Abstract
Background
Both chronic kidney disease (CKD) and acute kidney injury (AKI) are associated with adverse consequences among patients undergoing percutaneous coronary intervention (PCI). However, it remains undetermined whether the impact of CKD and post-PCI AKI are similar. We aimed to discriminate between the impact of CKD and post-PCI AKI on short- and long-term outcomes.
Methods
This retrospective cohort study included 1,100 patients undergoing PCI at a tertiary hospital. Based on baseline kidney function, patients were categorized as having preserved or impaired renal function, defining as an estimated glomerular filtration rate (eGFR) of ≥ and < 45 ml/min/1.73 m2, respectively. Post-PCI AKI was defined as ≥ 100% relative increase between baseline and post-procedural serum creatinine.
Results
Post-PCI AKI was associated with an increased risk of 90-day mortality [odds ratio (OR): 22.03, 95% confidence interval (CI): 10.36-46.88], long-term mortality [hazard ratio (HR): 6.63, 95% CI: 4.31-10.20], and composite endpoint of future end-stage renal disease (ESRD) and death (HR: 6.19, 95% CI: 4.06-9.42). Impaired kidney function at baseline was associated with an increased risk of future ESRD and composite endpoint of ESRD and death (for every 10 unit increase in eGFR, HR: 0.25, 95% CI: 0.14-0.43, and HR: 0.89, 95% CI: 0.82-0.97, respectively). The impact of post-PCI AKI outweighed that of impaired baseline renal function on short- and long-term prognosis. Patients with impaired baseline renal function who developed AKI following PCI had the worst prognosis.
Conclusions
Both post-PCI AKI and CKD were associated with a poor prognosis. Post-PCI AKI was more important than baseline renal function to predict long-term mortality and composite outcomes. The systemic pathophysiologic change accompanying AKI, rather than renal function per se, may play a crucial role.
Keywords: Acute kidney injury, Chronic kidney disease, End-stage renal disease, Mortality, Percutaneous coronary intervention
INTRODUCTION
Acute kidney injury (AKI) is a common complication among patients undergoing percutaneous coronary intervention (PCI) and is defined as an absolute increase of ≥ 0.3 mg/dL or a relative increase of ≥ 50% in serum creatinine from baseline to peak levels within 48 hours according to the Acute Kidney Injury Network (AKIN) criteria.1 Old age, baseline chronic kidney disease (CKD), diabetes mellitus (DM), acute myocardial infarction (AMI) and higher complexity of infarct-related artery are risk factors for AKI following PCI.2,3
Patients who develop AKI following PCI are associated with poor short-term outcomes, such as in-hospital major bleeding, AMI within 24 hours post-PCI, and in-hospital mortality.2 Among patients with ST-segment elevation myocardial infarction, those who develop AKI after PCI have been reported to have higher rates of major bleeding, death, reinfarction, target vessel revascularization, and stroke within 30 days.4 In addition, AKI after PCI has also been associated with long-term all-cause mortality, progression to end-stage renal disease (ESRD), and subsequent cardiovascular hospitalization or hospitalization with acute renal failure.5 AMI patients who develop AKI following PCI have been reported to have an increased risk of 1-year mortality, stent thrombosis, target lesion revascularization, and major adverse cardiovascular events.6 Among patients with acute coronary syndrome who receive reperfusion therapy such as PCI, thrombolysis, or coronary artery bypass grafting (CABG), those with AKI have been reported to have a higher risk of a composite outcome of death, recurrent nonfatal myocardial infarction and stroke compared to those without AKI.7
In addition to AKI, baseline renal function impairment has also been significantly associated with long-term adverse consequences among patients undergoing PCI.8 Among patients with diabetic nephropathy who receive PCI or CABG for left main coronary artery disease, baseline estimated glomerular filtration rate (eGFR) has been negatively correlated with the risk of long-term adverse consequences including all-cause death, myocardial infarction and stroke.9 The Global Registry of Acute Coronary Events (GRACE) risk score has been demonstrated to better predict outcomes in patients with AMI than the thrombolysis in myocardial infarction (TIMI) score.10 Incorporating renal function into the GRACE risk model is a possible explanation for the better predictive ability. However, whether acute or chronic renal dysfunction plays a similar role is unclear. Furthermore, a previous study reported that both CKD and non-CKD patients who develop AKI following PCI had higher rates of long-term mortality and ESRD, although the effect of AKI following PCI on long-term mortality among CKD patients was not shown after adjustments.11 In addition, it remains undetermined whether the impact of AKI following PCI on the prognosis is similar between patients with and without baseline renal function impairment. In addition, whether the impacts of CKD and post-PCI AKI are similar is also unknown.
The objective of this study was to discriminate between the impact of CKD and AKI following PCI on the prognosis. We explored the impact of post-PCI AKI on short- and long-term outcomes among patients with baseline preserved or impaired renal function who underwent PCI.
METHODS
Data source and study population
We enrolled 4,635 consecutive patients undergoing PCIs from July 01, 2012 through December 31, 2015 at a tertiary care center in southern Taiwan. We excluded patients who received dialysis before PCI (467 patients), those whose baseline (6 patients) or post-procedural (2,961 patients) serum creatinine levels were missing, those whose baseline eGFR was < 15 ml/min/m2 (30 patients), and those who died within 14 days after PCI (71 patients). The final analytic cohort included 1,100 subjects.
Study design
Baseline creatinine was defined as the pre-procedural serum creatinine level within 14 days prior to PCI, and post-procedural peak creatinine was defined as the highest serum creatinine level within 14 days after PCI. AKI was defined as ≥ 100% relative increase in serum creatinine between baseline and post-procedural peak serum creatinine levels, or the initiation of new dialysis after PCI. We used the Modification of Diet in Renal Disease (MDRD) formula to calculate the eGFR. Preserved renal function was defined as baseline eGFR ≥ 45 ml/min/1.73 m2, and impaired renal function was defined as baseline eGFR < 45 ml/min/1.73 m2. Comorbidities including DM, hypertension, dyslipidemia, previous coronary artery disease (prior CAD, including previous myocardial infarction, previous PCI, or previous CABG), previous congestive heart failure (prior CHF), previous cerebrovascular disease (prior CVD), previous peripheral vascular disease (prior PVD), and previous chronic lung disease (prior CLD) were recorded. Indications for PCI were classified as AMI and non-AMI. The short-term endpoint was patient death within 90 days after PCI. The long-term endpoint included ESRD, death, and a composite of ESRD or death. The patients were followed up until death or April 10, 2018.
This study adhered to the Declaration of Helsinki. Because this is a retrospective cohort study and there was no identifiable individual information, the need for informed consent was waived. The Research Ethics Committee of the Chi Mei Medical Center approved this study. All methods in this research were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
We used the chi-square test and t-test to investigate differences in categorical and continuous variables, respectively. Logistic regression analysis was used to explore the risk of 90-day mortality. Cox regression analysis was used to explore the long-term prognosis. We categorized the patients into four groups by baseline kidney function and AKI following PCI to explore the event rates and impact of post-PCI AKI on short- and long-term outcomes. Group 1 included subjects with preserved baseline renal function and without AKI after PCI. Group 2 included subjects with impaired baseline renal function and without AKI after PCI. Group 3 included subjects with preserved baseline renal function and with AKI after PCI. Group 4 included subjects with impaired baseline renal function and with AKI after PCI. We controlled covariates including gender, age, baseline kidney function, comorbidities (including DM, hypertension, dyslipidemia, prior CAD, prior CHF, prior CVD, prior PVD, prior CLD), and indication for PCI (AMI or non-AMI) to demonstrate the risk of short- and long-term adverse consequences. Statistical analysis was performed using SPSS version 22.
RESULTS
Table 1 shows the baseline characteristics of the study cohort and the differences between the patients with and without AKI following PCI. The mean age of the overall cohort was 68.30 years with male predominance (74.4%). The mean baseline eGFR was 51.75 ml/min/1.73 m2. Among the study subjects, 627 (57.0%) had preserved renal function (baseline eGFR ≥ 45 ml/min/1.73 m2) and 473 (43.0%) had impaired renal function (baseline eGFR < 45 ml/min/1.73 m2). The study subjects had a high prevalence of comorbidities including diabetes (52.2%), hypertension (73.3%), dyslipidemia (58.5%), and prior CAD (47.7%). Of the 1,100 patients in our study cohort, 54 (4.9%) experienced AKI after PCI. Compared to the patients without post-PCI AKI, those with AKI following PCI had a significantly worse baseline kidney function, and were more likely to have prior CAD. Patients who received PCI for the indication of AMI tended to develop AKI following PCI.
Table 1. Baseline characteristics of the study cohort and patients with [AKI (+)] and without AKI [AKI (−)].
| Total (n = 1100) | AKI (−) (n = 1046, 95.1%) | AKI (+) (n = 54, 4.9%) | p value | |
| Age (years) | 68.30 ± 12.01 | 68.27 ± 12.01 | 68.90 ± 11.99 | 0.709 |
| Male (n, %) | 818 (74.4%) | 788 (75.3%) | 30 (55.6%) | 0.001 |
| Baseline eGFR | 51.75 ± 21.98 | 52.26 ± 21.64 | 41.82 ± 26.08 | < 0.001 |
| Baseline kidney function | < 0.001 | |||
| Impaired (eGFR < 45) | 473 (43.0%) | 434 (41.5%) | 39 (72.2%) | |
| Preserved (eGFR ≥ 45) | 627 (57.0%) | 612 (58.5%) | 15 (27.8%) | |
| Indication | 0.01 | |||
| AMI (n, %) | 526 (47.8%) | 491 (46.9%) | 35 (64.8%) | |
| Non-AMI (n, %) | 574 (52.2%) | 555 (53.1%) | 19 (35.2%) | |
| Comorbidity (n, %) | ||||
| DM | 574 (52.2%) | 539 (51.5%) | 35 (64.8%) | 0.057 |
| HTN | 806 (73.3%) | 764 (73.0%) | 42 (77.8%) | 0.443 |
| Dyslipidemia | 643 (58.5%) | 618 (59.1%) | 25 (46.3%) | 0.063 |
| Prior CAD | 525 (47.7%) | 507 (48.5%) | 18 (33.3%) | 0.030 |
| Prior CHF | 150 (13.6%) | 141 (13.5%) | 9 (16.7%) | 0.506 |
| Prior CVD | 151 (13.7%) | 139 (13.3%) | 12 (22.2%) | 0.063 |
| Prior PVD | 37 (3.4%) | 35 (3.3%) | 2 (3.7%) | 0.887 |
| Prior CLD | 71 (6.5%) | 68 (6.5%) | 3 (5.6%) | 0.783 |
| Follow-up days (median, IQR) | 1075 (1068.5) | 1044 (616) | 476 (593) |
AMI, acute myocardial infarction; CAD, coronary artery disease; CHF, congestive heart failure; CLD, chronic lung disease; CVD, cerebrovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); HTN, hypertension; PVD, peripheral vascular disease.
The patients who developed AKI after PCI had higher rates of adverse consequences, including 90-day mortality (37.0% vs. 2.5%, p < 0.001), ESRD (7.4% vs. 1.8%, p < 0.01), and long-term mortality (55.6% vs. 13.9%, p < 0.001) compared to those without post-PCI AKI. After adjusting for gender, age, baseline eGFR, comorbidities (including diabetes, hypertension, dyslipidemia, prior CAD, prior CHF, prior CVD, prior PVD, prior CLD), and indication for PCI (AMI or non-AMI), AKI following PCI was associated with a significantly higher risk of 90-day mortality [adjusted odds ratio (aOR): 22.03, 95% confidence interval (CI): 10.36-46.88; p < 0.001], long-term mortality (aOR: 7.10, 95% CI: 3.83-13.17; p < 0.001), and composite endpoint of ESRD and long-term mortality (aOR: 6.43, 95% CI: 3.46-11.93; p < 0.001). Impaired kidney function at baseline was also associated with an increased risk of ESRD (aOR: 29.73, 95% CI: 3.82-231.09; p < 0.01), long-term mortality (aOR: 1.50, 95% CI: 1.03-2.17; p < 0.05), and composite endpoint of ESRD and long-term mortality (aOR: 1.86, 95% CI: 1.30-2.66; p < 0.001) after adjusting for gender, age, post-PCI AKI status, comorbidities, and indication for PCI.
Table 2 shows the event rate by baseline kidney function and post-PCI AKI status. Among the patients with impaired renal function (Group 2 & 4), those who developed AKI after PCI (Group 4) had higher rates of 90-day mortality (43.6% vs. 3.2%, p < 0.001), ESRD (10.3% vs. 4.1%, p = 0.083), and long-term mortality (66.7% vs. 18.9%, p < 0.001) compared to those without post-PCI AKI (Group 2). Similarly, among the patients with preserved renal function (Group 1 & 3), those who developed AKI after PCI (Group 3) had higher 90-day mortality (20.0% vs. 2.0%, p < 0.001) and long-term mortality (26.7% vs. 10.3%, p < 0.05) compared to those without post-PCI AKI (Group 1). Between the patients with impaired renal function at baseline (Group 2) and post-PCI AKI (Group 3), those with post-PCI AKI (Group 3) had a higher rate of 90-day mortality, but the rates of long-term outcome, including ESRD and mortality, were similar. Among the four groups, the patients with impaired kidney function at baseline who developing AKI after PCI had the highest rates of 90-day mortality, future ESRD, and long-term mortality.
Table 2. Numbers of outcome events of the four groups categorized by baseline kidney function and post-PCI AKI status.
| Group 1 (n = 612) | Group 2 (n = 434) | Group 3 (n = 15) | Group 4 (n = 39) | |
| 90-day mortality (n, %) | 12 (2.0%)* | 14 (3.2%)† | 3 (20.0%)‡ | 17 (43.6%)§ |
| ESRD (n, %) | 1 (0.2%) | 18 (4.1%) | 0 | 4 (10.3%)§ |
| Long-term mortality (n, %) | 63 (10.3%)# | 82 (18.9%)† | 4 (26.7%) | 26 (66.7%)§ |
Group 1: baseline eGFR ≥ 45 ml/min/1.73 m2 and no AKI following PCI; Group 2: baseline eGFR < 45 ml/min/1.73 m2 and no AKI following PCI; Group 3: baseline eGFR ≥ 45 ml/min/1.73 m2 and developing AKI following PCI; Group 4: baseline eGFR < 45 ml/min/1.73 m2 and developing AKI following PCI.
AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; PCI, percutaneous coronary intervention.
* Group 1 vs. 3, p value < 0.001. # Group 1 vs. 3, p value < 0.05. † Group 2 vs. 4, p value < 0.001. ‡ Group 2 vs. 3, p value < 0.001. § p value between 4 groups < 0.001.
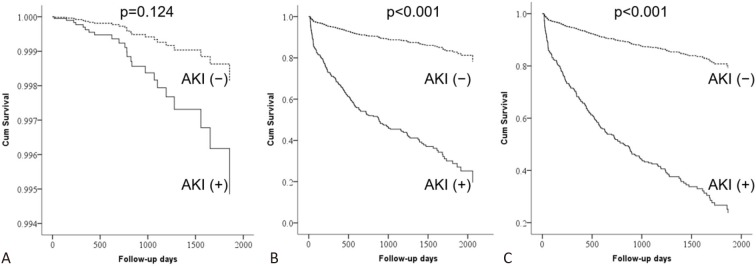
Table 3 shows the independent risk factors for future ESRD, long-term mortality, and composite endpoints. After a median follow-up duration of 1075 days, the patients with AKI following PCI had higher risks of long-term mortality [adjusted hazard ratio (aHR): 6.63, 95% CI: 4.31-10.20; p < 0.001] and composite endpoints of future ESRD and long-term mortality (aHR: 6.19, 95% CI: 4.06-9.42; p < 0.001). The risk of post-PCI AKI on future ESRD was also increased, but the difference did not reach significance (aHR: 2.79, 95% CI: 0.75-10.33; p = 0.124) (Figure 1). Baseline eGFR was also significantly associated with the risk of future ESRD and composite endpoint of future ESRD and long-term mortality.
Table 3. Independent risk factors of end-stage renal disease (ESRD), long-term mortality, and composite endpoints among the overall study cohort.
| AHR for ESRD (95% C.I.) | AHR for death (95% C.I.) | AHR for composite endpoint (95% C.I.) | |
| Male (vs. female) | 1.66 (0.62, 4.41) | 0.78 (0.56, 1.08) | 0.79 (0.57, 1.08) |
| Age (per 10 years) | 0.65 (0.41, 1.04) | 1.57 (1.34, 1.84)† | 1.43 (1.23, 1.66)† |
| eGFR (per 10 units) | 0.25 (0.14, 0.43)† | 0.96 (0.88, 1.04) | 0.89 (0.82, 0.97)# |
| AKI | 2.79 (0.75, 10.33) | 6.63 (4.31, 10.20)† | 6.19 (4.06, 9.42)† |
| DM | 8.81 (1.14, 68.08)* | 1.57 (1.13, 2.18)# | 1.69 (1.23, 2.33)# |
| Hypertension | 5.15 (0.64, 41.73) | 0.96 (0.65, 1.40) | 1.04 (0.71, 1.51) |
| Dyslipidemia | 1.02 (0.37, 2.83) | 0.74 (0.54, 1.00)* | 0.79 (0.59, 1.05) |
| Prior CAD | 0.55 (0.21, 1.45) | 1.26 (0.90, 1.77) | 1.20 (0.87, 1.66) |
| Prior CHF | 1.38 (0.45, 4.19) | 1.67 (1.16, 2.42)# | 1.56 (1.10, 2.22)* |
| Prior CVD | 1.00 (0.32, 3.14) | 1.62 (1.13, 2.33)# | 1.59 (1.12, 2.27)# |
| Prior PVD | 0.63 (0.07, 5.52) | 1.01 (0.49, 2.07) | 0.92 (0.45, 1.89) |
| Prior CLD | 0.85 (0.10, 7.47) | 1.10 (0.62, 1.93) | 1.16 (0.67, 1.99) |
| AMI (vs. non-AMI) | 0.29 (0.10, 0.81)* | 1.23 (0.89, 1.70) | 1.09 (0.80, 1.49) |
AHR, adjusted hazard ratios; AKI, acute kidney injury; AMI, acute myocardial infarction; CAD, coronary artery disease; CHF, congestive heart failure; CLD, chronic lung disease; CVD, cerebrovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); PVD, peripheral vascular disease; 95% C.I., 95% confidence interval.
* p < 0.05; # p < 0.01; † p < 0.001.
Figure 1.
Survival curves of patients with [AKI (+)] and without AKI [AKI (-)] for (A) end-stage renal disease (ESRD), (B) death, and (C) composite of ESRD or death. AKI, acute kidney injury.
Among the patients with impaired baseline renal function (Group 2 & 4), AKI following PCI (Group 4) was associated with higher risks of future ESRD (aHR: 3.40, 95% CI: 0.92-12.62; p = 0.067), long-term mortality (aHR: 6.13, 95% CI: 3.61-10.41; p < 0.001), and composite endpoint (aHR: 5.43, 95% CI: 3.29-8.96; p < 0.001). Similarly, among the patients with preserved baseline renal function (Group 1 & 3), AKI following PCI (Group 3) was associated with higher risks of long-term mortality (aHR: 3.80, 95% CI: 1.33-10.88; p = 0.013) and composite endpoint (aHR: 3.76, 95% CI: 1.32-10.74; p = 0.013). There were not enough events of ESRD in this group to calculate the risk.
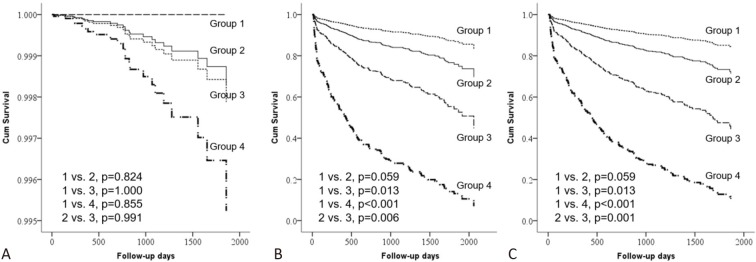
Among the four groups, the patients with impaired renal function at baseline who developed AKI after PCI (Group 4) had the worst long-term mortality and composite of ESRD and death (Table 4 and Figure 2). The risk of future ESRD also tended to be highest among Group 4, but without significance because of the small number of cases. The risks of long-term mortality (p = 0.006) and composite endpoint (p = 0.001) were higher in Group 3 than in Group 2, implicating that the impact of AKI following PCI on prognosis outweighed that of impaired renal function at baseline.
Table 4. Risk of end-stage renal disease (ESRD), long-term mortality, and composite endpoints among the four groups categorized by baseline kidney function and post-PCI AKI status.
| AHR for ESRD (95% C.I.) | AHR for death (95% C.I.) | AHR for composite endpoint (95% C.I.) | |
| Group 1 | Ref. | Ref. | Ref. |
| Group 2 | 0.80 (0.06, 10.72) | 1.96 (1.16, 3.32)* | 1.92 (1.16, 3.20)* |
| Group 3 | NA | 4.36 (1.56, 12.19)# | 4.61 (1.66, 12.85)# |
| Group 4 | 2.25 (0.12, 43.88) | 14.45 (7.15, 29.20)† | 12.73 (6.42, 25.24)† |
Group 1: baseline eGFR ≥ 45 ml/min/1.73 m2 and no AKI following PCI; Group 2: baseline eGFR < 45 ml/min/1.73 m2 and no AKI following PCI; Group 3: baseline eGFR ≥ 45 ml/min/1.73 m2 and AKI following PCI; Group 4: baseline eGFR < 45 ml/min/1.73 m2 and AKI following PCI.
AHR, adjusted hazard ratios; AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; NA, not applicable; PCI, percutaneous coronary intervention; 95% C.I., 95% confidence interval.
* p < 0.05; # p < 0.01; † p < 0.001.
Figure 2.
Survival curves of the four groups for (A) end-stage renal disease (ESRD), (B) death, and (C) composite of ESRD or death. Group 1: baseline eGFR ≥ 45 ml/min/1.73 m2 and no AKI following PCI; Group 2: baseline eGFR < 45 ml/min/1.73 m2 and no AKI following PCI; Group 3: baseline eGFR ≥ 45 ml/min/1.73 m2 and AKI following PCI; Group 4: baseline eGFR < 45 ml/min/1.73 m2 and AKI following PCI. AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention.
DISCUSSION
The main finding of this study is that patients with AKI following PCI were associated with significant risks of short- and long-term mortality, irrespective of the baseline kidney function. AKI following PCI and baseline CKD had additive effects on future ESRD and long-term mortality. Furthermore, the impact of AKI following PCI on short- and long-term mortality and composite endpoint of future ESRD and death outweighed that of baseline CKD.
In this study, we failed to demonstrate the significance of post-PCI AKI and baseline impairment of renal function on the risk of future ESRD. One possible explanation is that the number of cases was relatively small, leading to insufficient power to establish the significance. Another explanation is that the competing risks of future ESRD and death may confound the estimates of risk for each outcome. We found that there was a significantly increased risk of AKI following PCI and baseline renal function impairment on the composite endpoint of future ESRD and death.
Adverse short-term outcomes following post-PCI AKI have been frequently reported, and the greater the degree of the increase in serum creatinine after coronary angiography, the greater the risks of in-hospital mortality and longer length-of-stay.12 Among patients undergoing PCI, post-PCI AKI with increasing severity has been associated with graded higher rates of in-hospital mortality, major bleeding and recurrent myocardial infarction, and even higher for those requiring dialysis.2 Compared to patients without AKI, patients with AKI after PCI for acute coronary syndrome have been reported to have significantly higher rates of 30-day mortality, recurrent MI, target lesion revascularization, target vessel revascularization, and major bleeding.6 The deterioration in renal function may reflect an ongoing adverse event in the short term. In the current study, we excluded those who died within 14 days after PCI to eliminate the confounding effect of critical illness, which may have led to post-PCI AKI and short-term mortality. We found a higher short-term mortality risk within 90 days among the patients who developed AKI after PCI.
Although the association between post-PCI AKI and short-term adverse effects is well recognized, few studies have reported the long-term outcomes of AKI following PCI. Among non-ST-segment elevation myocardial infarction (NSTEMI) patients who undergo PCI, the association between post-PCI AKI and long-term mortality risk has not been established.13 In addition, one study reported that AKI following PCI was not an independent risk factor for long-term mortality or hospitalization for cardiovascular disease after excluding patients with shock, intra-aortic balloon pump or percutaneous cardiopulmonary support, endotracheal intubation, or catecholamine infusion within 12 hours of PCI.8
In contrast, AKI after coronary angiography has been associated with long-term mortality, ESRD, and cardiovascular and renal hospitalizations. The risk of long-term adverse consequences has also been reported to be increased in a graded manner with increasing severity of post-PCI AKI.5 Moreover, AKI following coronary angiography has been associated with a rapid decline in kidney function. A previous study reported that the rate of eGFR decline following AKI accelerated with the severity AKI compared to the pre-angiography rate of progression.14 Among patients undergoing PCI for acute coronary syndrome, those with AKI following PCI have been reported to be at higher risks of 1-year mortality, target lesion revascularization, target vessel revascularization, and major bleeding, compared to those without post-PCI AKI.6 In the current study, we also found a significant association between AKI following PCI and long-term mortality. The risk of future ESRD also tended to be higher, although significance was not demonstrated.
A previous study reported that AKI after coronary angiography was associated with increased risks of long-term mortality, cardiovascular events, ESRD, and prolonged hospitalization. However, after adjusting for baseline clinical characteristics, the association between AKI and adverse consequences was attenuated.15 Another study reported that among patients with baseline CKD, AKI following coronary angiography was not an independent risk factor for long-term adverse outcomes, including mortality and progression to ESRD.11 In contrast to their results, we found a significant association between post-PCI AKI and long-term adverse consequences both among the patients with preserved and impaired renal function at baseline. Furthermore, we found that the prognosis among patients with baseline preserved renal function who developed post-PCI AKI was worse than for those with baseline impaired renal function who did not develop AKI following PCI. The impact of AKI following PCI seemed to outweigh baseline CKD on long-term outcomes.
AKI is associated with increased long-term mortality, and non-recovery of kidney function is associated with an even greater risk of mortality and CKD outcomes.16 Patients with persistent renal dysfunction after a post-PCI AKI episode have been associated with a higher risk of 2-year mortality or dialysis compared to those with transient renal dysfunction or no AKI among AMI patients undergoing PCI.17 Persistent renal damage after coronary angiography has been associated with higher risks of death and combined endpoint of death, dialysis and cardiovascular events at 5 years.18 An absolute increase in creatinine of ≥ 0.5 mg/dl or a relative increase in creatinine ≥ 25% above baseline from 6 to 8 months after PCI among patients with acute coronary syndrome has been associated with a significantly higher 5-year mortality rate.19 In the current study, we did not examine the impact of AKI severity or persistency on the outcomes. Instead, we explored the interaction between baseline kidney function and post-PCI AKI on the long-term outcomes. We found that the patients with impaired baseline kidney function who developed AKI following PCI had the worst long-term outcomes.
Associations between AKI and risk of CKD progression or ESRD have been reported. Persistent renal ischemia, maladaptive repair, activation of fibroblasts and renal fibrosis are key components in the pathogenesis of AKI to CKD progression.20-22 On the other hand, the effects of AKI after PCI on the long-term risk of mortality are less clear. One possible explanation is that patients who develop AKI following PCI tend to have numerous comorbidities such as diabetes, hypertension, heart failure, and lower glomerular filtration rates, each of which may increase the risk of progression to kidney failure and death. However, the associations remained significant after adjusting for comorbidities, suggesting that confounding by these characteristics does not completely explain our findings. However, it is possible that some residual confounders may have existed and influenced the relationship between post-PCI AKI and long-term mortality. In addition, AKI may contribute to a persistent loss of kidney function,23 faster decline in kidney function,14 and future risk of ESRD.5 Renal dysfunction is associated with cardiovascular disease and may predispose patients to develop coronary heart disease and death.24
Strengths and limitations
This study expands the knowledge on the prognostic implications of AKI following PCI. We found that patients with baseline impaired renal function who developed AKI after PCI had the worst long-term outcomes. We also found that the impact of post-PCI AKI on short- and long-term prognosis outweighed that of baseline CKD. Our findings suggest the need for clinical follow-up and further management to reduce progressive renal failure and death in patients who develop AKI following PCI, especially in those with baseline renal insufficiency. However, this study is not without limitations. First, this is a retrospective cohort study, in which some of the information may be biased. For example, there were many patients without follow-up serum creatinine measurements after PCI who were excluded. Most of these patients were considered to not have AKI following PCI. In fact, the patients without serum creatinine measurements after PCI and were thus excluded from this study were younger, had fewer comorbidities, less complex disease, and shorter hospital length of stay compared to those who had post-PCI creatinine level measurements (data not shown). Excluding these subjects may have biased our findings to null. However, we still found a significant association between post-PCI AKI and short- and long-term adverse outcomes. In addition, we adapted our AKI definition from the AKIN criteria, which define AKI as an absolute increase of ≥ 0.3 mg/dL or a relative increase of ≥ 50% in serum creatinine from baseline to peak levels within 48 hours.1 Because of the retrospective design, we could not collect information on changes in serum creatinine in a 48-hour interval. Therefore, we defined AKI as ≥ 100% relative increase in serum creatinine between baseline and post-procedural peak levels, which was a more strict definition of AKI. Indeed, we found that even a small increase in serum creatinine was also associated with an increased risk of adverse short- and long-term outcomes (data not shown). Moreover, we could not address the causes of AKI following PCI among our patients. Contrast medium exposure is a major cause of AKI following PCI, however other comorbid conditions such as acute coronary syndrome-related hemodynamic compromise may also contribute to the development of AKI following PCI. It was difficult to determine the true etiology of AKI among these patients. Nevertheless, the focus of this study was on the consequences of post-PCI AKI rather than the causes. Moreover, the complexity of vascular lesions and PCI procedures, contrast volume, and left ventricular dysfunction may also influence the possibility of post-PCI AKI and subsequent outcomes. However, these data were not routinely recorded, and we were unable to collect complete data to control them as potential confounding factors. Prospectively collecting data from PCI patients would be helpful to estimate the true long-term risk of AKI among this population. Second, several patients received more than two PCI procedures during the inclusion period. The former data were censored, which may not be valid. Finally, as an observational study, the causal relationship between post-PCI AKI and long-term adverse consequence could not be established. Several confounders may have influenced the long-term outcomes among the PCI patients who developed AKI.
AKI following PCI is associated with increased risks of short- and long-term adverse consequences. The administration of pre- and post-procedural hydration,2 restriction of contrast volume, and correction of modifiable risk factors may reduce the risk of AKI following PCI.26 Clinicians should strive to prevent the occurrence of AKI among patients undergoing PCI. However, a delayed time to reperfusion may also increase the risk of AKI.27 Implementation of prevention strategies should not delay the time to reperfusion.
CONCLUSION
AKI following PCI was associated with adverse outcomes, including 90-day mortality, future ESRD, and long-term mortality. Patients with impaired renal function at baseline who developed AKI following PCI had even higher risks of short- and long-term adverse consequences. Furthermore, we found that post-PCI AKI had a higher impact on the prognosis than baseline renal function impairment. This indicates that systemic pathophysiologic changes accompanying AKI, rather than renal function per se, may play a more important role on the development of these adverse consequences. Further studies are needed to clarify the causal relationship and underlying mechanism between AKI following PCI and adverse outcomes.
Acknowledgments
The author would like to thank all colleagues who contributed to this study. Thanks for helping in data collection and analysis.
CONFLICT OF INTEREST
The only author, me, declares no competing interests. I have no financial or non-financial relationship with a commercial entity that has an interest in the subject of this manuscript.
REFERENCES
- 1.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai TT, Patel UD, Chang TI, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. doi: 10.1016/j.jcin.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu CW, Liao PC, Chen KC, et al. SYNTAX Score of infarct-related artery other than the number of coronary balloon inflations and deflations as an independent predictor of contrast-induced acute kidney injury in patients with ST-segment elevation myocardial infarction. Acta Cardiol Sin. 2017;33:362–376. doi: 10.6515/ACS20161130A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narula A, Mehran R, Weisz G, et al. Contrast-induced acute kidney injury after primary percutaneous coronary intervention: results from the HORIZONS-AMI substudy. Eur Heart J. 2014;35:1533–1540. doi: 10.1093/eurheartj/ehu063. [DOI] [PubMed] [Google Scholar]
- 5.James MT, Ghali WA, Knudtson ML, et al. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–416. doi: 10.1161/CIRCULATIONAHA.110.970160. [DOI] [PubMed] [Google Scholar]
- 6.Giacoppo D, Madhavan MV, Baber U, et al. Impact of contrast-induced acute kidney injury after percutaneous coronary intervention on short-and long-term outcomes: pooled analysis from the HORIZONS-AMI and ACUITY trials. Circ Cardiovasc Interv. 2015;8:e002475. doi: 10.1161/CIRCINTERVENTIONS.114.002475. [DOI] [PubMed] [Google Scholar]
- 7.Chen KC, Yin WH, Wu CC, et al. In-hospital implementation of evidence-based medications is associated with improved survival in diabetic patients with acute coronary syndrome - data from TSOC ACS-DM Registry. Acta Cardiol Sin. 2018;34:211–223. doi: 10.6515/ACS.201805_34(3).20180207B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura T, Obi Y, Yasuda K, et al. Effects of chronic kidney disease and post-angiographic acute kidney injury on long-term prognosis after coronary artery angiography. Nephrol Dial Transplant. 2010;26:1838–1846. doi: 10.1093/ndt/gfq631. [DOI] [PubMed] [Google Scholar]
- 9.Li HR, Hsu CP, Sung SH, et al. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with diabetic nephropathy and left main coronary artery disease. Acta Cardiol Sin. 2017;33:119–126. doi: 10.6515/ACS20160623A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YH, Huang SS, Lin SJ. TIMI and GRACE risk scores predict both short-term and long-term outcomes in Chinese patients with acute myocardial infarction. Acta Cardiol Sin. 2018;34:4–12. doi: 10.6515/ACS.201801_34(1).20170730B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neyra JA, Shah S, Mooney R, et al. Contrast-induced acute kidney injury following coronary angiography: a cohort study of hospitalized patients with or without chronic kidney disease. Nephrol Dial Transplant. 2013;28:1463–1471. doi: 10.1093/ndt/gft082. [DOI] [PubMed] [Google Scholar]
- 12.Weisbord SD, Chen H, Stone RA, et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol. 2006;17:2871–2877. doi: 10.1681/ASN.2006030301. [DOI] [PubMed] [Google Scholar]
- 13.Turan B, Erkol A, Gül M, et al. Effect of contrast-induced nephropathy on the long-term outcome of patients with non-ST segment elevation myocardial infarction. Cardiorenal Med. 2015;5:116–124. doi: 10.1159/000371900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James MT, Ghali WA, Tonelli M, et al. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78:803–809. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 15.James MT, Samuel SM, Manning MA, et al. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: a systematic review and meta-analysis. Circ Cardiovasc Interv. 2013;6:37–43. doi: 10.1161/CIRCINTERVENTIONS.112.974493. [DOI] [PubMed] [Google Scholar]
- 16.Sawhney S, Mitchell M, Marks A, et al. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open. 2015;5:e006497. doi: 10.1136/bmjopen-2014-006497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wi J, Ko YG, Kim JS, et al. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97:1753–1757. doi: 10.1136/hrt.2010.218677. [DOI] [PubMed] [Google Scholar]
- 18.Maioli M, Toso A, Leoncini M, et al. Persistent renal damage after contrast-induced acute kidney injury: incidence, evolution, risk factors and prognosis. Circulation. 2012;125:3099–3107. doi: 10.1161/CIRCULATIONAHA.111.085290. [DOI] [PubMed] [Google Scholar]
- 19.Nemoto N, Iwasaki M, Nakanishi M, et al. Impact of continuous deterioration of kidney function 6 to 8 months after percutaneous coronary intervention for acute coronary syndrome. Am J Cardiol. 2014;113:1647–1651. doi: 10.1016/j.amjcard.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Basile DP, Bonventre JV, Mehta R, et al. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol. 2016;27:687–697. doi: 10.1681/ASN.2015030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humphreys BD, Cantaluppi V, Portilla D, et al. Targeting endogenous repair pathways after AKI. J Am Soc Nephrol. 2016;27:990–998. doi: 10.1681/ASN.2015030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devarajan P, Jefferies JL. Progression of chronic kidney disease after acute kidney injury. Prog Pediatr Cardiol. 2016;41:33–40. doi: 10.1016/j.ppedcard.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu CY, Chertow GM, McCulloch CE, et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Angelantonio E, Chowdhury R, Sarwar N, et al. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ. 2010;341:c4986. doi: 10.1136/bmj.c4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 26.Gurm HS, Dixon SR, Smith DE, et al. Renal function-based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2011;58:907–914. doi: 10.1016/j.jacc.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Shacham Y, Leshem-Rubinow E, Gal-Oz A, et al. Acute cardio-renal syndrome as a cause for renal deterioration among myocardial infarction patients treated with primary percutaneous intervention. Can J Cardiol. 2015;31:1240–1244. doi: 10.1016/j.cjca.2015.03.031. [DOI] [PubMed] [Google Scholar]