Abstract
Active components were extracted from Angelica gigas Nakai by subcritical-water extraction (SWE) with the purpose of determining how the extraction conditions affect the SWE of antioxidant properties and active components (nodakenin and decursin), and to compare pilot-scale SWE (8 L) and conventional extraction methods. The extraction yields of nodakenin and decursin in the pilot-scale system were highest at 150 °C for 10 min and 190 °C for 15 min, respectively. The extraction yield of decursin increased as the stirring speed was increased to 200–250 rpm. Pearson’s correlation indicated that the radical-scavenging activities using DPPH and ABTS assays were more sensitive to the Maillard reaction (R2 = 0.822 and 0.933, respectively) than to the total phenolic contents (R2 = 0.486 and 0.724, respectively). The extraction yield of decursin was higher when using conventional extraction methods than for SWE.
Keywords: Subcritical water extraction, Angelica gigas Nakai, Pilot-scale system, Decursin, Nodakenin
Introduction
The dried roots of Angelica gigas Nakai (AN), which is also called Cham Danggui, have been used for medicinal purposes in Korea for more than 2000 years (Ahn et al., 1996). They have also been used to treat anemia, circulatory disorders, hemopoietic disease, enervation, and other promoting activities, especially by females (Choi et al., 2003; Li et al., 2011; Sarker and Nahar, 2004). Several studies have shown that AN can also exert immune-stimulatory, anticancer, and acetylcholinesterase (AChE)-inhibitory effects (Ahn et al., 1995; Kang et al., 2001).
Coumarin derivatives are representative components of AN such as decursin, decursinol angelate, nodakenin, umbelliferone, and marmesin (Ahn et al., 2008). Among them, decursin is a major active compound in AN that exerts positive effects against various diseases, oxidative stress, and inflammation. It is also known to be effective in treating obesity and diabetes (Hwang et al., 2012). Nodakenin is abundant in AN and shows effects on AChE-inhibitory activity that are as strong as those of decursinol (Kang et al., 2001). Nodakenin is a type of vegetable female hormone, and is known to be effective in treating uterine function, sedation, diarrhea, and vitamin E deficiency.
The traditional methods for extracting major compounds from AN employ organic solvents such as methanol and ethanol, because most of these compounds (including decursin, nodakenin, decursinol angelate, and other phenol compounds) are hydrophobic (Ahn et al., 2008; Li et al., 2011). However, using organic solvents to perform extraction has some disadvantages, including the extraction methods being time-consuming and their products being costly to dispose of, having negative impacts on the environment, and harming human health (Camel, 2001; Ramos et al., 2002). This situation has lead to a recent focus on alternative extraction methods.
Extraction techniques such as supercritical-fluid extraction and pressurized-liquid extraction are known to be more sustainable and efficient than those based on organic solvents, because they use ecofriendly solvents such as water and involve a shorter extraction process (Fernández-Ponce et al., 2016). One of the alternative extraction methods is subcritical-water extraction (SWE). Subcritical water is kept in the liquid state from 100 (the boiling temperature) to 374 °C (the critical temperature) by the application of pressure, and its dielectric constant (ε) varies with the temperature. Water is generally polar with strong hydrogen bonds and a large dielectric constant at room temperature. However, as the temperature is increased, the polarity of water decreases (from ε = 80 at 25 to ε = 27 at 250 °C) and its dielectric constant becomes similar to that of methanol and ethanol at room temperature (ε = 33 and 24, respectively) (Teo et al., 2010). At high temperatures the hydrogen bonds are weakened due to changes in the polarity of water, which makes it more suitable for extracting nonpolar compounds. These characteristics make it possible to selectively extract polar or nonpolar compounds by changing the temperature and pressure. Moreover, SWE can be expected to form new compounds by chemical reactions. According to Zhang et al. (2016), the heating that occurs in SWE could lead the appearance of nonenzymatic browning reactions (the Maillard reaction and caramelization), resulting in a brown color and the formation of antioxidant compounds called melanoidins.
SWE has advantages over using organic solvents in terms of providing a higher extraction yield, greater purity of the extract, and lower disposal costs (Ravber et al., 2015). Furthermore, it is an ecofriendly extraction method since only nontoxic solvents are used (Kwon and Chung, 2015). These advantages of SWE could make it an excellent method for extracting bioactive compounds from plants, vegetables, and fruits.
SWE systems can be categorized into two main modes: dynamic and static. In dynamic mode, the extraction solvent flows continuously through a sample vessel during the extraction time while controlling the flow rate. The extraction temperature, time, and flow rate can be important factors for the efficacy of SWE in this mode (Plaza and Turner, 2015). In static mode, the solvent remains in the sample vessel for a certain extraction time, and the important factors in this mode are the extraction temperature and time. SWE was conducted in the static mode in the present study.
Pilot-scale systems are often investigated before designing industrial-scale equipment. However, literature related to the scaling up of SWE systems is lacking, and so the purposes of this research were as follows: (1) to determine the effects of SWE conditions (extraction temperature, time, and stirring speed) on the antioxidant properties of nodakenin and decursin extracted from AN in a pilot-scale SWE system and (2) to compare the efficiencies of the SWE system with those of conventional extraction methods using methanol and ethanol.
Materials and methods
Sample preparation and chemicals
AN was obtained from Dongeherb (Gwangjin-Gu, Seoul, Korea). The samples were cut into small pieces and stored at 4 °C before extraction.
1,1-Diphenyl-2-picryl-hydrazyl-hydrate (DPPH, ≥ 100%), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, ≥ 98%), gallic acid (3,4,5-trihydroxybenzoic acid, ≥ 99%), Folin–Ciocalteu reagent, and potassium persulfate (≥ 99%) were purchased from Sigma-Aldrich (Yongin, Gyunggi-do, Korea). Standard samples of nodakenin and decursin for use in identification and quantification were also purchased from Sigma-Aldrich. Acetonitrile and water were used as high-performance liquid chromatography (HPLC) solvents and obtained from J.T. Baker (Phillipsburg, NJ, USA). Ethanol (95%; Samchun, Pyeongtaek, Gyunggi-do, Korea), 99.8% methanol, and sodium carbonate (Duksan, Ansan, Gyeonggi-do, Korea) were also used.
Pilot-scale SWE
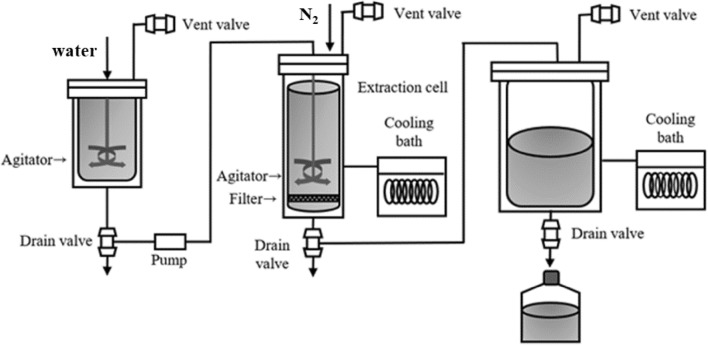
The pilot-scale system consisted of a water tank with a preheater, an extractor, and a collector (Fig. 1). The preheater and extractor included an agitator to ensure that the sample and solvent were mix thoroughly. The extraction procedure was performed according to Ko et al. (2016). The extraction cell (8 L) containing filter paper on its bottom was filled with AN (50 g) and placed in the extractor. Preheated water (1.1 L, at a temperature of 70–80 °C) was added to the extractor, which was then heated to the desired extraction temperature with the agitator turned on, and the extraction temperature was then maintained during the desired extraction time (around 5 MPa). After the extraction time had elapsed, the aqueous extracts were transferred to the collector automatically through the collector vent valve. The residual extracts in the extractor were then removed using nitrogen gas. Part of the extracts (40 mL) was lyophilized and kept at 4 °C for use in the subsequent experiments. The extraction was carried out at temperatures of 150 °C, 170 °C, 190 °C, and 210 °C for extraction times of 5, 10, 15, and 20 min.
Fig. 1.
Schematic diagram of pilot-scale SWE system
Conventional extraction methods
Nodakenin and decursin are usually extracted from AN with the aid of organic solvents due to the low solubility of water (Ahn et al., 2008; Choi et al., 2003). Therefore, to assess the suitability of applying SWE to AN, conventional extraction processes were also carried out using 95% ethanol and 99.8% methanol. Two grams of AN were extracted using 44 mL of solvent. The extractions were performed at 60 °C for 2 h using a water bath. After filtering using filter paper, the organic solvent extracts were evaporated under a vacuum and then the extracts were pretreated at a certain concentration using 99.8% methanol. Each extraction was performed in triplicate.
HPLC analysis
For the HPLC analysis, each freeze-dried sample (20 mg) extracted by SWE was pretreated with 99.8% methanol (10 mL) and sonicated for 2 h. The pretreated extract was then filtered through a 0.45-μm polyvinylidene fluoride filter (Whatman, Maidstone, Kent, UK). The amounts of nodakenin and decursin extracted were determined using HPLC (1200 series, Agilent Technologies, Santa Clara, CA, USA) with a Zorbax Eclipse Plus C18 column (4.6 mm × 100 mm, 5 μm pore size; Agilent Technologies). Gradient elution was performed with a mobile phase comprising water (solvent A) and acetonitrile (solvent B). The gradient program was as follows: 0–3 min, 20% solvent B; 3–18 min, 30% solvent B; 18–40 min, 50% solvent B; and 40–50 min, 90% solvent B. Ten microliters of the aliquots were injected, the mobile phase was pumped at a flow rate of 1.0 mL/min, and detection was conducted at 330 nm using a UV detector set. The extraction yield was calculated from calibration curves of the standard samples of nodakenin and decursin.
Total phenolic contents
The total phenolic contents were analyzed using the Folin–Ciocalteu method. The extract obtained by SWE (10 mg) was homogeneously mixed with 99.8% methanol (10 mL) and sonicated for 2 h. After mixing well, it was filtered through a 0.45-μm PVDF filter. Folin–Ciocalteu reagent (50%, 0.1 mL) and Na2CO3 (2%, 2 mL) were then added to 0.1 mL of the pretreated extract. The mixture was vortexed for 3 min and then incubated at room temperature for 30 min before measuring its absorbance at 700 nm using a spectrophotometer (Evolution 200, Thermo Scientific, Waltham, MA, USA). The total phenolic contents were calculated in units of gallic acid equivalents (milligrams of GAE per gram of AN) from the calibration curve (y = 1.081x + 0.047; R2 = 0.994).
DPPH-radical-scavenging activity
DPPH and ABTS assays were used to determine the antioxidant capacity of the extracts obtained by SWE. Five milligrams of the extract obtained by SWE were homogeneously mixed with 10 mL of 99.8% methanol, sonicated for 2 h, and filtered through a 0.45-μm PVDF filter. The pretreated extract (0.2 mL) was mixed with 1 mL of 100 μM DPPH. The mixture was then kept at room temperature for 15 min before measuring its absorbance at 517 nm using the spectrophotometer.
The activity was quantified as follows:
where Ablank is the absorbance of a blank (using methanol only) and Asample is the absorbance of the pretreated extract.
ABTS-radical-scavenging activity
ABTS solution (7 mM) was mixed with potassium persulfate to produce 2.45 mM potassium persulfate solution. The mixture was left in the dark at room temperature for 12 h to produce ABTS·+. The ABTS·+ solution was then dissolved in anhydrous ethanol so that it exhibited an absorbance of 0.70 ± 0.02 arbitrary units at 734 nm when measured using the spectrophotometer. The pretreated extract (0.1 mL) containing 5 mg in 10 mL of methanol was mixed with 0.9 mL of the ABTS·+ solution. The mixture was kept at room temperature for 6 min and then its absorbance was measured at 734 nm using the spectrophotometer.
The activity was quantified as follows:
where Ablank is the absorbance of a blank (using methanol only) and Asample is the absorbance of the pretreated extract.
Levels of the products of the Maillard reaction
The Maillard reaction and caramelization can be carried out under SWE conditions, and the final products of the Maillard reaction (MRPs) are called melanoidins (Yilmaz and Toledo, 2005). The amounts of melanoidins formed were estimated using the method of Plaza et al. (2010a; 2010b). The absorbance of each pretreated extract (5 mg in 10 mL of methanol) was measured at 420 nm using the spectrophotometer.
Statistical analysis
All data were expressed as mean ± standard-deviation values. One-way analysis of variance with Tukey’s test as well as Pearson’s correlation analysis were performed using SPSS software (version 24.0, IBM SPSS, Chicago, IL, USA).
Results and discussion
Effects of extraction temperature and time
The calibration curve was y = 28383x − 9.9525 (R2 = 0.999) for nodakenin and y = 27550x − 46.608 (R2 = 0.999) for decursin. The limits of detection and limits of quantification for the standards as determined at signal-to-noise ratios of about 3 and 10, respectively, were 0.000479 and 0.001452 mg/mL, respectively, for nodakenin, and 0.003410 and 0.010333 mg/mL, respectively, for decursin. The precision quantified as %RSD (percentage relative standard deviation) was estimated in triplicate within 1 day. A standard solution at a certain concentration was tested. %RSD was 4.76% for nodakenin and 3.44% for decursin.
The effects of extraction temperature and time on the amounts of nodakenin and decursin obtained from AN in the pilot-scale system are shown in Fig. 2A, B. The extraction yield of nodakenin was highest at 150 °C for 10 min (4.33 ± 0.18 mg/g AN), and decreased for extraction temperatures exceeding 170 °C and over time. The results indicated that although a high temperature improves the extraction efficiency, the extraction yield of nodakenin decreases due to degradation resulting from its solubility and thermal instability. The extraction yield of decursin was highest at 190 °C for 15 min (17.77 ± 2.39 mg/g AN). It increased for extraction times up to 15 min at temperatures of 190 °C and 210 °C, but then decreased for extraction times longer than 20 min at these temperatures. The solubility of decursin increases with temperature. A short extraction time might result in inadequate SWE efficiency, while extended extraction times (> 15 min) may cause decursin to decompose due to its thermal instability. These results were similar to a previous study which showed that decursin was more stable than nodakenin at high temperature (Ko et al., 2017a).
Fig. 2.
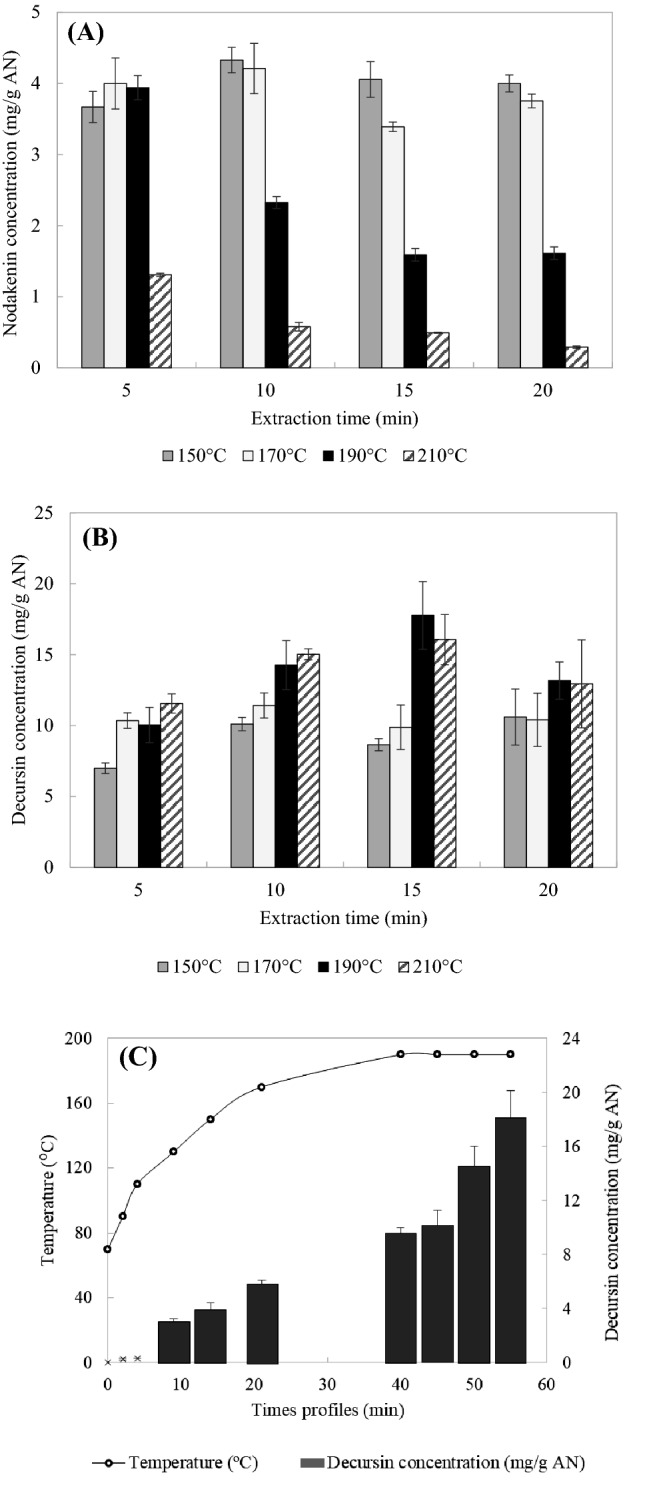
Nodakenin (A), decursin (B) concentrations in the extracts obtained from AN by SWE in the pilot-scale system. Extraction yield of decursin during the heating process in the pilot-scale SWE system at 190 °C for 15 min (C). Data are mean and standard-deviation values
The extraction temperature and time are clearly critical factors of SWE, with the extraction temperature being an especially important factor for optimum SWE. Increasing the extraction temperature of subcritical water enhances the extraction of target analytes by increasing the diffusion coefficient of the solvent, reducing surface tension, and allowing the solvent to penetrate the sample more deeply (Tomšika et al., 2017). Furthermore, when the water solvent is at a higher temperature it has a lower viscosity and a greater ability to disrupt the intermolecular interactions and remove the target analytes interacting with the solvent (Brunner, 2009; Pongnaravane et al., 2006). However, it is very important to control the temperature correctly when extracting compounds that are highly sensitive to temperature.
In some samples the target compounds will be present within pores or other structures. Increasing the extraction time at high temperature will allow the target compounds to be removed from the sample and diffuse into the solvent. However, a prolonged heating time could result in the compounds decomposing (Tomšika et al., 2017). It is therefore very important to optimize the extraction time. It is generally recommended that extraction should be completed within 20 min. If longer extraction times are required at higher extraction temperatures, the use of other solvents or consideration of additional factors such as the stirring speed, pressure, or other parameters of the static mode should be controlled.
The extraction yield of decursin in the pilot-scale system increased gradually during the heating time required to reach the target extraction temperature (Fig. 2C). The heating time required to reach the target extraction temperature in the pilot-scale system (> 40 min) is longer than it would be in an industrial system. The AN samples in the pilot-scale system were therefore exposed to the solvent for a longer time period, resulting in the extraction yield varying with the compound stability.
Effects of stirring speed
The stirring speed in the extractor was an important factor affecting the extraction process in the pilot-scale system. The presence of an agitator in the extractor can enhance mass transfer between the solvent and solute. Solutions under the optimum condition (190 °C for 15 min) for extracting decursin from AN were stirred at different speeds to investigate the effect of agitation. The amounts of decursin extracted from AN were 8.36 ± 0.26, 10.51 ± 0.60, 17.77 ± 2.39, and 6.66 ± 0.62 mg/g AN for stirring speeds of 0, 100–125, 200–250, and 300–375 rpm, respectively. This indicates that the extraction yield of decursin increased as the stirring speed increased to 200–250 rpm, and then decreased markedly at 300–375 rpm (Fig. 3A).
Fig. 3.
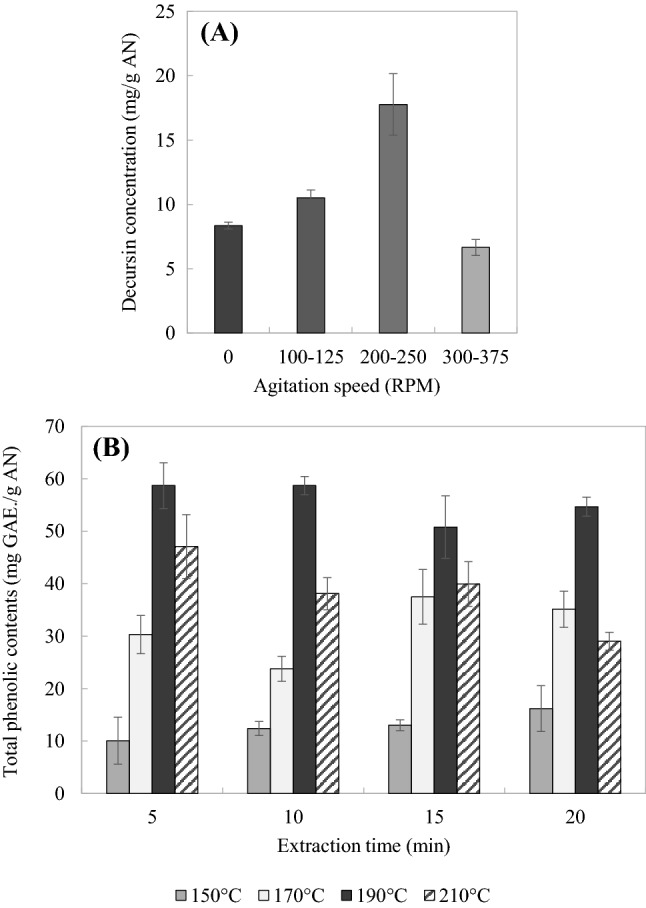
Effect of stirring speed (A), total phenolic contents (B) of the extracts obtained from AN by SWE in the pilot-scale system
Agitation in the extraction process induces physical changes. Increasing the stirring speed will increase the exposed surface area of the solids present and the rate of mass transfer. Increasing the stirring speed will therefore increase the extraction efficiency and so reduce the required extraction time (Xiao et al., 2006). However, when the stirring speed increases, the solvent travels in a circular motion due to a vortex being produced by the higher centrifugal forces. This does not facilitate mixing between the sample and solvent due to poor mixing between adjacent solvent and solute resulting from the very similar angular velocities of the agitator and solvent. This mechanism explains why the extraction yield of decursin decreased at stirring speeds of 300–375 rpm.
Total phenolic contents
It has been found that phenolic compounds are the main contributors to the antioxidant activities of plant-based foodstuffs (Velioglu et al., 1998). It is therefore important to measure the total phenolic contents obtained from AN according to the SWE conditions.
As shown in Fig. 3B, the highest total phenolic contents was 58.70 ± 1.73 mg GAE/g AN, as obtained at 190 °C for 10 min. The total phenolic contents increased with the extraction time at extraction temperatures of 150 °C and 170 °C. However, the contents decreased as the extraction time increased at an extraction temperature of 210 °C. The total phenolic contents increased at high temperature, which indicates an increased amount of free phenolic compounds being converted from bound phenolic compounds (Choi et al., 2006). However, the total phenolic contents decreased due to the heat degradation of phenolic compounds in this study. These results are similar to Lee et al. (2018) reporting that the total phenolic contents of red ginseng decreased as the extraction time increased.
Radical-scavenging activities
The DPPH- and ABTS-radical scavenging activities of the AN extracts obtained by SWE were analyzed (Fig. 4). The DPPH-radical-scavenging activity increased as the extraction temperature and time increased, peaking at 210 °C for 20 min (74.36 ± 3.95%). The results for the ABTS-radical-scavenging activity tended to be similar to those for the DPPH-radical-scavenging activity, in that it also increased with both the extraction temperature and time, peaking at 210 °C for 20 min (54.95 ± 1.67%).
Fig. 4.
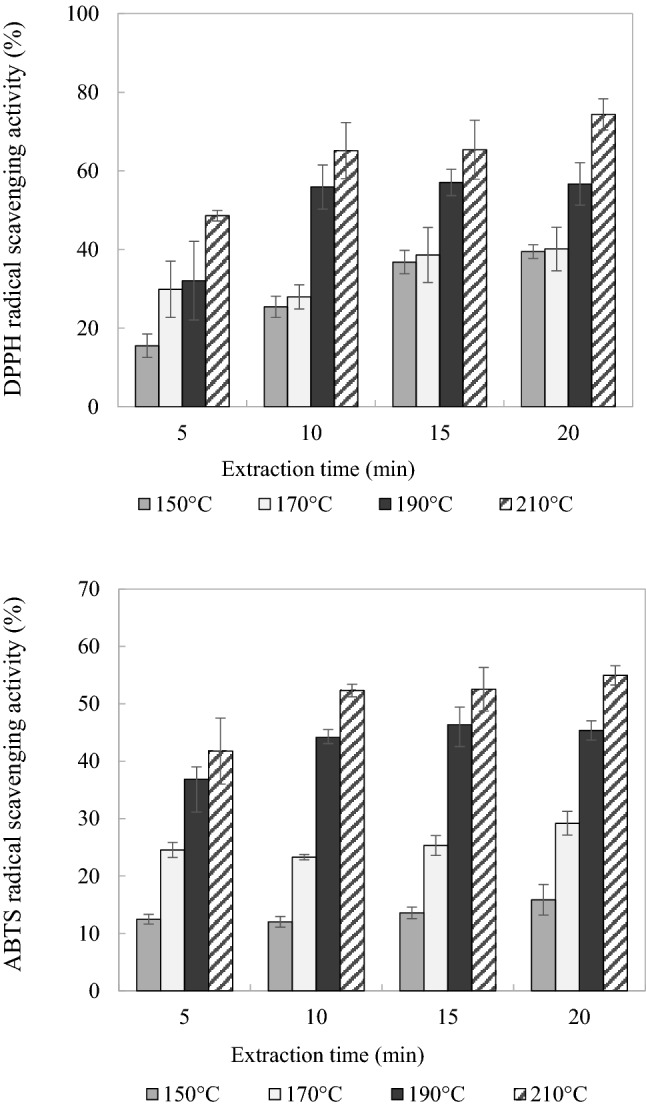
DPPH- and ABTS-radical-scavenging activities of the extracts obtained from AN by SWE in the pilot-scale system
Many studies have found a strong correlation between the total phenolic contents and radical-scavenging activities (Dudonné et al., 2009). However, we found that the radical-scavenging activities were only weakly correlated with the total phenolic contents by analyzing the data for the total phenolic contents and radical-scavenging activities (R2 = 0.486 for DPPH and R2 = 0.724 for ABTS). From these results it can be concluded that other compounds in AN affect the antioxidant activities. These results are also similar to those of Ko et al. (2017b) and show that differences in scavenging activity between the assays may be attributed to differences in the active compounds. The ABTS assay produced relatively strong correlations for the total phenolic compounds in the range of extraction times for extraction temperatures in SWE.
Levels of the MRPs
The Maillard reaction begins with the condensation between a carbonyl group of reducing sugars or aldehydes and an amine group of amino acids or proteins, forming brown nitrogenous compounds called melanoidins. During SWE, the components of the sample are released or new compounds can be formed by the Maillard reaction (Plaza et al., 2010a; 2010b).
The levels of the MRPs increased with the extraction time at temperatures ranging from 150 to 190 °C, as shown in Fig. 5. The rate of the Maillard reaction can be affected by various factors including pH, time, temperature, and the concentration and type of reactants. The reaction rate will be faster for higher temperatures and longer heating times, which significantly impacts the formation of melanoidins, and these factors would have affected the results obtained in the present study (Martins et al., 2000).
Fig. 5.
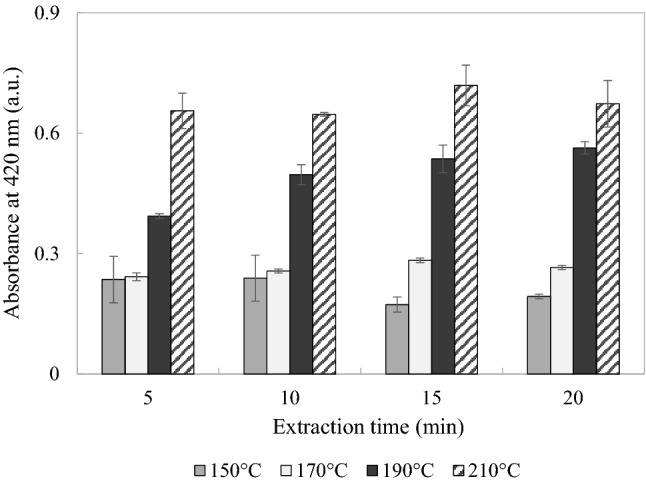
Levels of the MRPs in the extracts obtained from AN by SWE in the pilot-scale system
Correlation between antioxidant properties
Pearson’s correlation was used to analyze the association between total phenolic contents, radical-scavenging activities, and levels of the MRPs. The results revealed strong correlations between the DPPH- and ABTS-radical-scavenging activities (R2 = 0.873) and between the radical-scavenging activities and levels of the MRPs (R2 = 0.822 for DPPH and R2 = 0.933 for ABTS).
These results indicate that the DPPH- and ABTS-radical-scavenging activities were more sensitive to the Maillard reaction than to the total phenolic contents in this study. The MRPs, especially melanoidins, exhibit antioxidant activities through scavenging oxygen radicals, or chelating metals as reported by Yilmaz and Toledo (2005). Moreover, the MRPs synthesized from lysine and arginine are more reactive than other amino acids (Morales and Jimenez-Perez, 2001; Tan and Harris, 1995). The amino group of lysine and arginine, which is far from the carboxyl group, can readily interact with the carbonyl group of saccharide and its derivatives, and so melanoidins can be formed faster than other amino acids. The main amino acids of AN are arginine, proline, and lysine, which results in melanoidins being formed more rapidly, which in turn have a significant impact on the antioxidant capacity (Kil et al., 2015).
Comparison with conventional extraction methods
The decursin concentrations and total phenolic contents obtained by SWE and the conventional extraction methods using methanol and ethanol are shown in Fig. 6. The extraction yield of decursin was lower for SWE than when using conventional extraction methods with methanol (24.00 ± 1.21 mg/g AN) and ethanol (21.93 ± 2.10 mg/g AN). This was due to decursin being highly hydrophobic, and hence dissolving well in the organic solvents. However, the results were different for the total phenolic contents obtained using SWE and the conventional extraction methods. Using subcritical water produced higher total phenolic contents than using methanol (30.38 ± 1.84 mg GAE/g AN) or ethanol (20.29 ± 1.65 mg GAE/g AN). These results indicate that SWE is an efficient method for extracting phenolic compounds other than coumarins such as decursin and nodakenin. Kwon and Chung (2015) found that as the ethanol concentration was increased, the solubility increased and the dielectric constant of water decreased, resulting in an improved extraction yield for curcuminoids obtained by SWE. This result means that it can be recommended to use a mixture of water and organic solvents as a subcritical solvent to increase the extraction yield of coumarins in SWE.
Fig. 6.
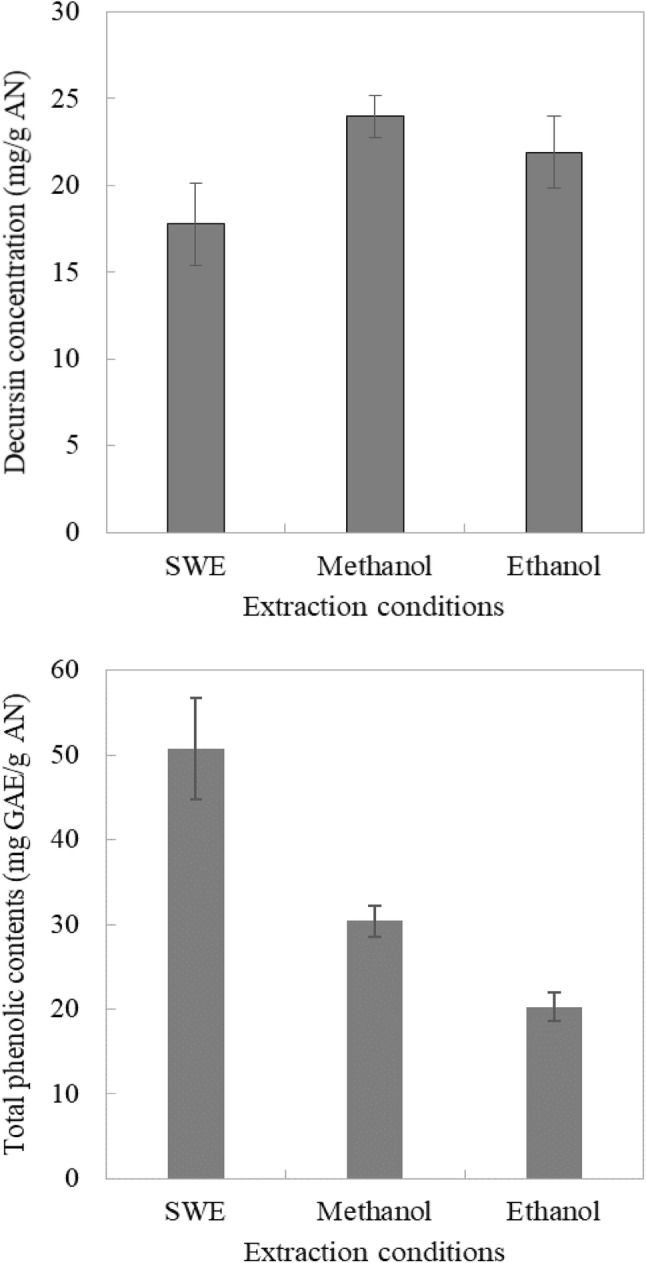
Comparison of amounts of decursin and total phenolic contents obtained by SWE (190 °C, 15 min) and the conventional extraction methods (60 °C, 2 h). Different letters indicate significant differences
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03930300).
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Min-Jung Ko, Email: mjko@hknu.ac.kr.
Mi-Ri Kwon, Email: aoxoa07@ewhain.net.
Myong-Soo Chung, Email: mschung@ewha.ac.kr.
References
- Ahn KS, Sim WS, Kim IH. Detection of anticancer activity from the root of Angelica gigas in vitro. J. Microbiol. Biotechnol. 1995;5:105–109. [Google Scholar]
- Ahn KS, Sim WS, Kim HM, Han SB, Kim IH. Immunostimulating components from the root of Angelica gigas Nakai. Korean J. Pharmacogn. 1996;27:254–261. [Google Scholar]
- Ahn MJ, Lee MK, Kim YC, Sung SH. The simultaneous determination of coumarins in Angelica gigas root by high performance liquid chromatography–diode array detector coupled with electrospray ionization/mass spectrometry. J. Pharmaceut. Biomed. 2008;46:258–266. doi: 10.1016/j.jpba.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Brunner G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J. Supercrit. Fluid. 47: 373-381 (2009)
- Camel V. Recent extraction techniques for solid matrices—supercritical fluid extraction, pressurized fluid extraction and microwave-assisted extraction: their potential and pitfalls. Analyst. 2001;126:1182–1193. doi: 10.1039/b008243k. [DOI] [PubMed] [Google Scholar]
- Choi SS, Han KJ, Lee HK, Han EJ, Suh HW. Antinociceptive profiles of crude extract from roots of Angelica gigas NAKAI in various pain models. Biol. Pharm. Bull. 2003;26:1283–1288. doi: 10.1248/bpb.26.1283. [DOI] [PubMed] [Google Scholar]
- Choi Y, Lee SM, Chun J, Lee HB, Lee J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006;99:381–387. doi: 10.1016/j.foodchem.2005.08.004. [DOI] [Google Scholar]
- Dudonné S, Vitrac X, Coutière P, Woillez M, Mérillon JM. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009;57:1768–1774. doi: 10.1021/jf803011r. [DOI] [PubMed] [Google Scholar]
- Fernández-Ponce MT, Parjikolaei BR, Lari HN, Casas L, Mantell C, Ossa E. Pilot-plant scale extraction of phenolic compounds from mango leaves using different green techniques: Kinetic and scale up study. Chem. Eng. J. 2016;299:420–430. doi: 10.1016/j.cej.2016.04.046. [DOI] [Google Scholar]
- Hwang JT, Kimg SH, Hur HJ, Kim HJ, Park JH, Sung MJ, Yang HJ, Ryu SY, Kim YS, Cha MR, Kim MS, Kwon DY. Decursin, an active compound isolated from Angelica gigas, inhibits fat accumulation, reduces adipocytokine secretion and improves glucose tolerance in mice fed a high-fat diet. Phytother. Res. 2012;26:633–638. doi: 10.1002/ptr.3612. [DOI] [PubMed] [Google Scholar]
- Kang SY, Lee KY, Sung SH, Park MJ, Kim YC. Coumarins isolated from Angelica gigas inhibit acetylcholinesterase: structure-activity relationships. J. Nat. Prod. 2001;64:683–685. doi: 10.1021/np000441w. [DOI] [PubMed] [Google Scholar]
- Kil HY, Seong ES, Sim JM, Choi SK, Heo K, Yu CY. Characterization of inorganic components, free sugars, amino acids, and fatty acids in Angelica gigas Nakai. Korean J. Med. Crop Sci. 2015;23:454–459. doi: 10.7783/KJMCS.2015.23.6.454. [DOI] [Google Scholar]
- Ko MJ, Kwon HL, Chung MS. Pilot-scale subcritical water extraction of flavonoids from satsuma mandarin (Citrus unshiu Markovich) peel. Innov. Food Sci. Emerg. 2016;38:175–181. doi: 10.1016/j.ifset.2016.10.008. [DOI] [Google Scholar]
- Ko MJ, Lee JH, Nam HH, Chung MS. Antioxidant activities of phenolic compounds from Angelica gigas Nakai extract using subcritical water. Korean J. Food Sci. Technol. 2017;49:44–49. doi: 10.9721/KJFST.2017.49.1.44. [DOI] [Google Scholar]
- Ko MJ, Lee JH, Nam HH, Chung MS. Subcritical water extraction of phytochemicals from Phlomis umbrosa Turcz. Innov. Food Sci. Emerg. 2017;42:1–7. doi: 10.1016/j.ifset.2017.05.009. [DOI] [Google Scholar]
- Kwon HL, Chung MS. Pilot-scale subcritical solvent extraction of curcuminoids from Curcuma long L. Food Chem. 2015;185:58–64. doi: 10.1016/j.foodchem.2015.03.114. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ko MJ, Chung MS. Subcritical water extraction of bioactive components from red ginseng (Panax ginseng C.A. Meyer). J. Supercrit. Fluid. 133: 177-183 (2018)
- Li L, Li W, Jung SW, Lee YW, Kim YH. Protective effects of decursin and decursinol angelate against amyloid β-protein-induced oxidative stress in the PC12 cell line: the role of Nrf2 and antioxidant enzymes. Biosci. Biotechnol. Biochem. 2011;75:434–442. doi: 10.1271/bbb.100606. [DOI] [PubMed] [Google Scholar]
- Martins S, Jongen W, Boekel M. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000;11:364–373. doi: 10.1016/S0924-2244(01)00022-X. [DOI] [Google Scholar]
- Morales FJ, Jimenez-Perez S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001;72:119–125. doi: 10.1016/S0308-8146(00)00239-9. [DOI] [Google Scholar]
- Plaza M, Turner C. Pressurized hot water extraction of bioactives. Trend. Anal. Chem. 2015;71:39–54. doi: 10.1016/j.trac.2015.02.022. [DOI] [Google Scholar]
- Plaza M, Amigo-Benavent M, Castillo MD, Ibáñez E, Herrero M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res. Int. 2010;43:2341–2348. doi: 10.1016/j.foodres.2010.07.036. [DOI] [Google Scholar]
- Plaza M, Amigo-Benavent M, Castillo MD, Ibáñez E, Herrero M. Neoformation of antioxidants in glycation model systems treated under subcritical water extraction conditions. Food Res. Int. 2010;43:1123–1129. doi: 10.1016/j.foodres.2010.02.005. [DOI] [Google Scholar]
- Pongnaravane B, Goto M, Sasaki M, Anekpankul T, Pavasant P, Shotipruk A. Extraction of anthraquinones from roots of Morinda citrifolia by pressurized hot water: Antioxidant activity of extracts. J. Supercrit. Fluid. 2006;37:390–396. doi: 10.1016/j.supflu.2005.12.013. [DOI] [Google Scholar]
- Ramos L, Kristenson EM, Brinkman UAT. Current use of pressurised liquid extraction and subcritical water extraction in environmental analysis. J. Chromatogr. A. 2002;975:3–29. doi: 10.1016/S0021-9673(02)01336-5. [DOI] [PubMed] [Google Scholar]
- Ravber M, Knez Z, Skerget M. Optimization of hydrolysis of rutin in subcritical water using response surface methodology. J. Supercrit. Fluid. 2015;104:145–152. doi: 10.1016/j.supflu.2015.05.028. [DOI] [Google Scholar]
- Sarker SD, Nahar L. Natural medicine: the genus Angelica. Curr. Med. Chem. 2004;11:1479–1500. doi: 10.2174/0929867043365189. [DOI] [PubMed] [Google Scholar]
- Tan BK, Harris ND. Maillard reaction products inhibit apple polyphenoloxidase. Food Chem. 1995;53:267–273. doi: 10.1016/0308-8146(95)93932-H. [DOI] [Google Scholar]
- Teo CC, Tan SN, Yong JWH, Hew CS, Ong ES. Pressurized hot water extraction (PHWE) J. Chromatogr. A. 2010;1217:2484–2494. doi: 10.1016/j.chroma.2009.12.050. [DOI] [PubMed] [Google Scholar]
- Tomšika A, Pavlić B, Vladić J, Cindrić M, Jovanov P, Sakač M, Mandić A, Vidović S. Subcritical water extraction of wild garlic (Allium ursinum L.) and process optimization by response surface methodology. J. Supercrit. Fluid. 128: 79-88 (2017)
- Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Xiao Q, Hu B, Yu C, Xia L, Jiang Z. Optimization of a single-drop microextraction procedure for the determination of organophosphorus pesticides in water and fruit juice with gas chromatography-flame photometric detection. Talanta. 2006;69:848–855. doi: 10.1016/j.talanta.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Yilmaz Y, Toledo R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005;93:273–278. doi: 10.1016/j.foodchem.2004.09.043. [DOI] [Google Scholar]
- Zhang X, Li N, Lu X, Liu P, Qiao X. Effects of temperature on the quality of black garlic. J. Sci. Food Agr. 2016;96:2366–2372. doi: 10.1002/jsfa.7351. [DOI] [PubMed] [Google Scholar]