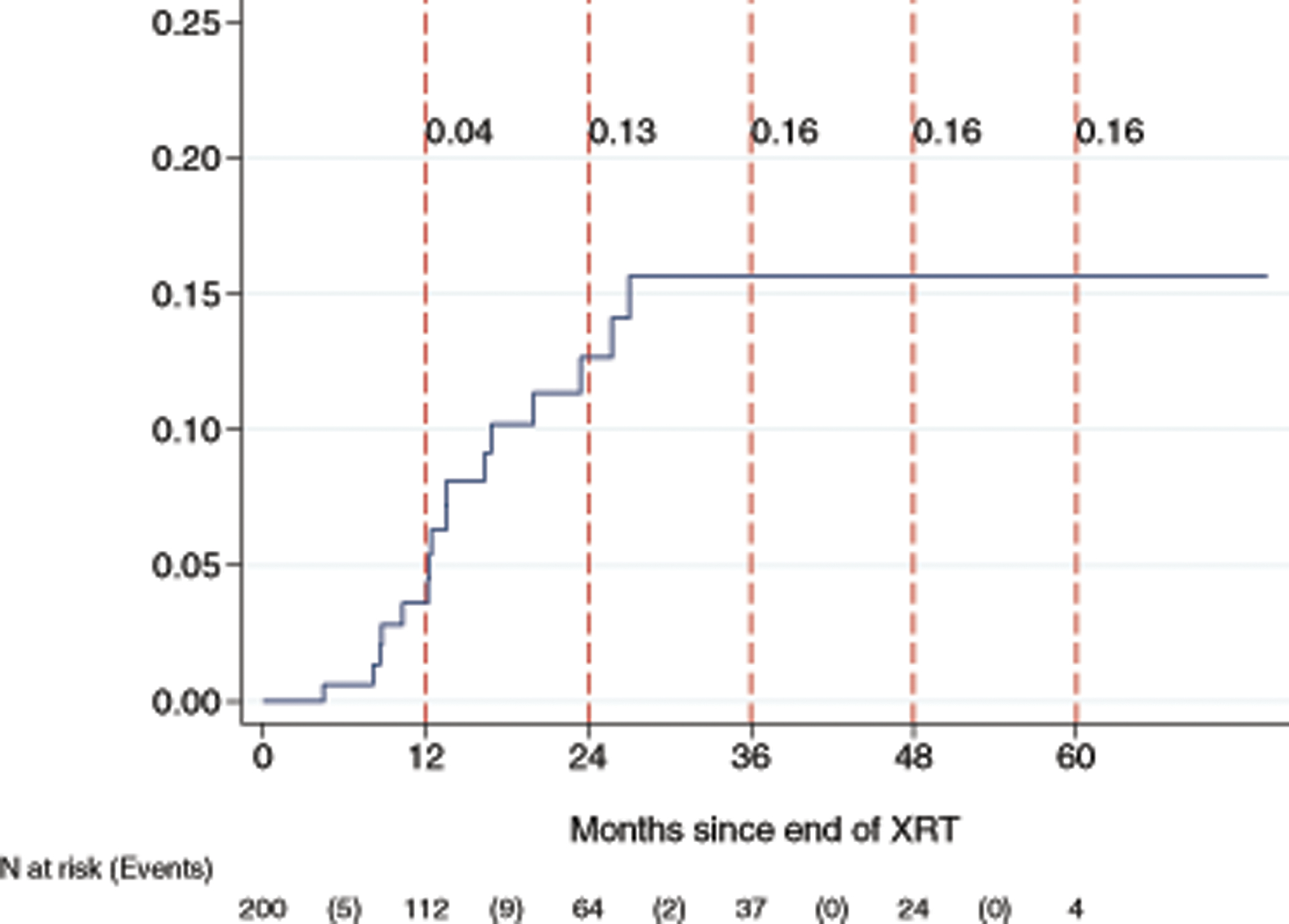
Cumulative incidence of pelvic fracture following radiotherapy
Table 3:
Fracture sites
| Characteristic | N | % |
|---|---|---|
| Any Fracture | ||
| No | 189 | 92.2 |
| Yes | 16 | 7.8 |
| Fractures site | ||
| Sacrum | 12 | 75.0 |
| Lumbar spine | 2 | 12.5 |
| Sacrum and lumbar spine | 1 | 6.3 |
| Sacrum and pubis | 1 | 6.3 |
The Cox regression models assessing predictive risk factors of pelvic fracture are shown in Table 4. The development of a pelvic fracture was associated with older age (HR: 1.06; 95% CI: 1.02 – 1.11; p=0.007), lower BMI (HR: 90; 95% CI: 0.81 −0.99; p = 0.035), menopausal status (HR: 3.55; 95% CI: 1.00 – 12.58; p = 0.049); higher baseline BAP level (HR: 1.16; 95% CI: 1.08 – 1.25; p<0.001) and osteoporosis at baseline (HR: 11.06 95% CI: 3.39 – 36.11; p<0.001). There was insufficient evidence that radiation modality, administration of concurrent and/or adjuvant chemotherapy, smoking and vitamin D deficiency were associated with development of a fracture.
Table 4.
Cox proportional hazards regression models assessing prognostic factors association with fracture
| Characteristic | N | Events | log-rank | HR | LB | UB | p-value |
|---|---|---|---|---|---|---|---|
| Age at Diagnosis | 200 | 16 | 1.06 | 1.02 | 1.11 | 0.007 | |
| BMI | 200 | 16 | 0.90 | 0.81 | 0.99 | 0.035 | |
| Baseline BAP | 196 | 16 | 1.16 | 1.08 | 1.25 | <0.001 | |
| Baseline CTX | 194 | 16 | 1.00 | 1.00 | 1.00 | 0.036 | |
| Diagnosis | |||||||
| Cervical | 123 | 9 | 0.813 | 1.00 | 1.00 | 1.00 | |
| Endometrial | 63 | 6 | 1.40 | 0.50 | 3.94 | 0.522 | |
| Vaginal | 14 | 1 | 1.10 | 0.14 | 8.67 | 0.931 | |
| Race | |||||||
| Non-white | 30 | 2 | 0.810 | 1.00 | 1.00 | 1.00 | |
| White | 167 | 14 | 1.20 | 0.27 | 5.28 | 0.810 | |
| Menopausal at Study Entry? | |||||||
| No | 87 | 3 | 0.036 | 1.00 | 1.00 | 1.00 | |
| Yes | 108 | 12 | 3.55 | 1.00 | 12.58 | 0.050 | |
| Currently Smoking? | |||||||
| No | 44 | 3 | 0.912 | 1.00 | 1.00 | 1.00 | |
| Yes | 22 | 1 | 0.88 | 0.09 | 8.51 | 0.912 | |
| Concurrent Chemo? | |||||||
| No | 29 | 2 | 0.981 | 1.00 | 1.00 | 1.00 | |
| Yes | 171 | 14 | 1.02 | 0.23 | 4.50 | 0.981 | |
| Boost | |||||||
| No | 91 | 9 | 0.465 | 1.00 | 1.00 | 1.00 | |
| Yes | 108 | 7 | 0.69 | 0.26 | 1.86 | 0.468 | |
| Vitamin D Deficiency? (< 20) | |||||||
| No | 119 | 11 | 0.596 | 1.00 | 1.00 | 1.00 | |
| Yes | 77 | 5 | 0.75 | 0.26 | 2.17 | 0.597 | |
| Osteoporosis | |||||||
| Normal/Osteopenia | 185 | 12 | <0.001 | 1.00 | 1.00 | 1.00 | |
| Osteoperosis | 11 | 4 | 11.06 | 3.39 | 36.11 | <0.001 | |
| Pelvis | |||||||
| 4-field | 113 | 10 | 0.505 | 1.00 | 1.00 | 1.00 | |
| AP/PA | 15 | 2 | 1.64 | 0.36 | 7.48 | 0.525 | |
| IMRT | 72 | 4 | 0.63 | 0.20 | 1.99 | 0.427 | |
| Extended field | |||||||
| no | 149 | 12 | 0.896 | 1.00 | 1.00 | 1.00 | |
| yes | 51 | 4 | 0.93 | 0.30 | 2.88 | 0.896 | |
| Extended field Modality | |||||||
| 4-field | 22 | 1 | 0.608 | 1.00 | 1.00 | 1.00 | |
| IMRT | 29 | 3 | 1.79 | 0.19 | 17.28 | 0.613 |
BAP: Bone Specific Alkaline Phosphatase
CTX: Collagen Type IC Telepeptide
Osteopenia/osteoporosis for all patients increased over time from 50% at baseline to 58%, 59%, and 70% at three months, one year and two years, respectively. There were significant changes in the BAP biomarker of bone turnover at three months (p = 0.002), one year (p <0.001) and two years (p < 0.001), compared to baseline (Supplemental Table 1 and Supplemental Table 2). For CTX, there was a significant increase from baseline to three months (p = 0.003), however the differences at one year (p 0.239) and two years (0.318) were not significantly different from baseline.
Discussion
Our prospective study showed a pelvic fracture rate of 7.8% among women receiving definitive RT for cervical, endometrial or vaginal cancer, with the majority of fractures occurring within two years of completing treatment. Associated risk factors included baseline osteoporosis, higher baseline BAP level, menopausal status, lower BMI and older age.
Previous studies have reported the incidence of pelvic fractures to vary widely from 1.7 to 89%.6–9,12–14 A recent meta-analysis of 3,929 patients from 21 studies showed a pelvic fracture incidence rate of 14% among women undergoing RT for a gynecologic cancer.14 A large study by Baxter and colleagues13 analyzed 6,428 women ≥65 years of age with pelvic malignancies (including 1,605 women with cervical cancer) using the Surveillance, Epidemiology, and End Results (SEER) cancer registry data. Among the women with cervical cancer, they noted the cumulative 5-year fracture rate to be 8.2% among women who received RT compared with 5.9% among the women who did not receive RT. The authors concluded that pelvic RT in older women raises the risk of pelvic fracture.13 A subsequent prospective study in Japan evaluated 59 patients with cervical cancer treated with RT (external-beam and brachytherapy). The median age was 73 years and they had a median follow-up of 24 months. The two-year cumulative incidence of insufficiency fractures was 36.9%.15 In a subsequent study in Japan, Yamamoto et al.16 retrospectively evaluated post-treatment image studies on 533 women treated with RT for cervical cancer. Pelvic insufficiency fractures were noted in 15.8% of the patients, with 80% occurring within three years of completion of treatment.16 The higher rates of pelvic fractures reported in these studies compared with the current study may be due to Japanese women having lower BMIs and higher rates of osteoporosis compared with American women. It is estimated that the prevalence of osteoporosis (total hip or spine) in women >50 years of age is 16.0% in the United States compared with 38.0% in Japan.17 Furthermore, the mean BMI is 29.2 kg/m2 among women in the United States compared with 22.1 kg/m2 in Japan18 and it is known that low BMI is associated with a higher risk of osteoporosis.19
Our group previously performed a retrospective analysis of 300 women treated with curative-intent radiotherapy for cervical cancer.9 Pelvic fractures were noted in 9.7% of the patients, and were diagnosed a median of 14.1 months (range, 2.1–63.1) from the completion of radiotherapy. Thirty-eight percent of fractures were diagnosed within one year and 83% within two years of completing treatment. Fracture sites included sacrum (n = 24; 83%), sacrum and pubis (n = 3; 10%), iliac crest (n = 1; 3%), and sacrum and acetabulum (n = 1; 3%).9 In our cohort, we had similar findings with most fractures occurring within two years of completing treatment and the majority located in the sacrum.
In the current study, 52.2% of the patients reported pain at the time of fracture diagnosis. This finding is similar to that of our previous retrospective study, where 13 patients (45%) were symptomatic, with pain being the most common presenting symptom.9 In contrast, Huh and colleagues7 evaluated 463 patients treated with RT for cervical cancer in Korea, and pelvic fractures were noted in only eight patients (1.7%), with a median follow up of 38 months. However, all eight patients reported pelvic pain at the time of diagnosis. It is unknown if imaging was only performed in symptomatic patients for this study, potentially explaining the low reported fracture rate compared with the current and previous studies.
In the current study, 35.2% of participants received pelvic IMRT and it was not found to be associated with a decreased pelvic fracture rate. A previous study by Ioffe and colleagues20 analyzed 83 patients treated with IMRT for locally advanced cervical cancer and compared the outcomes with 83 controls treated with conventional RT. In the IMRT group, 4% developed a sacral fracture compared with 11% in the control group (OR 4.49, p=0.01). In the conventional RT group, there were two cases of osteonecrosis and three cases of osteomyelitis. Similar to our findings, all fractures were located in the sacrum.20
Misra et al.21 performed a randomized phase III trial comparing the late toxicity between chemoRT and RT in 180 patients with locally advanced cervical cancer. The median follow-up among the surviving patients was 10.5 years. They found the incidence of fractures to be higher in the chemoRT arm (5%) compared with the RT alone arm (0%), p=0.018.21 In the current study, 84.4% of patients received concurrent chemotherapy and 18.7% received adjuvant chemotherapy, with no differences noted in the fracture rate between those that did and did not receive chemotherapy.
In our cohort, pelvic fractures were associated with osteoporosis, higher BAP, menopausal status, lower BMI and older age at baseline prior to beginning RT. Similarly, a study by Ramlov and colleagues22 evaluated 126 women with locally-advanced cervical cancer treated with RT and found age, but not BMI, to be a significant risk factor for pelvic fractures. And a previous study in Japan evaluating risk factors for pelvic fractures among 126 cervical cancer patients found that older age, postmenopausal state and decreased bone density predisposed women to fracture after RT.23
In our study, 50% of women were diagnosed with osteopenia/osteoporosis at baseline, with an increase to 70% at 2 years. A recent publication from the American Society of Clinical Oncology (ASCO) provides guidelines that suggest oral bisphosphonates, intravenous bisphosphonates, and subcutaneous denosumab as efficacious options for patients with nonmetastatic cancer with osteoporosis or at increased risk of osteoporotic fractures.24 Additional preventive strategies suggested include: 1) consume a diet with adequate calcium and vitamin D; 2) exercise; 3) stop smoking; and 4) limit alcohol consumption. Furthermore, any patient with risk for osteoporotic fracture should be offered BMD testing with central/axial dual-energy x-ray.24
Dybnik et al.25 compared total hip replacement rates between women with gynecologic cancer undergoing pelvic RT (n=962) and women with breast cancer treated with breast RT (n=7,545). The rate of total hip replacement was 3% in both groups with no significant differences in the 8-year cohort. Idiopathic osteoarthritis was the most common indication for total hip replacement in both groups.24 In the current study, none of the patients required a total hip replacement but one patient underwent kyphoplasty due to L5 compression fracture.
To date, there are limited data regarding the effects of radiotherapy on the bone turnover process. Bone remodeling or turnover is a complex process involving osteoblasts (bone formation), osteoclasts (bone resorption). RT has been shown to directly affect this process, resulting in a reduction in overall bone mass.26 The radiotherapy may affect the vascular supply of bone and compromise osteoblast function.27 Increase in bone turnover is associated with an increased risk of fracture independent of BMD.28 It has also been suggested that chemotherapy may be a confounding factor.27 In the current study, higher baseline BAP was associated with pelvic fractures.
Our study is limited as the data are from a single institution which is a highly specialized cancer center, leading to possible referral bias. In addition, we treated all patients as soon as we detected vitamin D deficiency or osteopenia/osteoporosis, possibly reducing the number of fractures or increasing the bone density during the study. In addition, our study is limited by a short follow-up period, and therefore the long-term morbidity associated with pelvic fractures and osteoporosis could not be reported. In addition, we did not measure quality of life or patient reported outcomes in our cohort. It is well known that the toxicities and long-term effects of treatment can have profound effects on cancer survivors.21,28,29 The bone toxicity we describe in our study requires further evaluation to determine the impact on gynecologic cancer survivorship and generalizability to broader populations.
The strengths of our study include a large number of patients with cervical, endometrial or vaginal cancer and prospective data collection including BMD measurements. Our results suggest that pelvic fractures and changes in BMD were detected in a substantial proportion of women following RT for gynecologic malignancies. Preventive strategies such as BMD screening and medical regimens at preventing osteoporosis should be considered to improve survivorship for these high-risk women. Changes in radiation technique for high-risk individuals to minimize the radiation dose to the bone are currently being investigated.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672, the MD Anderson Cancer Center Institutional Research Grant (IRG) and the Dunaway Family Fund.
Footnotes
Conflict of Interest: The authors have the following disclosures: Anil K. Sood, MD: consulting (Merk and Kivatec), research funding (M-Trap) and shareholder (BioPath); these conflicts are not related to the current study. The remaining authors do not have any conflicts of interest to disclose.
Trial Registration: The study was registered with clinicaltrials.gov (NCT00800644).
References
- 1.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999. March 13;353:878–82. doi: 10.1016/S0140-6736(98)09075-8 [DOI] [PubMed] [Google Scholar]
- 2.Riggs BL, Melton LJ. The worldwide problem of osteoporosis: insights afforded by epidemiology. Bone. 1995. November;17(5 Suppl):505S–511S. [DOI] [PubMed] [Google Scholar]
- 3.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996. May 18;312(7041):1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siris ES, Miller PD, Barret-Connor E, et al. : Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001. December 12;286(22):2815–22. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BJ, Brooks ER, Langman CB. Osteoporosis screening of postmenopausal women in the primary care setting: a case-based approach. Gend Med. 2004. December;1(2):70–85. [DOI] [PubMed] [Google Scholar]
- 6.Blomlie V, Rofstad EK, Talle K, Sundfor K, Winderen M, Lien HH. Incidence of radiation-induced insufficiency fractures of the female pelvis: evaluation with MR imaging. AJR Am J Roentgenol. 1996. November;167(5):1205–10. doi: 10.2214/ajr.167.5.8911181 [DOI] [PubMed] [Google Scholar]
- 7.Huh SJ, Kim B, Kang MK, et al. Pelvic insufficiency fracture after pelvic irradiation in uterine cervix cancer. Gynecol Oncol. 2002. September;86(3):264–268 doi: 10.1006/gyno.2002.6756. [DOI] [PubMed] [Google Scholar]
- 8.van den Blink QU, Garcez K, Henson CC, Davidson SE, Higham CE. Pharmacological interventions for the prevention of insufficiency fractures and avascular necrosis associated with pelvic radiotherapy in adults. Cochrane Database Syst Rev. 2018. April 23;4:CD010604. doi: 10.1002/14651858.CD010604.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmeler KM, Jhingran A, Iyer RB, et al. Pelvic Fractures After Radiotherapy for Cervical Cancer: Implications for Survivors. Cancer. 2010. February 1; 116(3): 625–630. doi: 10.1002/cncr.24811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization (WHO) 2007. WHO Scientific Group on the assessment of osteoporosis at primary health care level Summary Meeting Report Brussels, Belgium, 5–7 May 2004. https://www.who.int/chp/topics/Osteoporosis.pdf Acessed June 28, 2019 [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bliss P, Parsons CA, Blake PR. Incidence and possible aetiological factors in the development of pelvic insufficiency fractures following radical radiotherapy. Br J Radiol. 1996. June;69(822):548–54. doi: 10.1259/0007-1285-69-822-548 [DOI] [PubMed] [Google Scholar]
- 13.Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005. November 23;294(20):2587–93. doi: 10.1001/jama.294.20.2587 [DOI] [PubMed] [Google Scholar]
- 14.Sapienza LG, Salcedo MP, Ning MS, et al. Pelvic insufficiency fractures after external beam radiotherapy for gynecologic cancers: a meta-analysis and meta-regression of 3,929 patients. Int J Radiat Oncol Biol Phys. 2019. September 30 pii: S0360–3016(19)33836–2. doi: 10.1016/j.ijrobp.2019.09.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Tokumaru S, Toita T, Ogushi M, et al. Insufficiency fractures after pelvic radiation therapy for uterine cervical cancer: an analysis of subjects in a prospective multi-institutional trial, and cooperative study of the Japan Radiation Oncology Group (JAROG) and Japanese Radiation Oncology Study Group (JROSG). Int J Radiat Oncol Biol Phys. 2012. October 1;84(2):e195–200. doi: 10.1016/j.ijrobp.2012.03.042. Epub 2012 May 12. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto K, Nagao S, Suzuki K, et al. Pelvic fractures after definitive and postoperative radiotherapy for cervical cancer: A retrospective analysis of risk factors. Gynecol Oncol. 2017. December;147(3):585–588. doi: 10.1016/j.ygyno.2017.09.035. Epub 2017 Oct 18. [DOI] [PubMed] [Google Scholar]
- 17.Wade SW, Strader C, Fitzpatrick LA Anthony MS, O’Malley CD. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos. 2014;9:182. doi: 10.1007/s11657-014-0182-3. Epub 2014 May 16. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) 2017. Global Health Observatory data repository. 27 September, 2017. http://apps.who.int/gho/data/view.main.BMIMEANADULTCv?lang=en. Accessed June 4, 2019.
- 19.Asomanin K, Bertone-Johnson ER, Nasca PC, Hooven F, Pekow PS. The association between body mass index and osteoporosis in patients referred for a bone mineral density examination. J Womens Health (Larchmt). 2006. November;15(9):1028–34. [DOI] [PubMed] [Google Scholar]
- 20.Ioffe YJ, Hillen TJ, Zhou G, et al. Postradiation damage to the pelvic girdle in cervical cancer patients: is intensity-modulated radiation therapy safer than conventional radiation? Int J Gynecol Cancer. 2014. May;24(4):806–12. doi: 10.1097/IGC.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 21.Misra S, Lal P, Kumar EpS, et al. Comparative assessment of late toxicity in patients of carcinoma cervix treated by radiotherapy versus chemo-radiotherapy - Minimum 5 years follow up. Cancer Treat Res Commun. 2018;14:30–36. doi: 10.1016/j.ctarc.2017.11.007. Epub 2017 Dec 1. [DOI] [PubMed] [Google Scholar]
- 22.Ramlov A, Pedersen EM, Rohl L, et al. Risk Factors for Pelvic Insufficiency Fractures in Locally Advanced Cervical Cancer Following Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2017. April 1;97(5):1032–1039. doi: 10.1016/j.ijrobp.2017.01.026. Epub 2017 Mar 15. [DOI] [PubMed] [Google Scholar]
- 23.Uezono H, Tsujino K, Moriki K, et al. Pelvic insufficiency fracture after definitive radiotherapy for uterine cervical cancer: retrospective analysis of risk factors. J Radiat Res. 2013. November 1;54(6):1102–9. doi: 10.1093/jrr/rrt055. Epub 2013 May 17. doi: 10.1093/jrr/rrt055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro CL, Van Poznak C, Lacchetti C et al. Management of Osteoporosis in Survivors of Adult Cancers With Nonmetastatic Disease: ASCO Clinical Practice Guideline. J Clin Oncol. 2019. November 1;37(31):2916–2946. doi: 10.1200/JCO.19.01696. Epub 2019 Sep 18. [DOI] [PubMed] [Google Scholar]
- 25.Dybvik E, Furnes O, Fosså SD, Trovik C, Lie SA. Pelvic irradiation does not increase the risk of hip replacement in patients with gynecological cancer. A cohort study based on 8,507 patients. Acta Orthop. 2014. December;85(6):652–6. doi: 10.3109/17453674.2014.963784. Epub 2014 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller PD, Baran DT, Bilezikian JP, et al. Practical clinical application of biochemical markers of bone turnover: Consensus of an expert panel. J Clin Densitom. 1999. Fall;2(3):323–42. [DOI] [PubMed] [Google Scholar]
- 27.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003. September;41(3):208–11. doi: 10.1002/mpo.10338 [DOI] [PubMed] [Google Scholar]
- 28.Nekhlyudov L, Ganz PA, Arora NK and Rowland JH. Going Beyond Being Lost in Transition: A Decade of Progress in Cancer Survivorship. J Clin Oncol. 2017. June 20;35(18):1978–1981. doi: 10.1200/JCO.2016.72.1373. Epub 2017 Apr 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lokich E Gynecologic Cancer Survivorship. Obstet Gynecol Clin North Am. 2019. March;46(1):165–178. DOI: 10.1016/j.ogc.2018.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


