Abstract
Background
Moderate autophagy plays a positive role in the prevention of atherosclerosis. Aberrant promoter methylation of autophagic genes can affect autophagy. Shen-Yuan-Dan Capsule (SYDC), a traditional Chinese medicine, can prevent atherosclerosis in high-fat-fed mice. However, its precise mechanism remains unclear. This study investigated the mechanism of SYDC in ameliorating atherosclerosis in a mice model.
Methods
After 6 weeks of a high-fat diet, apolipoprotein E knockout (apoE–/–) mice were randomly grouped into control, Lipitor, and SYDC groups (n = 10). The mice were intragastrically administered with the respective drugs for 6 weeks. The expressions of Beclin1 and Atg5-Atg12 complex in atherosclerotic plaques of the mice were measured. The levels of 5-mC and DNA methyltransferase 1 (DNMT1) in the plasma of the mice were determined. The average methylation rate of CpG islands in the promoter region of autophagy-related protein (Atg13) and the mRNA expression of Atg13 in the aortas of the mice were determined.
Results
SYDC up-regulated the expressions of Atg5-Atg12 complex and Beclin-1 in atherosclerotic plaques (p < 0.01). Moreover, SYDC decreased the 5-mC and DNMT1 levels in plasma and atherosclerotic plaques of the mice (p < 0.01), and also decreased the average methylation rate of CpG islands in the promoter region of Atg13 and increased the mRNA levels of Atg13 in the aortas of atherosclerotic mice (p < 0.01).
Conclusions
SYDC attenuates atherosclerosis by promoting autophagy, probably through regulating genomic DNA methylation and Atg13 promoter demethylation.
Keywords: Atherosclerosis, Atg13, Autophagy, DNA methylation, Shen-Yuan-Dan Capsule
INTRODUCTION
Atherosclerosis is involved in altering gene expressions and the corresponding activities of these gene products. Epigenetic changes, especially aberrant deoxyribonucleic acid (DNA) methylation, is an important mechanism that controls gene expression in the development of atherosclerosis.1 Alterations in DNA methylation profiles have been shown to be early markers of atherosclerosis in apolipoprotein E knockout (apoE–/–) mice, and atherogenic lipoproteins have been shown to induce DNA hypermethylation in cultured cells.2 In addition, inhibition of DNA methyltransferase 1 (DNMT1) by siRNA has been shown to inhibit endothelial inflammation and lesion formation.3 Taken together, these findings suggest that DNA methylation is involved in the pathogenesis of atherosclerosis.
Autophagy is the process by which lysosomes can degrade damaged organelles and macromolecules in cells under the regulation of autophagic genes.4 In the early stage of atherosclerotic lesions, autophagy degrades the damaged structures in cells to adapt to environmental changes such as inflammation and hypoxia, and delay the development of atherosclerotic plaques.5,6 Therefore, inducing autophagy may be an effective approach to attenuate atherosclerosis.
Aberrant DNA methylation of autophagic genes can regulate autophagy. Beclin1 is a key regulator of autophagy and an essential molecule for the formation of autophagic bodies. Aberrant methylation status in the Beclin1 promoter region has been shown in human umbilical vein endothelial cells injured by oxidized low-density lipoprotein treatment.7 In addition, Sutter et al. showed that DNA methylation modification of protein phosphatase 2 was regulated, and affected the mRNA expressions of autophagic genes such as Beclin1.8 Therefore, DNA methylation can affect autophagy and induce the formation and development of atherosclerosis.
Shen-Yuan-Dan Capsule (SYDC) is a traditional Chinese medicine (TCM) compound that has been confirmed to effectively treat coronary heart disease and angina pectoris.9,10 It has also been shown to protect against myocardial ischemia/reperfusion injury, ameliorate oxygen-free radical injury in ischemic myocardium, and improve the antioxidant ability of myocardial tissue.11-13 Our previous studies have shown that SYDC can prevent atherosclerosis by inhibiting the phosphatidylinositol 3′-kinase/Akt/nuclear factor-kappa B pathway in apoE–/– mice fed a high-fat diet.14 However, the precise mechanism for the anti-atherosclerotic properties of SYDC has yet to be elucidated. Therefore, the aim of this study was to evaluate the role of SYDC on autophagy and DNA methylation in atherosclerotic mice.
MATERIALS AND METHODS
Animals and ethics
Male ApoE–/– mice in the C57BL/6J background (n = 30, 8 weeks of age, weight 18-20 g) and 6 wild-type (WT) C57BL/6J mice were purchased from Beijing WeitongLiHua Experimental Technology Co. Ltd. (Beijing, China). All animal research conformed to the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23) and was approved by the Ethics Review Board for Animal Studies of Peking University Health Science Center (Permit Number: IMM-GuYC-1).
Reagents
SYDC was provided by the manufacturing laboratory of Beijing TCM Hospital (Beijing, China, Z20053327). Atorvastatin calcium was purchased from Pfizer Pharmaceutical Co., Ltd. (Shanghai, China, H20051408). Kits for assessing DNMT1 were purchased from R&D Systems (CK-E433652M, USA). Antibodies against Beclin-1 (Cat#: ab62557), 5-mC (Cat#: ab10805) and DNMT1(Cat#: ab 4870) were purchased from Abcam (Cambridge, UK). Antibody against Atg5-Atg12 complex (Cat#: LS-B9976/101817) was purchased from Life Span Biosciences, Inc. (USA; Lot#101817).
Establishment of the atherosclerotic model and drug treatment
All of the ApoE–/– mice were fed with a high-fat diet containing 21% (wt/wt) fat supplemented with 0.15% (wt/wt) cholesterol15 from Beijing Ke’aoXieli Feed Co. Ltd. (Beijing, China) for 12 weeks. After 6 weeks of high-fat diet feeding, the ApoE–/– mice were randomized into control, atorvastatin (positive-control group, 3.34 mg/kg), and SYDC (SYDC, 80 mg/kg) groups (n = 10). All mice were orally gavaged for 6 weeks in combination with a high-fat diet. The dose of SYDC was selected as the clinically relevant dose in humans. The medical dose in mice is 9.01 times than that used for humans.16 The six WT C57BL/6J mice were fed with a standard chow diet for 12 weeks.
Histology
After 6 weeks of treatment, all mice were euthanized with 0.1% pentobarbital sodium. The heart of each mouse was removed, and one-third of the apical heart including the aortic sinus was fixed in 10% formaldehyde, embedded in paraffin, and sectioned. Aorta samples, except for the aortic root in each mouse, were removed and stored at -80 °C. Blood samples were collected from the left ventricle of male ApoE–/– mice after feeding with a high-fat diet for 12 weeks.
Assessment of genomic DNA methylation and DNMT1 levels
Genomic DNA methylation levels in mouse plasma were assessed using high-performance liquid chromatography (HPLC). An improved SDS method was used to extract DNA. In a 100 μL DNA solution containing 25 μg DNA, 50 μL of 70% perchloric acid was added, and the pH of the mixture was adjusted to 3.0-5.0 with 1 mol/L KOH. After high-speed centrifugation, the filtered supernatant was used for HPLC analysis. The retention times corresponding to C and 5-methylcytosine (5-mC) were 7.2 and 12.7 min, respectively. Using the formula, 5-mC (%) = C5-mC/(C5-mC + CC) × 100%, the amount of 5-mC was determined. The percent of 5-mC represented the level of methylated DNA. The DNMT1 levels in mouse plasma were measured using enzyme-linked immunosorbent assay (ELISA) kits.
Immunocytochemistry
Immunocytochemistry was performed using the same method as in our previous study.14 The sections were incubated overnight at 4 °C with anti-Atg5-Atg12 complex (1:200), Beclin-1 (1:400), 5-mC (1:200) and DNMT1 (1:400) antibody.
Real-time quantitative polymerase chain reaction (PCR)
Total ribonucleic acid (RNA) was isolated using TRIzol reagent (Invitrogen). Reverse transcription (RT) was performed with 2 μg of total RNA using a first-strand cDNA kit (Takara). PCR amplification was performed for 2 min at 95 °C, followed by 40 cycles of 94 °C for 10 s, and annealing/extension at 60 °C for 10 s on an ABI 7300 Thermocycler (Applied Biosystems), using a SYBRPremix Ex Taq kit (Takara). The specific gene expression primers used in quantitative RT-PCR are listed in Table 1. Data analysis was performed using the 2–ΔΔCT method for relative quantification. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Proteintech Group) was used as the reference gene.
Table 1. The primers in RT-PCR of this study.
| Gene | Forward | Reverse |
| Atg3 | 5′-ATGAGCAACGGCAGCCTTTA-3′ | 5′-ATCACTTCAGCATGCCTGCA-3′ |
| Atg13 | 5′-ATTTCAGAACCCCCCTCAGC-3′ | 5′-TCATGCACAGCCAGCTTCTC-3′ |
| Atg4a | 5′-AAACCCCTGCTGCTCATTGT-3′ | 5′-GCCCCTAAAGACTGTGGCAT-3′ |
| GAPDH | 5′-GGCAAATTCAACGGCACAGT-3′ | 5′-ACGACATACTCAGCACCGGC-3′ |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; RT-PCR, reverse transcription polymerase chain reaction.
Validation of differentially methylated genomic regions using bisulfite sequencing
The CpG-rich regions of Atg13 promoter were selected for bisulfite sequencing. Briefly, 1 μg of genomic DNA was bisulfite converted using a DNA Methylation Kit (EZ-96; Zymo Research) in accordance with the manufacturer’s protocol. Primers (5′-TTTGAAGATTTTGATG TATAGAAATATT-3′,5′-ATTATTACCCTCAAAAATAAATACCT-3′; length 448 bp) were designed using MethPrimer (http://www.urogene.org/cgi-bin/methprimer/methprimer.cgi). The PCR products were purified using SK8141 PCR Purification (Qiagen), cloned into a pUC18-T Vector System, and subsequently transformed into SK9307 cells. Independent white colonies were selected and sequenced using a GenomeLabGeXP Genetic Analysis System (Beckman Coulter). Bisulfite sequencing DNA methylation analysis software was used to analyze the sequencing data. The average methylation percentage at each CpG site in each group was used for the analysis.
Statistical analysis
Data were presented as mean ± standard deviation. All statistical procedures were performed using SPSS 13.0. Normally distributed data were analyzed using one-way analysis of variance with a Bonferroni post hoc test to evaluate the statistical significance of intergroup differences in all the tested variables. The CpG methylation rates of Atg13 gene promoter were analyzed using the chi-square test. A p value < 0.05 was considered to indicate statistical significance.
RESULTS
Establishment of the atherosclerotic mouse model
The morphology of aortic roots was assessed using H&E staining to evaluate whether the atherosclerotic mouse model had been successfully established after the ApoE–/– mice had been fed a high-fat diet for 12 weeks. Obvious atherosclerotic plaques were observed in aortic valve attachment sites in the ApoE–/– mice (Figure 1A and 1B).
Figure 1.
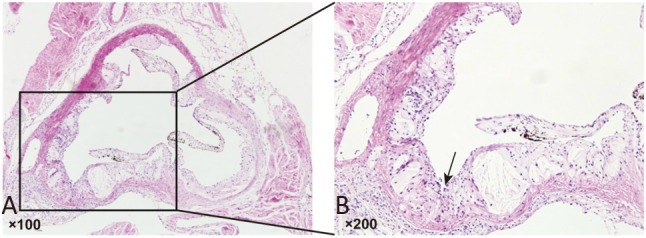
Establishment of the atherosclerotic mouse model. (A) Representative image of haemotoxylin and eosin (H&E) staining. Tissues were examined using a light microscope (100× magnification). (B) A representative image of the local magnification; black arrow indicates the atherosclerotic plaque. Tissues were examined using a light microscope (200× magnification).
Validation of candidate genes based on gene expression profiles and methylation microarrays
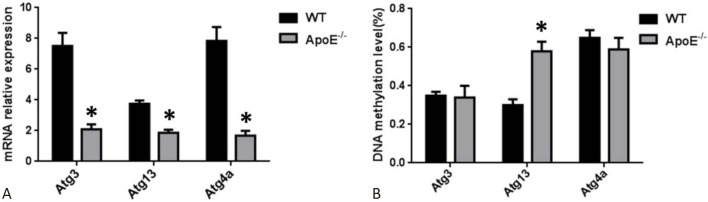
Gene expression and methylation profiling of aortic samples of the ApoE–/– mice with atherosclerotic plaques were assessed using high-throughput microarrays to identify candidate atherosclerosis-related genes with aberrant methylation in their promoter regions. The results showed that three autophagy-related genes including Atg3, Atg13 and Atg4a were filtered (Table 2). Next, microarray data for the gene expression and methylation status of candidate genes were validated using RT-PCR and bisulfite sequencing PCR (BSP), respectively. The results demonstrated that the mRNA levels of Atg3, Atg13 and Atg4a in the ApoE–/– mouse aortic samples were significantly decreased, but DNA methylation levels in the promoter region of only Atg13 were significantly increased compared with the WT control groups (p < 0.01; Figure 2).
Table 2. The autophagic genes based on the DNA methylation array.
| Chr | Gene_name | Avg_p value | Fold_change |
| chr16 | Atg3 | 0.0002182 | 1.7288389 |
| chr2 | Atg13 | 6.61E-05 | 1.4279148 |
| chrX | Atg4a | 0.002267 | 1.1917074 |
Figure 2.
Validation of expression levels and promoter methylation levels of filtered autophagy-related genes. (A) The mRNA level of filtered candidate autophagy-related genes in aortas of the mice in the wild-type (WT) and ApoE–/– groups. (B) The promoter methylation levels of filtered candidate autophagy-related genes in aortas of the mice in the WT and ApoE–/– groups. Data are expressed as the mean ± SD (n = 6). * p < 0.01 vs. WT group. WT, wild type C57BL/6J mice fed standard chow diet; ApoE–/–, ApoE–/– mice fed a western-type diet.
SYDC promoted autophagy in ApoE–/– mice
The protein expressions of Atg5-Atg12 complex and Beclin-1 in plaques of the atherosclerotic mice were measured to assess the effect of SYDC on autophagy in the atherosclerotic mice. As shown in Figure 3A, 3B, 3C, the protein levels of Atg5-Atg12 complex in the atherosclerotic plaques of the ApoE–/– mice in the SYDC group and Beclin-1 in the atorvastatin and SYDC groups were significantly increased compared to the control group (p < 0.01). The protein expressions of Atg5-Atg12 complex in the atherosclerotic plaque of mice in the SYDC group were significantly increased compared to the atorvastatin group (p < 0.01).
Figure 3.
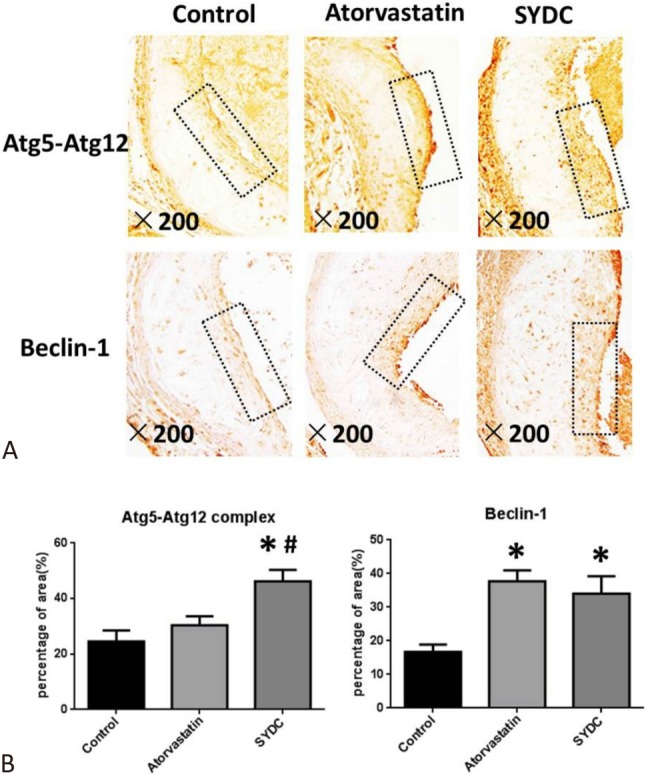
Effects of Shen-Yuan-Dan Capsule (SYDC) on Atg5-Atg12 complex and Beclin-1 expressions in atherosclerotic plaque. (A) Representative images of the immunohistochemistry showing the expression of Atg5-Atg12 complex and Beclin-1 in aortic samples. Black arrows indicate atherosclerotic plaques of the mice in the control, atorvastatin, and SYDC groups. Tissues were examined using a light microscope (200× magnification). (B) Quantitation of expression levels of Atg5-Atg12 complex and Beclin-1 in atherosclerotic plaques of the mice in the control, atorvastatin, and SYDC groups. Data are expressed as the mean ± standard deviation (SD) (n = 8). * p < 0.01 vs. control group, # p < 0.01 vs. atorvastatin group.
Effect of SYDC on genomic DNA methylation and Atg13 methylation levels
Genomic DNA methylation and DNMT1 levels in mouse plasma were measured using HPLC and ELISA to assess the effect of SYDC on genomic DNA methylation in the atherosclerotic mice. The results showed that 5-mC and DNMT1 levels in the mouse plasma in the atorvastatin and SYDC groups were significantly decreased compared with the control group (p < 0.01). However, no significant difference was found between the SYDC treatment groups and the positive-control (atorvastatin) group (p > 0.05; Figure 4A and 4B). Moreover, the protein expression of 5-mC in atherosclerotic plaques was detected using immunohistochemical analysis. The data showed that the 5-mC and DNMT1 protein expression levels in the atherosclerotic plaques of the mice in the SYDC and atorvastatin groups were significantly reduced compared with those in the control group (p < 0.01) (Figure 4C and 4D).
Figure 4.
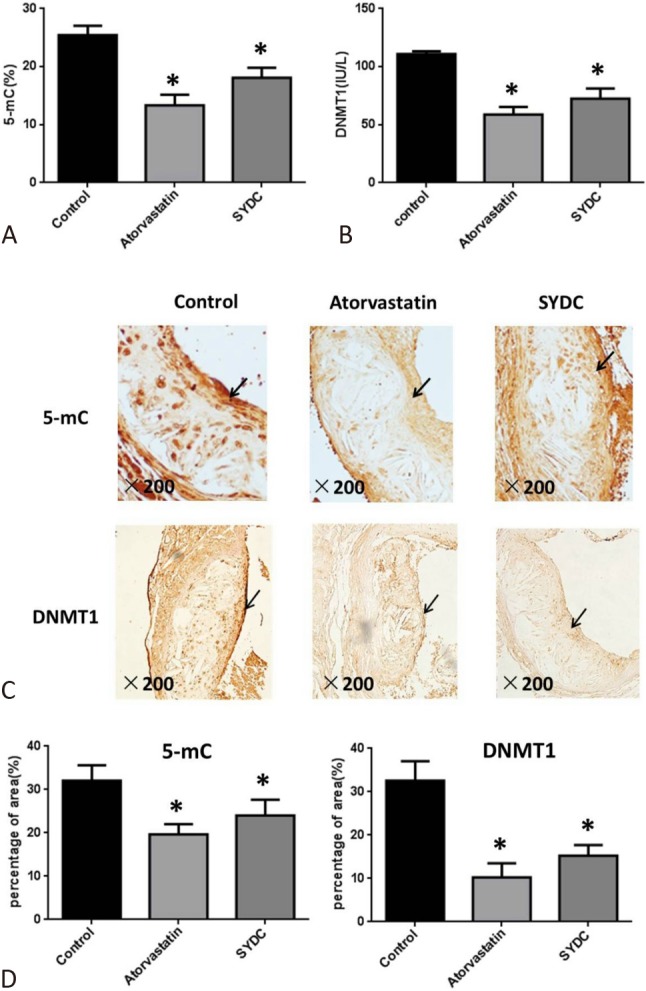
Effects of Shen-Yuan-Dan Capsule (SYDC) on genomic DNA methylation level and DNA methyltransferase 1 (DNMT1) in atherosclerotic mice. (A) The 5-mC levels in plasma of the mice in the control, atorvastatin, and SYDC groups. (B) The DNMT1 levels in plasma of the mice in the control, atorvastatin, and SYDC groups. Data are expressed as the mean ± standard deviation (SD) (n = 10). * p < 0.01 vs. control group. (C) Representative images of the immunohistochemistry showing the protein expressions of 5-mC and DNMT1 in the atherosclerotic plaque of the mice in the control, atorvastatin, and SYDC groups.
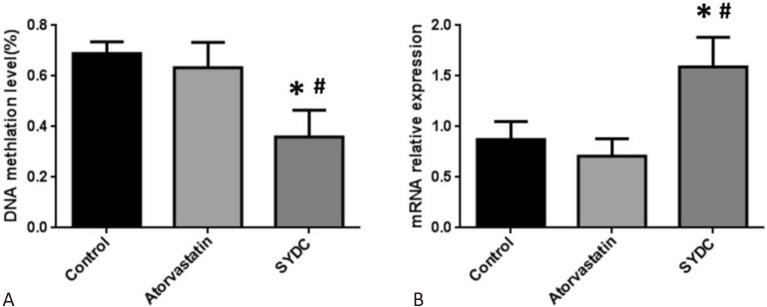
Furthermore, the effect of SYDC on the DNA methylation level in the promoter region of Atg13 in the mice aortas in the different groups was evaluated using BSP. The results demonstrated that the average methylation rate of CpG islands in the promoter region of Atg13 in the aortas from the SYDC group was significantly reduced compared to the control group (p < 0.01), while the mRNA levels of Atg13 in the mice aortas from the SYDC group were significantly increased compared to the control group (p < 0.01). In addition, the average methylation rate of CpG islands in the promoter region of Atg13 in the aortas of the mice from the SYDC group were significantly decreased compared to the atorvastatin group (p < 0.01), and the mRNA levels of Atg13 in the mice aortas from the SYDC group were significantly increased compared to the atorvastatin group (p < 0.01; Figure 5A and 5B).
Figure 5.
Effects of Shen-Yuan-Dan Capsule (SYDC) on the average methylation rate of the CpG island of Atg13 and mRNA expression of Atg13 in atherosclerotic mice. (A) The average methylation rate of the CpG island of Atg13 in aortas of the mice in the control, atorvastatin, and SYDC groups. (B) The mRNA expression of Atg13 in aortas of the mice in the control, atorvastatin, and SYDC groups. Data are expressed as the mean ± standard deviation (SD) (n = 6). * p < 0.01 vs. control group, # p < 0.01 vs. atorvastatin group.
DISCUSSION
Autophagy and aberrant DNA methylation are vital in the pathogenesis of atherosclerosis. Our previous study revealed that SYDC exerted an anti-atherosclerotic effect in ApoE–/– mice fed with a high-fat diet,14 however its precise mechanism has not been fully elucidated. The findings of the present study indicated that SYDC attenuated atherosclerosis by promoting autophagy, probably by regulating the genomic DNA methylation status and Atg13 promoter demethylation.
DNA methylation has been associated with the pathogenesis of atherosclerosis. Previous studies have demonstrated the presence of atherosclerosis in familial aggregation, suggesting the role of genetic factors in atherogenesis.17 Recent evidence supports an association between DNA methylation and cardiovascular diseases such as atherosclerosis and vascular remodeling.18-20 Dunn et al.3 reported that inhibition of DNA methylation attenuated atherosclerotic burden by ameliorating endothelial dysfunction and restoring sensitive endothelial gene expression. In our previous study, we used DNA methylation and gene arrays to identify crucial target genes altered in an atherosclerotic mouse model (data not shown). The findings showed that three autophagic genes, including Atg3, Atg13, and Atg4a were filtered. In this study, we used RT-PCR and BSP methods to validate the three candidate autophagic genes, and Atg13 was finally confirmed to be the autophagic target gene with hypermethylation level in the promoter region in the aortas of the atherosclerotic mice.
SYDC is a TCM compound which contains eight crude Chinese medicinal agents named Salvia miltiorrhiza Bunge, Astragalus robustus Bunge, Codonopsis pilosula (Franch) Nannf, Scrophularia aestivalis Griseb, leech, Lumbricus, Eupolyphaga Steleophaga and Corydalis tuberipisiformis Z.Y. Su. Clinically, SYDC has been widely used for treating coronary heart disease, especially unstable angina pectoris, and atherosclerosis is the pathological basis resulting in angina pectoris. Our previous study revealed that SYDC exerted an anti-atherosclerotic effect in ApoE–/– mice fed with a high-fat diet,14 however its precise mechanism was unclear. Accumulating data have indicated a potential role of autophagy as a diagnostic and prognostic indicator for atherosclerosis.21-23 The factors that induce atherosclerosis, such as oxidized lipids, inflammation, and metabolic stress, can also stimulate autophagy.23 Beclin1 is an important autophagic gene that is mainly responsible for autophagosome assembly and the recruitment of other Atgs,24 while Atg5-Atg12 complex belongs to the Atgs necessary for the early autophagosome formation.25 The results of the present study suggested that SYDC may have induced autophagy by up-regulating the protein expressions of Atg5-Atg12 complex and Beclin1 in the atherosclerotic plaques in the aortas from the atherosclerotic mice.
5-mC level is an epigenetic marker in both animals and plants, and it has been shown to bevital in various biological processes, including gene silencing and imprinting.26 Elevated 5-mC levels have been reported in the intima of atherosclerotic mice, and high 5-mC levels have also been linked to low-density lipoprotein receptors in vascular cells and endothelial inflammation.3 DNMT1 is the most important catalytic enzyme of DNA methylation, and it is an important target gene in DNA methylation studies of an atherosclerotic mechanism. Previous studies have shown that levels of DNMT1 and genomic DNA methylation are highly expressed in atherosclerotic endothelium, and that silencing the DNMT1 gene can obviously improve vascular endothelial dysfunction caused by vibration and shear stimulation.27 In addition, treatment with 5-aza, a DNMT1 inhibitor, has been shown to inhibit atherosclerotic progression in high-fat fed ApoE–/– mice.28 The use of the DNMT1 inhibitor 5-Aza-Cd has also been shown to promote the demethylation of Beclin1 in the promoter region and up-regulate the expression of Beclin1 in MCF-7 cells to induce autophagy.29 In the present study, SYDC could significantly inhibit 5-mC and DNMT1 levels in the plasma of atherosclerotic mice and reduce the 5-mC and DNMT1 expressions in the atherosclerotic plaques in ApoE–/– mice. This suggests that SYDC can inhibit genomic DNA methylation and DNMT1 levels to exert its anti-atherosclerotic effect and promote autophagy.
On the basis of the integrated analysis of DNA methylation and gene arrays, Atg13 was selected as the crucial target gene involved in the methylation-related regulation of atherosclerosis. In mammalian cells, Atg13 is directly controlled by mTOR and is a critical part of the autophagy machinery in response to nutritional status.30,31 In the present study, the effect of SYDC on atherosclerosis-related genes with aberrant promoter DNA methylation was analyzed in an atherosclerotic mouse model. The Atg13 gene promoter methylation levels and its mRNA expression in the aortas of atherosclerotic mice were detected. The results showed that SYDC significantly decreased the DNA methylation level in the promoter region of Atg13 and subsequently increased the mRNA expression of Atg13 in the aortas of the atherosclerotic mice.
In summary, our findings suggested that SYDC prevented atherosclerosis by inhibiting not only global genomic methylation levels but also the methylation level in the promoter regions of Atg13, a crucial autophagy-related gene involved in the methylation-related regulation of atherosclerosis.
CONCLUSIONS
This novel study demonstrated that SYDC could dramatically promote autophagy in atherosclerotic mice, probably by regulating methylation levels of Atg13 in the promoter region and genomic DNA methylation levels.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81673744), and the Beijing Natural Science Foundation (7162043).
CONFLICTS OF INTEREST
All the authors declare no conflict of interest.
DATA AVAILABILITY
All the readers can freely access the data of this manuscript published online.
REFERENCES
- 1.Liu C, Wang Q, Guo H, et al. Plasma S-adenosylhomocysteine is a better biomarker of As than homocysteine in apolipoprotein E-deficient mice fed high dietary methionine. J Nutr. 2008;138:311–315. doi: 10.1093/jn/138.2.311. [DOI] [PubMed] [Google Scholar]
- 2.Lund G, Andersson L, Lauria M, et al. DNA methylation polymorphisms precede any histological sign of As in mice lacking apolipoprotein E. J Biol Chem. 2004;279:29147–29154. doi: 10.1074/jbc.M403618200. [DOI] [PubMed] [Google Scholar]
- 3.Dunn J, Qiu H, Kim S, et al. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J Clin Invest. 2014;124:3187–3199. doi: 10.1172/JCI74792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umut Somuncu M, Bulut U, Karakurt H, et al. The relationship between obstructive sleep apnea and coronary plaque: a coronary computed tomographic angiography study. Acta Cardiol Sin. 2019;35:325–334. doi: 10.6515/ACS.201905_35(3).20181029A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding JW, Zhou T, Zheng XX, et al. The effects of high mobility group Box-1 protein on peripheral Treg/Th17 balance in patients with atherosclerosis. Acta Cardiol Sin. 2018;34:399–408. doi: 10.6515/ACS.201809_34(5).20180419A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu M, Thompson MD, Cohen RA, XiaoYong T. Autophagy and oxidative stress in cardiovascular diseases. Biochim Biophys Acta. 2015;185:243–251. doi: 10.1016/j.bbadis.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng J, Yang Q, Li AF, et al. Tet methylcytosine dioxygenase 2 inhibits As via upregulation of autophagy in ApoE-/- mice. Oncotarget. 2016;7:76423–76436. doi: 10.18632/oncotarget.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutter BM, Wu X, Laxman S, Tu BP. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell. 2013;154:403–415. doi: 10.1016/j.cell.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Honexu JM, Zhenvu W. One-hundred and thirteen cases of unstable angina pectoris (blood stasis syndrome) treated with Shen-yuan-dan decoction. J Tradit Chin Med. 1999;40:219–221. [Google Scholar]
- 10.Shang JJL, H. X, Wang ZY. Clinical observation of effects of Shengyuandan in treatment of unstable angina. Beijing J Tradit Chin Med. 2006;25:67–69. [Google Scholar]
- 11.Shang JJ, La Y, Yang HZ, et al. Effect of Shenyuandan pharmacology preconditioning on rat’s ischemia-reperfusion myocardial infarction size, protein kinase C and heat shock protein 70. China J Tradit Chin Med Pharm. 2011;26:1730–1733. [Google Scholar]
- 12.Wen QX, Yh Z, Shang JJ. Effect of Shenyuandan on myocardial infarct size, nitric oxide synthase and protein kinase C of myocardial ischemic preconditioning rat. Chin J Inf Tradit Chin Med. 2010;17:33–35. [Google Scholar]
- 13.Liu Hongxu LA, Xie XR. Effects of ShenYundan post conditioning on content of serum SOD, MDA in rats with myocardial ischemia/reperfusion. Chin J Microcir. 2011;21:1–25. [Google Scholar]
- 14.Zhou M, Li P, Kang Q, et al. Shen-Yuan-Dan Capsule inhibiting inflammatory reaction by regulating insulin receptor substrate 1/PI3K/Akt/NF-kappaB signaling pathway in apoliprotein E knockout mice fed with a high-fat diet. Acta Cardiol Sin. 2017;33:285–291. doi: 10.6515/ACS20160901B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang XR, Wat E, Wang YP, et al. Effect of dietary cocoa tea (camellia ptilophylla) supplementation on high-fat diet-induced obesity, hepatic steatosis, and hyperlipidemia in mice. Evid Based Complement Alternat Med. 2013;2013:783860. doi: 10.1155/2013/783860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou M, Xu H, Pan L, et al. Rosiglitazone promotes atherosclerotic plaque stability in fat-fed ApoE-knockout mice. Eur J Pharmacol. 2008;590:297–302. doi: 10.1016/j.ejphar.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 17.SK Y. Several important calculated parameters of blood lipids and its clinical significance. 2008;Proceeding of the 4th National Symposium about Lipid Analysis and 9th National Clinical Lipoprotein Conference Papers Series 4 [Google Scholar]
- 18.Superko HR, Roberts R, Agatston A, et al. Genetic testing for early detection of individuals at risk of coronary heart disease and monitoring response to therapy: challenges and promises. Curr Atheroscler Rep. 2011;13:396–404. doi: 10.1007/s11883-011-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bressler J, Shimmin LC, Boerwinkle E, Hixson JE. Global DNA methylation and risk of subclinical As in young adults: the Pathobiological Determinants of As in Youth (PDAY) study. Atherosclerosis. 2011;219:958–962. doi: 10.1016/j.atherosclerosis.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluckman PD, Hanson MA, Buklijas T, et al. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nature reviews. Nat Rev Endocrinol. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 21.Razani B, Feng C, Coleman T, et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 2012;15:534–544. doi: 10.1016/j.cmet.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shao BZ, Han BZ, Zeng YX, et al. The roles of macrophage autophagy in atherosclerosis. Acta Pharmacol Sin. 2016;37:150–156. doi: 10.1038/aps.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao K, Xu XS, Meng X, et al. Autophagy of monocytes attenuates the vulnerability of coronary atherosclerotic plaques. Coron Artery Dis. 2013;24:651–656. doi: 10.1097/MCA.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 24.Cuervo AM. Autophagy in neurons: it is not all about food. Trends Mol Med. 2006;12:461–464. doi: 10.1016/j.molmed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Ott C, Konig J, Hohn A, et al. Macroautophagy is impaired in old murine brain tissue as well as in senescent human fibroblasts. Redox Biol. 2016;10:266–273. doi: 10.1016/j.redox.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stroud H, Feng S, Morey Kinney S, et al. 5-hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells. Genome Biol. 2011;12:R54. doi: 10.1186/gb-2011-12-6-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng N, Meng N, Wang S, et al. An activator of mTOR inhibits oxLDL-induced autophagy and apoptosis in vascular endothelial cells and restricts atherosclerosis in apolipoprotein E-/- mice. Sci Rep. 2014;4:5519. doi: 10.1038/srep05519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhuang JH, Luan PP, Li HL, et al. The Yin-Yang dynamics of DNA methylation is the key regulator for smooth muscle cell phenotype switch and vascular remodeling. Arterioscler Thromb Vasc Biol. 2017;37:84–97. doi: 10.1161/ATVBAHA.116.307923. [DOI] [PubMed] [Google Scholar]
- 29.Park SE, Yi HJ, Suh NY, et al. Inhibition of EHMT2/G9a epigenetically increases the transcription of Beclin1 via an increase in ROS and activation of NF-κB. Oncotarget. 2016;7:39796–39808. doi: 10.18632/oncotarget.9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hara T, Takamura A, Kishi C, et al. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung CH, Jun CB, Ro SH, et al. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell. 2009;20:1992–2003. doi: 10.1091/mbc.E08-12-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the readers can freely access the data of this manuscript published online.