The rampant global spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has resulted in more than 2.7 million confirmed coronavirus disease 2019 (COVID-19) cases and more than 190 thousand deaths (~7%) in 185 countries up to April 24, 2020.1 According to data from 1099 patients with laboratory-confirmed COVID-19 in mainland China till January 29, 2020,2 the presence of cardiovascular risk factors and diseases was more prevalent in patients with severe disease compared to that in patients with non-severe disease: approximately two-fold for hypertension (23.7% vs. 13.4%) and cerebrovascular disease (2.3% vs. 1.2%), three-fold for diabetes (16.2% vs. 5.7%) and coronary heart disease (5.8% vs. 1.8%) and 3.5-fold for chronic kidney disease (1.7% vs. 0.5%). This observation not only suggests patients with cardiovascular comorbidities might be more vulnerable while catching COVID-19, but also raises concerns regarding whether the medications commonly used in these patients pose certain risks.
All these cardiovascular comorbidities are often associated with renin-angiotensin system (RAS) activation. Treatment with the RAS inhibitors, including angiotensin-converting enzyme inhibitors (ACE-I), angiotensin receptor blockers (ARB), and angiotensin receptor neprilysin inhibitor (ARNI), in patients with these cardiovascular risk factors or diseases had been demonstrated to reduce morbidities and mortality in numerous sizable randomized clinical trials and is widely recommended by various relevant national/regional guidelines. After identification of the angiotensin-converting enzyme 2 (ACE2) protein as the receptor that facilitates SARS-CoV-2 entry into cells,4 the RAS inhibitors have been once again in the spotlight, given the intuitive assumptions that the use of RAS inhibitors might enhance the expression of ACE2, the entry of SARS-CoV-2, and the severity of COVID-19.5 There are limited data showing that certain RAS inhibitors increased plasma ACE2 activities, but not tissue ACE2 levels, in humans.6,7 However, recently published data from 1128 hospitalized COVID-19 patients with hypertension in Hubei, China showed that the 28-day mortality rate was 3.7% in 188 patients treated with ACE-I/ARB during hospitalization and 9.8% in 940 patients not receiving ACE-I/ARB (mixed-effect Cox model, adjusted HR, 0.42; 95% CI, 0.19-0.92; p = 0.03).8 Another study including 362 hospitalized COVID-19 patients with hypertension also in Hubei, China showed that there was a numerically lower percentage of patients taking ACE-I/ARB during hospitalization between in-hospital non-survivors and survivors (unadjusted univariate analysis, 27.3% vs. 33.0%; p = 0.34), despite those taking ACE-I/ARB had significantly higher prevalence of cardiovascular diseases.9 In addition to these reassuring pieces of clinical evidence, the truth regarding the use of RAS inhibitors in patients with COVID-19 might be opposite to the above-mentioned intuitive assumptions, according to the following lines of evidence from the in-depth molecular mechanistic perspective.
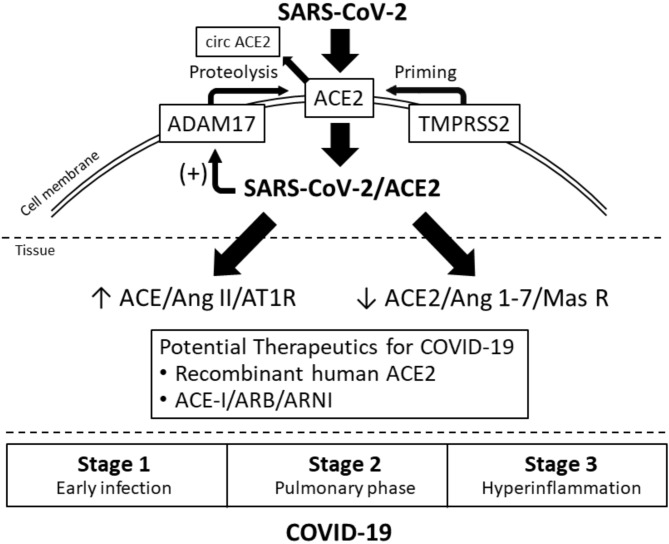
First, ACE2 is the carrier, but not the only player that commits the cell entry of SARS-CoV-2 (Figure 1, top). After the combination of the spike protein of SARS-CoV-2 and the extra-cellular domain of ACE2, another cell surface molecule, transmembrane protease serine 2 (TMPRSS2), conducts priming of the SARS-CoV-2/ACE2 complex and facilitates its cell entry.4 Camostat mesylate, a TMPRSS2 inhibitor, can inhibit cell entry of SARS-CoV-2 ex vivo and has been studied about its therapeutic potential for COVID-19.6 The fact that ACE2 is not the only player commiting the cell entry of SARS-CoV-2 may partly explain why lung is the major target organ involved in COVID-19 where ACE2 is weakly expressed, rather than organs like kidney and heart where ACE2 is highly expressed.10 This intriguing inconsistency between the tissue expression of ACE2 and organ-specific vulnerability to SARS-CoV-2 implies that we should not focus on ACE2 alone in considering issues like the effects of RAS inhibitors on COVID-19.
Figure 1.
ACE2, renin-angiotensin system, SARS-CoV-2 and COVID-19. ACE, angiotensin-converting enzyme; ADAM17, a disintegrin and metalloproteinase 17; Ang, angiotensin; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; AT1R, angiotensin 1 receptor; COVID-19, coronavirus disease 2019; SARS-CoV-2, severe adult respiratory syndrome coronavirus-2; TMPRSS2, transmembrane protease serine 2.
Second, the entry of SARS-CoV-2/ACE2 into cells then causes significant deletion of ACE2 at cell surface by activating membrane-bound A disintegrin and metalloproteinase 17 (ADAM17),6 which mediates proteolysis and shedding of ACE2 (Figure 1, top). In animal and human tissues infected by severe adult respiratory syndrome coronaviruses (SARS-CoV), which share the same cell entry pathway with SARS-CoV-2, the tissue expression of ACE2 decreased by > 80% and the ACE2 protein levels became almost not detectable.11-13 Accordingly, tissue concentration of angiotensin II was greatly increased,11 and the balance between the ACE/angiotensin II/angiotensin receptor type 1 and ACE2/angiotensin 1-7/Mas receptor axes would heavily lean towards the pro-inflammatory ACE/angiotensin II/angiotensin receptor type 1 axis (Figure 1, middle). The administration of ARB could effectively mitigate acute lung injury by counteracting the deviated balance between ACE and ACE2 axes in SARS-CoV-infected mice.11 In this context, the assumed increased expression of ACE2 by RAS inhibitors is either negligible in terms of ADMA17-mediated ACE2 shedding following entry of SARS-CoV-2/ACE2 or beneficial in terms of counteracting the activated RAS. Several clinical trials are ongoing to test the safety and efficacy of RAS modulators, including ARB and recombinant human ACE2, in COVID-19 (Figure 1, bottom).14
Third, abrupt withdrawal of RAS inhibitors in patients with heart failure can induce clinical instability and adverse events. In the TRED-HF trial, 40% of patients with previous dilated cardiomyopathy who were asymptomatic, with left ventricular ejection fraction ≥ 50% and an N-terminal pro-B-type natriuretic peptide < 250 ng/L with guideline-directed medical therapies, relapsed within 6 months following scheduled stepwise treatment withdrawal.15
Given the above lines of evidence, the Taiwan Hypertension Society recommends:
• Continuation of ACE-I, ARB, and ARNI is recommended in COVID-19 patients.
• In the case of shock, all blood pressure-lowering agents should be discontinued.
These recommendations are in line with those from major hypertension/cardiology societies globally.14 Whether RAS modulators could improve the outcomes of COVID-19 patients, irrespective of hypertension status and stages of COVID-19, awaits verification (Figure 1, bottom).
In this issue of the Journal, we published one article assessing the correlation between apelin-13 and coronary artery ectasia.16 Apelin acting through the apelin receptors specifically increased ACE2 promoter activity leading to an increase in ACE2 mRNA and protein.6 Different apelins have been shown to reduce angiotensin II-induced myocardial hypertrophy, dysfunction, and fibrosis and abdominal aortic rupture in animal models. In this article, Sun X et al. showed an even lower level of apelin-13 in patients with coronary artery ectasia, compared to that in patients with coronary artery disease or no coronary stenosis. Given the common pathophysiologic mechanisms between coronary artery ectasia and abdominal aortic aneurysm, this study provides a serologic link between these two entities.
In the recent 2 years, our Journal published many research articles regarding acute coronary syndrome and heart failure, which cast challenges upon how to deliver optimized care during the COVID-19 pandemic. We summarized the articles as cited herein for the readers’ interest.17-30 We wish you enjoy this issue of the Acta Cardiologica Sinica.
Acknowledgments
None.
REFERENCES
- 1.Johns Hopkins Coronavirus Resource Center. Available at: https://coronavirus.jhu.edu. Accessed April 24, 2020. [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;[E-pub ahead of print]; doi: 10.1056/NEJMoa2002032 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang CC, Wu CK, Tsai ML, et al. 2019 focused update of the guidelines of the Taiwan Society of Cardiology for the diagnosis and treatment of heart failure. Acta Cardiol Sin. 2019;35:244–283. doi: 10.6515/ACS.201905_35(3).20190422A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280, e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res. 2020;[E-pub ahead of print]; doi: 10.1161/CIRCRESAHA.120.317015 doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28:15–21. doi: 10.1093/ajh/hpu086. [DOI] [PubMed] [Google Scholar]
- 8.Tipnis SR, Hooper NM, Hyde R, et al. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 9.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020 Apr 17;[E-pub ahead of print]; doi: 10.1161/CIRCRESAHA.120.317134 doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Wang X, Chen J, et al. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020 Apr 23;[E-pub ahead of print]; doi: 10.1001/jamacardio.2020.1624 doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glowacka I, Bertram S, Herzog P, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;[E-pub ahead of print]; doi: 10.1161/HYPERTENSIONAHA.120.15082:HYPERTENSIONAHA12015082 doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halliday BP, Wassall R, Lota AS, et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): an open-label, pilot, randomised trial. Lancet. 2019;393:61–73. doi: 10.1016/S0140-6736(18)32484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, Zhang Y, Qi X, et al. Impact of apelin-13 on the development of coronary artery ectasia. Acta Cardiol Sin. 2020;36:216–222. doi: 10.6515/ACS.202005_36(3).20190901A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YB, Hao GZ, Jiang YF, et al. Effects of levosimendan on right ventricular function in patients with acute decompensated heart failure. Acta Cardiol Sin. 2019;35:585–591. doi: 10.6515/ACS.201911_35(6).20190327A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeh JK, Liu WH, Wang CY, et al. Targeted next generation sequencing for genetic mutations of dilated cardiomyopathy. Acta Cardiol Sin. 2019;35:571–584. doi: 10.6515/ACS.201911_35(6).20190402A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Fu J, Wan H, et al. Protective roles and mechanisms of taurine on myocardial hypoxia/reoxygenation-induced apoptosis. Acta Cardiol Sin. 2019;35:415–424. doi: 10.6515/ACS.201907_35(4).20181218A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai YJ, Huang WC, Weng TP, et al. Early phase II comprehensive cardiac rehabilitation after acute myocardial infarction. Acta Cardiol Sin. 2019;35:425–429. doi: 10.6515/ACS.201907_35(4).20190330A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doganay B, Okutucu S, Cetin M, et al. Association of serum copeptin levels with patency of infarct-related arteries in patients with ST-segment elevation myocardial infarction. Acta Cardiol Sin. 2019;35:360–368. doi: 10.6515/ACS.201907_35(4).20181101A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiou CC, Tsai TH, Lee CH, et al. Impact of pharmacist interventions on the long-term clinical outcomes in patients with myocardial infarction. Acta Cardiol Sin. 2019;35:290–300. doi: 10.6515/ACS.201905_35(3).20181122B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen WT, Shie CB, Yang CC, et al. Blockade of cardiac proton pump impairs ventricular remodeling through a superoxide-DDAH-dependent pathway in infarcted rats. Acta Cardiol Sin. 2019;35:165–178. doi: 10.6515/ACS.201903_35(2).20180917A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YH, Chiu YW, Cheng JJ, et al. Changing practice pattern of acute coronary syndromes in Taiwan from 2008 to 2015. Acta Cardiol Sin. 2019;35:1–10. doi: 10.6515/ACS.201901_35(1).20180716B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Zhong Z, Luo S, et al. Efficacy of antihypertensive therapy in the acute stage of cerebral infarction - a prospective, randomized control trial. Acta Cardiol Sin. 2018;34:502–510. doi: 10.6515/ACS.201811_34(6).20180622B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang WT, Hsu CH, Huang TL, et al. MicroRNA-21 is associated with the severity of right ventricular dysfunction in patients with hypoxia-induced pulmonary hypertension. Acta Cardiol Sin. 2018;34:511–517. doi: 10.6515/ACS.201811_34(6).20180613A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karadeniz M, Sarak T, Duran M, et al. Hyperhomocysteinemia predicts the severity of coronary artery disease as determined by the Syntax score in patients with acute coronary syndrome. Acta Cardiol Sin. 2018;34:458–463. doi: 10.6515/ACS.201811_34(6).20180528B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kageyama M, Toyoda S, Sakuma M, et al. Theophylline-induced left ventricular relaxation disturbance in magnesium-deficient rats: improvement by k201, a novel 1,4-benzothiazepine derivative. Acta Cardiol Sin. 2018;34:432–439. doi: 10.6515/ACS.201809_34(5).20180513A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serdar Kuyumcu M, Kuyumcu A, Yayla C, et al. The relationship between nesfatin-1 levels and Syntax score in patients with non-ST segment elevation myocardial infarction. Acta Cardiol Sin. 2018;34:386–393. doi: 10.6515/ACS.201809_34(5).20180423A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cetin S, Gunduz A, Sababli Cetin A, et al. Evaluation of subtle left ventricular systolic dysfunction by longitudinal systolic strain in patients with human immunodeficiency virus. Acta Cardiol Sin. 2018;34:321–327. doi: 10.6515/ACS.201807_34(4).20180222B. [DOI] [PMC free article] [PubMed] [Google Scholar]