Abstract
In this work, the fermentation conditions and the antibacterial characteristics of Eucommia ulmoides leaves vinegar (EV) were studied. By single factor orthogonal test, it was found that under optimal fermentation conditions (bran addition 10%, sugar addition 8%, leaven addition 0.3% and acetic acid bacteria solution 12%), the acetic acid content and CA content of EV were 45.5 ± 2.8 mg/mL and 0.98 ± 0.08 mg/mL, respectively. Then, by the disc diffusion method, it was concluded that the antibacterial effect of EV was significantly higher than that of Eucommia ulmoides leaves enzymatic hydrolysate and Zhenjiang aromatic vinegar (P<0.05). An investigation into action mode of EV against Bacillus subtilis indicated that, under the combined action of CA and acetic acid, EV exerted its antibacterial effect by damaging bacterial cell wall and cell membrane, increasing the cell permeability which resulted in the structural lesions and release of cell components, thus led to cell death.
Keywords: Eucommia ulmoides leaves vinegar, Chlorogenic acid content, Antibacterial characteristic, Bacillus subtilis
Introduction
Vinegar stands as a highly appreciated fermented food product due to several functional properties and multiple applications (Eduardo et al., 2017). In China, vinegar is traditionally produced by cereal for more than 3000 years, and different regions of the country possess their own kinds of vinegar. Among them, Zhenjiang Aromatic Vinegar (ZAV) is extraordinary famous of their traditional vinegar (Wu et al., 2012) which possesses a variety of physiological functions, such as anti-bacteria, anti-infection, anti-oxidation, blood glucose control, lipid metabolism regulation, weight loss, and anticancer activities. The antibacterial and anti-infective properties of vinegar are mainly due to the presence of organic acids, polyphenols, and melanoidins (Budak et al., 2014; Ozturk et al., 2015). Therefore, more and more attention is paid to the health benefits of vinegar, and different degrees of human and financial resources are invested to develop the health vinegar products (Eduardo et al., 2017; Kaur et al., 2011). Recently, the study of the main composition and function of plant has been a hot spot (Ge et al., 2017; Wang et al., 2017). The Eucommia ulmoides is a very valuable medicine in China. Eucommia ulmoides leaves contain many similar active ingredients and function as the barks. Studies also have proved that the extract of Eucommia ulmoides leaves have strong bacteriostatic action, oxidation resistance and inhibit different free radicals (Shao et al., 2015; Shingo et al., 2015). Therefore, the application and function of Eucommia ulmoides leaves in food are getting more and more attention.
The research and development of natural bacteriostatic agent has become a hot research topic (Kobbi et al., 2015; Yan et al., 2018). It has a morphological effect on various bacterial cells (Booyens et al., 2014; Diao et al., 2014). Natural antimicrobials inhibit the growth and reproduction of microorganisms by interfering with and destroying their cellular structure, or related physiological and biochemical reactions and metabolic activities, and then lead to the death of microorganisms. The main manifestations of antimicrobial activity are the destruction of cell wall structure, the change of cell membrane permeability, the destruction of enzyme protein, and the decomposition of cell protoplasts, etcetera (Liu et al., 2015; Tang et al., 2017; Wang et al., 2018). There are more and more studies on the antibacterial activity and mechanism of natural bacteriostatic agents against Bacillus subtilis (Escobosa et al., 2014).
At present, there are no reports about Eucommia ulmoides leaves vinegar (EV), especially its antibacterial characteristics. So, in this study, we optimized the Eucommia ulmoides leaves vinegar process and evaluated the antimicrobial efficacy of EV on different microbes. Then, we investigated the antimicrobial mechanism of EV on the growth curve, the cell wall, the cell membrane, and the cellular morphology of Bacillus subtilis. This study would serve as a theoretical and technical underpinning for the industrial production and the antibacterial characteristic of Eucommia ulmoides leaves vinegar.
Materials and methods
Materials
ZAV was bought in supermarkets, it was produced in Zhenjiang, Jiangsu province, China on September 27, 2013, the brand was Hengshun and the sour taste is mild an fragrant. Acetic acid bacterium strain AS.14l was obtained from the Institute of Microorganisms of Chinese Academy of Sciences. Eucommia ulmoides leaves were collected in autumn from Hanzhong City, Shanxi Province, China. Cellulase (10,000 U/g) was purchased from Beijing Oboxing Company. CA standard products, glacial acetic acid, acetonitrile, anhydrous ethanol are of chromatographic purity, and purchased from Shanghai Ronghe Medical Science and Technology Co Ltd. Four microorganisms (Bacillus subtilis, Escherichia coli, Aspergillus niger and Yeast) were obtained from the Institute of Microbiology, Chinese Academy of Sciences, and were preserved at the Department of biochemistry, Baoding University. An Alkaline Phosphatase (AKP) assay kit was purchased from Nanjing Jian Cheng Bioengineering Institute (Nanjing, China).
Microbial strains and growth conditions
Acetic acid bacterium strain AS.14l was obtained from the Institute of Microorganisms of Chinese Academy of Sciences which was preserved at the Department of biochemistry, Baoding University. It was cultured in Acetic acid bacteria broth (0.1% Yeast extract, 0.3% glucose, 0.4% alcohol, w/v) at 200 rpm, 32 °C for 24 h.
Four microorganisms (Bacillus subtilis, Escherichia coli, Aspergillus niger and Yeast) were obtained from the Institute of Microbiology, Chinese Academy of Sciences, and they were preserved at the Department of Biochemistry, Baoding University. The strain B. subtilis and E. coli were cultured in nutrient agar broth (0.3% beef extract, 1.0% tryptone, 0.5% NaCl and 1.5% Agar, w/v) at pH 7.2 at 37 °C for 12 h. The strains A. niger and Yeast were cultured in potato dextrose agar broth (2% glucose, 1.5% agar and potato extract) at 28 °C for 48 h (Fu et al., 2009). The concentration of four microorganisms was measured at 600 nm and adjusted to 1 × 106 cfu/mL through turbidimetry (Han et al., 2013). The four microbial suspensions were be used to test antimicrobial activity.
The optimization of fermentation process of Eucommia ulmoides Leaves vinegar
According to the report (Jia et al., 2013), Eucommia ulmoides leaves were enzymatic hydrolysed to obtain Eucommia ulmoides leaves enzymatic hydrolysate (EEH). Then, EEH was mixed with bran and sugar. After that, sterilized at 121 °C for 30 min. After cooled, leaven were added into the sterilized solution to ferment at 28 °C for 3 days. Then, the acetic acid bacteria solution was added into the fermentation liquor. After that, it was fermented at 200 rpm, 32 °C for 4 days. Finally the Eucommia ulmoides leaves vinegar was obtained after collecting and treating the fermentation broth.
The acetic acid content and CA content were the double indexes in the optimization of fermentation process of EV. In order to determine the proper scope of bran addition (A), sugar addition (B), leaven addition (C) and the addition of acetic acid bacteria solution (D) for acetic acid production, at first, single factor experiments were tested as follows: various ratio of bran addition from 0 to 20%, sugar addition at a range of percentage from 0 to 10%, leaven addition at different concentrations ranging from 0.05 to 0.4%, the addition of acetic acid bacteria solution at a range of percentage from 6 to 16%. Various raw materials were added into the EEH according to the above EEH percentage, and then the mixture was fermented to produce vinegar. Then, based on the results of single factor test and previous reports (Escobosa et al., 2014; Ma et al., 2014), the orthogonal test L9 (34) was designed to examine comprehensive influence of bran addition, sugar addition, leaven addition and the addition of acetic acid bacteria solution on acetic acid content and CA content of EV. The result was showed in Table 1.
Table 1.
The single factor orthogonal array design and results
| Test numbers | Factors | Acidity (mg/mL) | CA content (mg/mL) | |||
|---|---|---|---|---|---|---|
| A (%) | B (%) | C (%) | D (%) | |||
| 1 | 1 (5) | 1 (6) | 1 (0.2) | 1 (10) | 16.11 ± 0.19 | 0.78 ± 0.01 |
| 2 | 1 (5) | 2 (8) | 2 (0.3) | 2 (12) | 47.21 ± 0.27 | 1.07 ± 0.01 |
| 3 | 1 (5) | 3 (10) | 3 (0.4) | 3 (14) | 43.13 ± 0.18 | 1.03 ± 0.01 |
| 4 | 2 (10) | 1 (6) | 2 (0.3) | 3 (14) | 32.22 ± 0.26 | 0.98 ± 0.01 |
| 5 | 2 (10) | 2 (8) | 3 (0.4) | 1 (10) | 34.31 ± 0.22 | 0.88 ± 0.01 |
| 6 | 2 (10) | 3 (10) | 1 (0.2) | 2 (12) | 44.24 ± 0.23 | 0.89 ± 0.01 |
| 7 | 3 (15) | 1 (6) | 3 (0.4) | 2 (12) | 42.16 ± 0.24 | 1.01 ± 0.02 |
| 8 | 3 (15) | 2 (8) | 1 (0.2) | 3 (14) | 40.14 ± 0.23 | 0.85 ± 0.01 |
| 9 | 3 (15) | 3 (10) | 2 (0.3) | 1 (10) | 36.12 ± 0.16 | 0.88 ± 0.01 |
| Acidity (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| K1 | 109.21 | 99.89 | 108.49 | 96.54 | ||
| K2 | 110.17 | 121.66 | 116.95 | 129.61 | ||
| K3 | 116.42 | 114.25 | 110.36 | 109.65 | ||
| k1 | 36.4 | 33.3 | 36.16 | 32.18 | ||
| k2 | 36.72 | 40.55 | 38.98 | 43.2 | ||
| k3 | 38.81 | 38.08 | 36.79 | 36.55 | ||
| R | 2.4 | 7.26 | 2.82 | 11.02 | ||
| Order | D > B>C > A | |||||
| Optimal level | A3 | B2 | C2 | D2 | ||
| CA content (mg/mL) | ||||||
|---|---|---|---|---|---|---|
| K1 | 2.88 | 2.61 | 2.53 | 2.53 | ||
| K2 | 2.75 | 2.8 | 2.93 | 2.98 | ||
| K3 | 2.74 | 2.8 | 2.91 | 2.86 | ||
| k1 | 0.96 | 0.87 | 0.84 | 0.84 | ||
| k2 | 0.92 | 0.93 | 0.98 | 0.99 | ||
| k3 | 0.91 | 0.93 | 0.97 | 0.95 | ||
| R | 0.05 | 0.06 | 0.14 | 0.15 | ||
| Order | D > C>B > A | |||||
| Optimal level | A1 | B2 | C2 | D2 | ||
A: bran addition, B: sugar addition, C: leaven addition, D: acetic acid bacteria solution. Data represent mean ± standard deviation of three independent experiments
Detection method of vinegar acidity
According to the study of Chen and Chen (2009), acid–base titration was used to detect the acidity of vinegar. After diluting 0.1 mL vinegar by 4.9 mL distilled water, 1 ~ 2 drops of phenolphthalein was added. Then, the sample was titrated with 0.05 mol/L NaOH until the solution turned red.
Detection method of CA
The samples were centrifuged at 10,000 rpm for 1 min, and then filtrated by 0.25 μm micro-porous membrane. Then, the CA of samples were tested by high performance liquid chromatography (HPLC) method. The instrument and experimental parameters are as follows: High performance liquid chromatograph (LC-20AT, SPD-M20A Detector, Class VP chromatographic work station, Shimadzu, Japan), Dima C18 column (250 mm × 4.6 mm, 5 μm), acetonitrile/water/glacial acetic acid (12:88:1, v/v/v) as mobile phase, determine wavelength: 325 nm, Column temperature: 30 °C, Flow velocity: 1.0 mL/min, Sample volume: 5 μm, Retention times 11.57 min.
Antimicrobial activity of EV
Antimicrobial activity of EV was evaluated by the disc diffusion method (Ma et al., 2014; Wu et al., 2014). Sterile nutrient agar plates and sterile potato dextrose agar plates were prepared for the four microbial suspensions and inoculated respectively by a spread plate method under aseptic conditions. The filter paper disc of 5 mm diameter was prepared and sterilized. 10 μL EEH, 10 μL EV and 10 μL ZAV were added into each disc respectively. The sterile impregnated disc with EEH, EV and ZAV were placed on the agar surface with framed forceps and gently pressed down to ensure complete contact of the disc with the agar surface. Filter paper discs soaked in sterile water were used for negative controls. The B. subtilis and E. coli plates were incubated at 37 °C for 24 h. The A. niger and Yeast plates were incubated at 28 °C for 48 h. After incubation, the size (diameter) of the inhibition zones was measured.
The effect of EV on growth curve of B. subtilis
The effect of EV on the growth of B. subtilis was examined according to the previous method (Ge et al., 2015; Nakayama et al., 2015). 0.1 mL 1 × 106 cfu/mL B. subtilis solution and 10 mL EV nutrient medium were added to each test tube to be cultured at 160 rpm, 37 °C. The nutrient medium (0.3% beef extract, 1.0% tryptone, 0.5% NaCl, w/v, pH 7.2) was blank control. During the culture process, three tubes of the same sample were taken out respectively at 0, 1, 2, 4, 6, 8, 10, 12, 14, 16, 20, 24, 28, 32, 34 h. Then, the tubes were tested by turbidimetry at 600 nm. The OD-T curve was plotted with time on X-axis and OD value on Y-axis. The samples of nutrient medium without EV were the control group.
The effect of EV on cell wall of B. subtilis
The effect of EV on the cell wall of B. subtilis can be concluded by determination of alkaline phosphatase (AKP) content of the culture medium (Hou et al., 2012; Zhang et al., 2017). B. subtilis was cultured to logarithmic phase. 0.1 mL 1 × 106 cfu/mL the B. subtilis solution and 10 mL EV nutrient medium were added into each test tube to be cultured at 160 rpm, 37 °C. During the culture process, three tubes of the same sample were taken out at 0, 1, 2, 4, 6, 8 h, respectively. Then, the tubes were placed in a 0 ~ 4 °C refrigerator for test at same time. After the samples were centrifuged at 3500 rpm for 10 min, the supernatants were collected. The AKP activity was measured by visible spectrophotometer according to the instruction manual of AKP assay kit. The samples of nutrient medium without EV were the control group.
The effect of EV on cell membrane of B. subtilis
The effect of EV on the cell membrane of B. subtilis can be concluded by determination of electrical conductivity of the culture medium. B. subtilis was cultured to logarithmic phase. 0.1 mL 1 × 106 cfu/mL the B. subtilis solution and 10 mL EV nutrient medium were added to each test tube to be cultured at 160 rpm and 37 °C. During the culture process, three tubes of the same sample were taken out respectively at 0, 1, 2, 3, 4, 6, 8 h. Then, the tubes were placed in a 0 ~ 4 °C refrigerator for test by conductivity meter at same time and 25 °C. The tubes that B. subtilis was cultured in the nutrient medium were blank control (Wang et al., 2014). The samples of nutrient medium without EV were the control group.
The effect of EV on morphology of B. subtilis
The effect of EV on the morphology of B. subtilis was determined by Transmission Electron Microscopy (TEM) according to the report (Booyens et al., 2014). B. subtilis was cultured to logarithmic phase. Then, 1% bacterial suspensions containing 1 × 106 cfu/mL of B. subtilis were inoculated into EV nutrient medium and the nutrient medium to be cultured at 160 rpm, 37 °C for 6 h. The samples of nutrient medium without EV were the control group. The bacterial cells were centrifuged at 8000 r/min for 2 min, then, were fixed using 2.5% glutaraldehyde in 0.075 and 0.1 M phosphate buffer (pH 7.4). It was then rinsed three times in 0.1 M phosphate buffer and fixed in 0.5% osmium tetroxide. The sample was rinsed with distilled H2O and dehydrated in a graded series of ethanol (50, 70, 90, and 100%) before being infiltrated with Quetol epoxy resin and allowed to polymerize for 39 h at 60 °C. Ultrathin sections were cut and stained with aqueous uranyl acetate. The sections were then counterstained with lead acetate and rinsed in distilled H2O. Monitor sections of 0.5 μm were cut and stained in toluidine blue. The samples were then viewed using a JEOL JEM-2100F microscope.
Statistical analysis
The datas were processed by SPSS 17.0. The data was performed with Sigma Plot 10.0. Results were expressed as mean ± standard deviation (n = 3) through one-way analysis of variance (ANOVA) and the Duncan’s test was used to test the difference between means.
Results and discussion
The CA concentration of EEH
Using to above methods, under optimal conditions, the CA content of EEH was 1.8 ± 0.11 mg/mL. The results were consistent with previous researches in which the CA concentration of Eucommia ulmoides leaves was 1% ~ 5.5%.
The result of single factor orthogonal test
As showed in Table 1, it could be concluded that, optimal combination was bran addition (A3) 15%, sugar addition (B2) 8%, leaven addition (C2) 0.3% and acetic acid bacteria solution (D2) 10%. The greatest influence on CA concentration was factor D, and the smallest is factor A. But, the optimal level of bran addition (A1) was 5% which was different from that influence on acidity. At last, according single factor test, 10% bran addition (A2) was selected to be the optimal level. The optimum fermentation time of ethanol was 3d at 28 °C. The optimum acetic acid fermentation time was 4 days at 32 °C, 200 rpm. Under optimal conditions, the acetic acid content of EV was 45.5 ± 2.8 mg/mL and CA content of EV was 0.98 ± 0.08 mg/mL.
The antimicrobial activity of EV
As Table 2 showed, by correlation analysis, it was found that the inhibitory activity of EV against B. subtilis, E. coli and Yeast was significantly correlated with ZAV and EEH (P<0.05). By significant difference analysis, it was found that the activity of EV inhibiting B. subtilis, E. coli and Yeast was significantly higher than that of ZAV and EEH (P<0.05). Then, which can be seen is that EV antibacterial activity was the result of the combination of vinegar and Eucommia ulmoides leaves, which enhanced the antibacterial effect. So, the addition of Eucommia ulmoides leaves increased the antimicrobial activity of the vinegar, and EV had higher antimicrobial activity. Vinegar and EEH have their own health beneficial effects such as antibacterial activity and when vinegar is produced using EEH, it is expected that the fermentation product, EV, would have synergistic effects on the health beneficial activities. Furthermore, Eucommia ulmoides leaves are easily available and low cost and this will enable the production of EV more economical. Vinegars, containing considerable amounts of acetic acid, have been known to have strong antimicrobial activity against bacteria (Medina et al., 2007; Ozturk et al., 2015). CA effectively inhibited the growth of Bacillus subtilis 9372 (Lou et al., 2011). CA is stable in acid environment, but not in alkaline and neutral environment. Therefore, it was speculated that it can better play its bacteriostatic function in vinegar. So, it was agreed with EV antibacterial activity was the result of the combination of vinegar and Eucommia ulmoides leaves.
Table 2.
The antibacterial activity of EV
| Samples | E. coli | B. subtilis | Yeast | A. niger | Sterile water |
|---|---|---|---|---|---|
| ZAV | 7.12 ± 0.10Aa | 8.03 ± 0.10Aa | 5.71 ± 0.06Aa | 5.00 ± 0.00Aa | 5.00 ± 0.00Aa |
| EV | 7.78 ± 0.06Bb | 9.00 ± 0.08Bb | 6.40 ± 0.07Bb | 5.00 ± 0.00Aa | 5.00 ± 0.00Aa |
| EEH | 7.20 ± 0.09Cc | 5.60 ± 0.07Cc | 5.60 ± 0.05Cc | 5.00 ± 0.00Aa | 5.00 ± 0.00Aa |
Data represent mean ± standard deviation of three independent experiments. The diameter unit of inhibition zone is millimeter
A,B,CMeans within the same column, values with different letters superscripts were significantly different (P < 0.05), while the same letters superscripts mean no significant difference
a,b,cMeans within the same column, values with different letters superscripts were significant correlation (P < 0.05), while the same letters superscripts mean no significant correlation
It also could be seen from Table 2 as flows, EV had strongest antibacterial activity against B. subtilis and E. coli, strong against Yeast, but did not have antibacterial activity against A. niger. So, EV showed the strongest antibacterial activity against bacteria. By comparing the inhibition zone diameter, EV showed better antibacterial effect to B. subtilis than E. coli. Similarly, Ozturk et al. (2015) found that sensitivity of the bacteria to the vinegars was highly variable, B. cereus was observed as the most sensitive strain. It could be concluded that antibacterial effect of EV was better to gram-positive bacteria than gram-negative ones, which showed same antibacterial effect than previous studies (Zhang et al., 2017).
The effect of EV on growth curve of B. subtilis
The growth curves of the tested B. subtilis incubated with EV were shown in Fig. 1. As shown in Fig. 1, bacterial growth in the control group followed the model s-shaped growth curve. B. subtilis began to be at logarithmic phase after 2 h, and then stationary phase after 6 h. Bacterium grow significantly slower during the logarithmic phase when EV was added into the medium. Absorptions at 600 nm were almost significantly lower than the control in each growth phase after 2 h. So, EV showed an antibacterial activity after 2 h. The strongest anti-bacterial activity happened during 2 to 10 h. So, the sample of transmission electron microscopy was B. subtilis treated with EV for 6 h. The inhibition effect gradually decreased after 10 h, and reached to the minimum until 34 h. So, EV exhibited an inhibitory effect on B. subtilis.
Fig. 1.
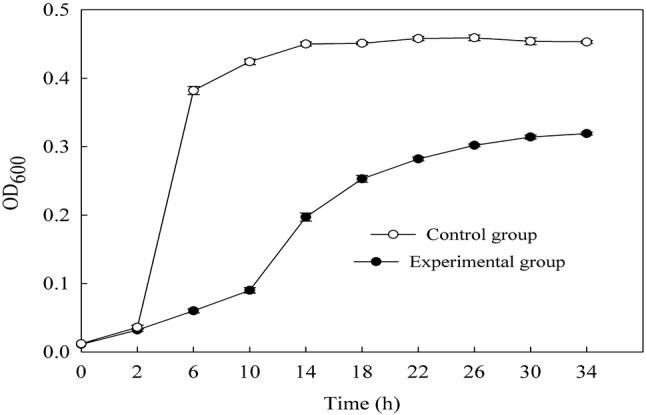
Changes of antibacterial curve of B. subtilis treated with EV
This phenomenon was similar to that observed by Ge et al. (2015) and Li et al. (2014). They concluded that the time-kill curves of bacteriostatic agent exhibited the inhibitory effect. Wang et al. (2015) reported that CA was a new class of potent inhibitors against the S. aureus growth through inhibitors against the activity of sortase A of cell wall. Lou et al. (2011) supported that CA bound to the outer membrane, disrupted the membrane, exhausted the intracellular potential, and released cytoplasm macromolecules, which led to cell death. However, which part of the cell was damaged by EV to inhibit the growth of B. subtilis still needed further study.
The effect of EV on cell wall of B. subtilis
Cell walls are located in the outermost layer of cells to keep cell shape. AKP exists between the cell wall and the cell membrane. Normally AKP does not penetrate from cell wall to out of the cell. But, when cell wall is damaged, the cell wall permeability increases, AKP will penetrate into the out of the cell. Therefore, AKP activity of culture medium reflects the damage of cell wall by anti-bacterial compounds.
The cell wall is an important anti-bacterial target of anti-bacterial compounds. According to the instruction manual of AKP assay kit, after incubation with EV for 8 h, AKP activity of the control group and the experimental group were measured respectively as showed in Fig. 2. AKP activity of the experimental group was significantly higher than that of the control group. The strongest damage happened during 0 to 2 h. Then, the damage gradually decreased after 2 h, and reached to the minimum until 8 h. Therefore, it indicated that EV badly damaged the cell wall of B. subtilis.
Fig. 2.
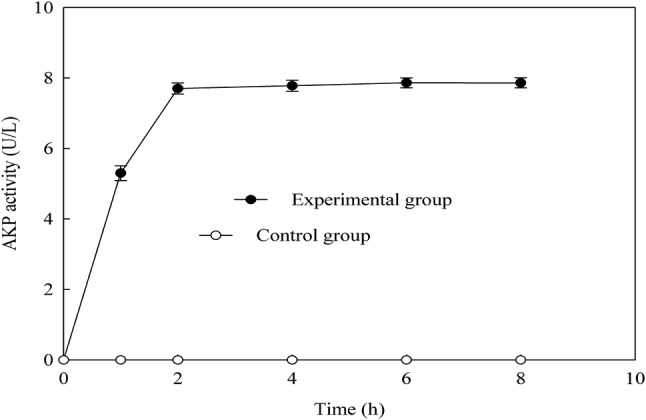
Changes of cell wall permeability of B. subtilis treated with EV
Zhang et al. (2017) observed that AKP activity of polysaccharide group was 127 U, almost 3-times as the control of 57 U. Therefore, it indicated that Cordyceps cicadae polysaccharide could damage the cell wall of E.coli. Therefore, according to the results of this experiment, the bacterial cell wall was destroyed by the EV.
The effect of EV on cell membrane of B. subtilis
Electric conductivity is a digital show method of conduction current capacity. At a certain temperature, the conductivity is related to the content of inorganic acid, alkali, salt or organic charged colloid. Cell membrane is the protective barrier of cell. When cell is in unfavorable environment or is poisoned by some drug, the fluidity of cell membrane reduce and the semipermeable property is lost, then, a lot of internal electrolytes (such as k+) permeate into the culture solution, so that the conductivity of the culture solution is increased (Hou et al., 2012; Xi et al., 2014). Therefore, Electric conductivity of culture medium reflects the damage of cell membrane by anti-bacterial compounds.
The changes of the specific conductance of B. subtilis culture medium treated with EV were indicated in Fig. 3. The experimental group showed significantly higher than the control group in the beginning. This may be caused by a large amount of electrolyte of EV. After cultured for 2 h, the electric conductivity of the experimental group reached its peak. So, during the first 2 h of cultivation, it was the highest damage value of EV to the cell membrane. At 2 h, the experimental group showed significantly higher electric conductivity than that in the beginning. In a word, it was concluded that EV badly damaged the cell membrane of B. subtilis.
Fig. 3.
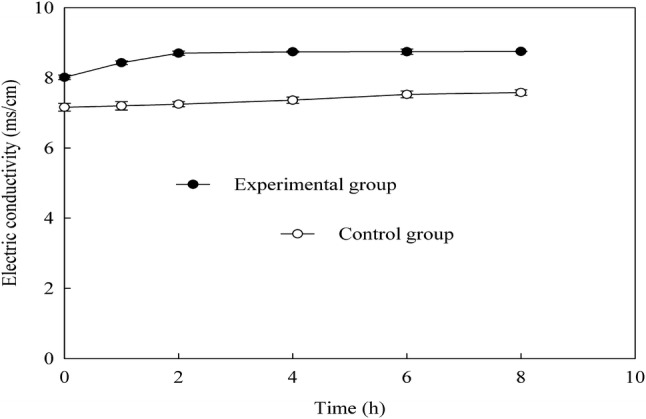
Changes of electric conductivity of B. subtilis treated with EV
Bacterial outer membranes possess several ATPase enzymes, including ATP-dependent transport proteins and F1F0 ATPase, which have a role in ATP generation and cellular pH stability (Shabala et al., 2002). Therefore, the exposure to low pH can disrupt the proton motive force leading to inactivation of H+-ATPase and also can make the bacterial outer membranes less resistant to the antibacterial agents (Lin et al., 2004). In the present work, the pH of EV was lower. So, it had a significant hurting effect on the cell membrane of B. subtili. Lou et al. (2008) reported that CA bound to the outer membrane, disrupted the membrane, exhausted the intracellular potential, and released cytoplasm macromolecules, which led to cell death. We hypothesize that the reductions in the B. subtili strains were affected by the combination of action of CA and acetic acid than the single pH action of organic acids. This assumption is also based on the findings of previous work (Wu et al., 2008).
The effect of EV on morphology of B. subtilis
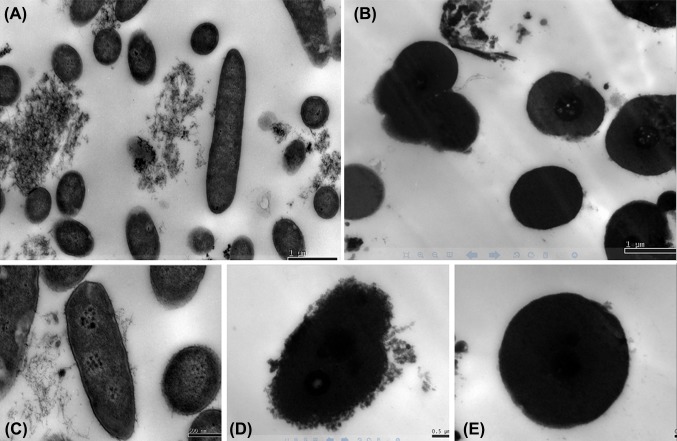
The intracellular organization changes of B. subtilis with or without EV treatment were visualized by TEM (Fig. 4). Transmission electron microscopy micrographs revealed intact cells with uniform cytoplasmic appearance and well defined walls and membranes for untreated B. subtilis (Fig. 4A, C). On the contrary, B. subtilis cells treated with EV were clearly damaged. After 6 h treatment with EV, the cytoplasmic content was observed (Fig. 4B). Furthermore, severely damages were noticed for B. subtilis cells treated such as membrane contraction, lower density, vacuolization and cell wall lysised with presence of debris evident in the lysed cells’ surrounding environment (Fig. 4D, E). This phenomenon was similar to that described in the reports, which was black pepper chloroform extract damages cell integrity and causes cell rupture and aggregate (Belguith et al., 2009; Taranto et al., 2006; Zou et al., 2015). Recently, Hatoum et al. (2013) also reported the TEM micrographs showing cell membrane perforation, cell lysis, and leakage of cellular material in Listeria monocytogenes due to antimicrobial effects of compounds released by yeast cells. Loss of cell contents evident from the SEM and TEM micrographs indicates irreversible damage to the cytoplasmic membranes. It is possibly as a result of accumulation of antimicrobial agent in the cell membrane, which is associated with an increase in permeability of the cell. The cells ultimately die due to the leakage of cytoplasmic contents and impairment of enzyme systems (Lv et al., 2011). Similar observations for polyphenols (CA) related the compounds have not only better bacteriostatic effects but also bactericidal effects (Faisal et al., 2014; Tang et al., 2017).
Fig. 4.
Transmission electron microscopy images of B.subtilis treated with EV. (A) untreated 20,000×; (B) treated 20,000× ; (C) untreated 60,000× ;(D) treated 40,000× ;(E) treated 50,000×
According to the result of the effect of EV on morphology of B. subtilis was in consistent with the effect of EV on cell wall and cell membrane. So, this further demonstrated that EV could induce seriously changes of intracellular organization of B. subtilis and showed their antibacterial action by the disruption of cell wall and cell membrane.
Acknowledgements
This study was carried out with the support of “China National Spark Project (2010GA600015)”.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chun-Feng Jia, Email: 87976976@qq.com.
Wang-Ning Yu, Email: 289214262@qq.com.
Bo-Lin Zhang, Email: zhangbolin888@163.com.
References
- Belguith H, Kthiri F, Ben Ammar A, Jaafoura H, Ben Hamida J, Landoulsi A. Morphological and biochemical changes of Salmonella hadar exposed to aqueous garlic extract. Int. J. Morphol. 2009;27:705–713. doi: 10.4067/S0717-95022009000300013. [DOI] [Google Scholar]
- Booyens J, Labuschagne MC, Thantsha MS. In vitro antibacterial mechanism of action of crude garlic (allium sativum) clove extract on selected probiotic bifidobacterium species as revealed by SEM, TEM, and SDS-PAGE analysis. Probiotics Antimicro. 2014;6:82–87. doi: 10.1007/s12602-013-9145-z. [DOI] [PubMed] [Google Scholar]
- Budak NH, Aykin E, Seydim AC, Greene AK, Guzel-Seydim ZB. Functional properties of vinegar. J. Food Sci. 2014;79:757–764. doi: 10.1111/1750-3841.12434. [DOI] [PubMed] [Google Scholar]
- Chen CX, Chen FS. Study on the conditions to brew rice vinegar with high content of γ-amino butyric acid by response surface methodology. Food Bioprod. Process. 2009;87:334–340. doi: 10.1016/j.fbp.2009.03.003. [DOI] [Google Scholar]
- Diao WR, Zhang LL, Feng SS, Xu JG. Chemical composition, antibacterial activity, and mechanism of action of the essential oil from Amomum kravanh. J. Food Prot. 2014;77:1740–1746. doi: 10.4315/0362-028X.JFP-14-014. [DOI] [PubMed] [Google Scholar]
- Eduardo C, Zlatina G, José MO, José AT, Lucília D. Vinegar production from fruit concentrates: effect on volatile composition and antioxidant activity. J. Food Sci. Tech. 2017;54:4112–4122. doi: 10.1007/s13197-017-2783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobosa ARC, Ojeda AG, Wrobel K, Magana AA, Wrobel K. Methylglyoxal is associated with bacteriostatic activity of high fructose agave syrups. Food Chem. 2014;165:444–450. doi: 10.1016/j.foodchem.2014.05.140. [DOI] [PubMed] [Google Scholar]
- Faisal K, Shigeru K, Noriko T, Soichiro N. Antimicrobial effects of chlorogenic acid and related compounds. Appl. Biol. Chem. 2014;57:359–365. doi: 10.3839/jabc.2014.057. [DOI] [Google Scholar]
- Fu XW, Zhang MZ, Huang B, Liu J, Hu HJ, Feng FQ. Enhancement of antimicrobial activities by the food-grade monolaurin microemulsion system. J. Food Process Eng. 2009;32:104–111. doi: 10.1111/j.1745-4530.2007.00209.x. [DOI] [Google Scholar]
- Ge QS, Zhang HM, Liu X, Wang SY, Lv DC, Li XD. Crude extract of maggots: Antibacterial effects against Escherichia coli, underlying mechanisms, separation and purification. World J. Gastroentero. 2015;21:1510–1517. doi: 10.3748/wjg.v21.i5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge WL, Suryoprabowo S, Zheng QK, Kuang H. Development of an immunechromategraphic test strip for the detection of papaverine in pure ginger powder. Food Agr. Immunol. 2017;28:1304–1314. doi: 10.1080/09540105.2017.1337086. [DOI] [Google Scholar]
- Han CY, Wang JQ, Li Y, Cui Y. In vitro antimicrobial activity and effect on E. Coli integrity of cinnamon essential oil and rhubarb ethanol extract. Food Sci. Technol. Res. 19: 1155-1163 (2013)
- Hatoum R, Labrie S, Fliss I. Identification and partial characterization of antilisterial compounds produced by dairy yeasts. Probiotics Antimicrob. Prot. 2013;5:8–17. doi: 10.1007/s12602-012-9109-8. [DOI] [PubMed] [Google Scholar]
- Hou WF, Xie J, Lan WQ, Zhu JW. Antimicrobial mechanisms of phytic acid against Escherichia coli. Jiangsu J. of Agr. Sci. 2012;28:443–447. [Google Scholar]
- Jia CF, Zhang BL, Rong KX, Hao JX. Study on process parameters of efficient release of chlorogenic acid from Eucommia ulmoides Oliv. Food Ind. 2013;34:57–60. [Google Scholar]
- Kaur P, Kocher GS, Phutela RP. Production of tea vinegar by batch and semicontinuous fermentation. J. Food Sci. Tech. 2011;48:755–758. doi: 10.1007/s13197-010-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobbi S, Balti R, Bougatef A, Flem GL, Firdaous L, Bigan M, Chataigné G, Chaabouni S, Dhulster P, Nedjar N. Antibacterial activity of novel peptides isolated from protein hydrolysates of RuBisCO purified from green juice alfalfa. J. Funct. Foods. 2015;18:703–713. doi: 10.1016/j.jff.2015.09.007. [DOI] [Google Scholar]
- Li L, Li ZW, Yin ZQ, Wei Q, Jia RY, Zhou LJ, Xu J, Song X, Zhou Y, Du YH, Peng LC, Kang S, Yu W. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med. 2014;7:1721–1727. [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Labbe RG, Shetty K. Inhibition of Listeria monocytogenes in fish and meat systems by use of oregano and cranberry phytochemical synergies. Appl. Environ. Microb. 2004;70:5672–5678. doi: 10.1128/AEM.70.9.5672-5678.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Liu M, Du LH, Wang DY, Geng ZM, Zhang MH, Sun C, Xu XX, Zhu YZ, Xu WM. Synergistic antibacterial effect of the combination of ε-polylysine and nisin against enterococcus faecalis. J. Food Prot. 2015;78:2200–2206. doi: 10.4315/0362-028X.JFP-15-220. [DOI] [PubMed] [Google Scholar]
- Lou ZX, Wang HX, Zhu S, Ma CY, Wang ZP. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011;76:398–403. doi: 10.1111/j.1750-3841.2011.02213.x. [DOI] [PubMed] [Google Scholar]
- Lv F, Liang H, Yuan Q, Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011;44:3057–3064. doi: 10.1016/j.foodres.2011.07.030. [DOI] [Google Scholar]
- Ma YH, Ma JG, Yang T, Cheng WH, Lu Y, Cao YH, Wang JD, Feng S. Components, antioxidant and antibacterial activity of tomato seed oil. Food Sci. Technol. Res. 2014;20:1–6. doi: 10.3136/fstr.20.1. [DOI] [Google Scholar]
- Medina E, Romero C, Brenes M, de Castro A. Antimicrobial activity of olive oil, vinegar, and various beverages against foodborne pathogens. J. Food Prot. 2007;70:1194–1199. doi: 10.4315/0362-028X-70.5.1194. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Tomiyama D, Ikeda K, Katasuki M, Nonaka A, Miyamoto T. Antibacterial effects of monoglycerol fatty acid esters and sucrose fatty acid esters on bacillus spp. Food Sci. Technol. Res. 2015;21:431–437. doi: 10.3136/fstr.21.431. [DOI] [Google Scholar]
- Ozturk I, Caliskan O, Tornuk F, Ozcan N, Yalcin H, Baslar M, Sagdic O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT Food Sci. Technol. 2015;63:144–151. doi: 10.1016/j.lwt.2015.03.003. [DOI] [Google Scholar]
- Shabala L, Budde B, Ross T, Siegumfeldt H, Jakobsen M, McMeekin T. Responses of Listeria monocytogenes to acid stress and glucose availability revealed by a novel combination of fluorescence microscopy and microelectrode ion-selective techniques. Appl. Environ. Microb. 2002;68:1794–1802. doi: 10.1128/AEM.68.4.1794-1802.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao P, Zhang JF, Chen XX, Sun PL. Microwave-assisted extraction and purification of chlorogenic acid from by-products of Eucommia Ulmoides Oliver and its potential anti-tumor activity. J. Food Sci. Tech. 2015;52:4925–4934. doi: 10.1007/s13197-014-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo H, Masahiro K, Mai K, Tetsuya H, Yasuyo Y, Hiroo Ya, Atsunori W, Keiji W, Sansei N, Kozo N. The restorative effects of Eucommia ulmoides Oliver leaf extract on vascular function in spontaneously hypertensive rats. Molecules. 20: 21971-21981 (2015) [DOI] [PMC free article] [PubMed]
- Tang C, Xie BJ, Sun ZD. Antibacterial activity and mechanism of B-type oligomeric procyanidins from lotus seedpod on enterotoxigenic Escherichia coli. J. Funct. Foods. 2017;38:454–463. doi: 10.1016/j.jff.2017.09.046. [DOI] [Google Scholar]
- Taranto MP, Perez-Martinez G, de Valdez GF. Effect of bile acid on the cell membrane functionality of lactic acid bacteria for oral administration. Res Microbiol. 2006;157:720–725. doi: 10.1016/j.resmic.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Wang EB, Jin BF, Li X, Liu RL, Xie XR, Guo WF, Zheng HX, Zhao ZB. Comparative analysis between aerial parts and roots (astragali radix) of astragalus membranaceus by NMR-based metabolomics. Food Agr. Immunol. 2017;2:1126–1141. doi: 10.1080/09540105.2017.1332007. [DOI] [Google Scholar]
- Wang HT, Zou D, Xie KP, Xie MJ. Antibacterial mechanism of fraxetin against Staphylococcus aureus. Mol. Med. Rep. 2014;10:2341–2345. doi: 10.3892/mmr.2014.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Bi C, Cai H, Liu B, Zhong X, Deng X, Wang T, Xiang H, Niu X, Wang D. The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front. Microbiol. 2015;6:1031. doi: 10.3389/fmicb.2015.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SG, Yao JY, Zhou B, Yang JX, Chaudry MT, Wang M, Xiao FL, Li Y, Yin WZ. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 2018;81:68–78. doi: 10.4315/0362-028X.JFP-17-214. [DOI] [PubMed] [Google Scholar]
- Wu H, Zhu JX, Yang L, Wang R, Wang CR. Ultrasonic-assisted enzymatic extraction of phenolics from broccoli (Brassica oleracea L. var. italica) inflorescences and evaluation of antioxidant activity in vitro. J. Food Sci. Technol. 21: 306-319 (2014) [DOI] [PubMed]
- Wu JJ, Ma YK, Zhang FF, Chen FS. Biodiversity of yeasts, lactic acid bacteria and acetic acid bacteria in the fermentation of “shanxi aged vinegar”, a traditional Chinese vinegar. Food Microbiol. 2012;30:289–297. doi: 10.1016/j.fm.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Wu VCH, Qiu X, Bushway A, Harper L. Antibacterial effects of American cranberry (Vaccinium macrocarpon) concentrate on foodborne pathogens. LWT Food Sci. Technol. 2008;41:1834–1841. doi: 10.1016/j.lwt.2008.01.001. [DOI] [Google Scholar]
- Xi D, Wang XM, Teng D, Mao RY, Zhang Y, Wang XJ, Wang JH. Mechanism of action of the trihybrid antimicrobial peptide LHP7 from lactoferricin, HP and plectasin on Staphylococcus aureus. Biometals. 2014;27:957–968. doi: 10.1007/s10534-014-9768-x. [DOI] [PubMed] [Google Scholar]
- Yan SS, Shao HJ, Zhou ZH, Wang Q, Zhao LH, Yang XB. Non-extractable polyphenols of green tea and their antioxidant, anti-α-glucosidase capacity, and release during in vitro digestion. J. Funct. Foods. 2018;42:129–136. doi: 10.1016/j.jff.2018.01.006. [DOI] [Google Scholar]
- Zhang Y, Wu YT, Zheng W, Han XX, Jiang YH, Hu PL, Tang ZX, Shi LE. The antibacterial activity and antibacterial mechanism of a polysaccharide from cordyceps cicadae. J. Funct. Foods. 2017;38:273–279. doi: 10.1016/j.jff.2017.09.047. [DOI] [Google Scholar]
- Zou L, Hu YY, Chen WX. Antibacterial mechanism and activities of black pepper chloroform extract. J. Food Sci. Technol. 2015;52:8196–8203. doi: 10.1007/s13197-015-1914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]