Abstract
The instability and strong flavor or odor of essential oils (EO) limit their direct incorporation into food products. In this study, the antioxidant and antimicrobial Heracleum lasiopetalum essential oil (HLEO) was added to Lepidium sativum seed mucilage (LSSM) solution at four concentrations (0, 0.5, 1, and 1.5%) to develop a novel edible coating and expand its food application. HLEO-loaded LSSM coating was then used to improve the shelf life and quality of beef as a model food system. The coated and control beef samples were periodically analyzed for physicochemical analysis, microbiological, and sensory characteristics over a period of 9 days at 4 °C. The HLEO-enriched LSSM coating, particularly 1.5% loaded one resulted in a significant (p < 0.05) increase in oxidative and microbiological stability and overall acceptance of the beef samples, compared to the control counterpart. HLEO-loaded LSSM coating, therefore, provides a promising alternative to preserve the meat products under cold storage.
Keywords: Lepidium sativum seed mucilage, Antimicrobial, Edible coating, Beef shelf life, Modeling
Introduction
Oxidation and foodborne pathogens are two important factors that influence the quality properties of foods during processing and storage (Wu et al., 2019). Edible coatings are currently gaining much attention as novel food packaging to improve the food quality and shelf life through inhibiting biological, chemical and physical deteriorations (Ojagh et al., 2010). In the last decade, there has been an increasing trend in the development of polysaccharide-based biodegradable and edible coatings (Huang et al., 2017; Hui et al., 2016).
Lepidium sativum (LS) plant is commonly recognized as garden cress and its seed comprises about 6.5–15% mucilage with good biodegradable, hydrophilic, and rheological properties along with low production cost. These characteristics make LS seed mucilage (LSSM) an interesting substitute to typical synthetic plastic-based materials in fabricating edible films and coatings (Behrouzian et al., 2014). However, polysaccharide-based coatings have hardly antimicrobial or antioxidant effect themselves (Wu et al., 2019), and therefore food-grade ingredients, such as antioxidant and antimicrobial compounds, are commonly incorporated into edible coatings to amend their technological functionality (Sapper et al., 2019).
Essential oils (EO) are one of the widely used principal natural and generally recognized as safe (GRAS) compounds in food preservation technologies, due to their antioxidant and antimicrobial compounds (Sánchez-González et al., 2011). Heracleum lasiopetalum is a perennial aromatic herb, the fruit of which had been employed by indigenous people as a spice and flavoring agent for food products, particularly meat, and as traditional medicines, owing to their antimicrobial and antiseptic characteristics (Pirbalouti et al., 2013a). It was observed that H. lasiopetalum essential oil (HLEO) indicated good antimicrobial and antioxidant properties (Pirbalouti et al., 2013b). However, EO have generally strong flavor or odor, which limits their direct application in food products (Wu et al., 2019). In this context, edible coatings can be utilized to encapsulate EO and decrease their negative effects on sensory characteristics and consumer acceptance of related foods. The EO-loaded edible coatings could be therefore used to decrease the chemical and microbial spoilage of food products, especially fresh beef, which is highly sensitive to lipid oxidation and microbial deterioration (Behbahani et al., 2017a, b).
There are no researches in the literature concerning the potential effect of HLEO-enriched LSSM-based edible coatings on the quality and shelf life of beef during refrigeration storage. The present study was therefore aimed to investigate the inhibitory effect of HLEO-loaded LSSM edible coating towards lipid oxidation and microbial spoilage of beef during cold storage conditions. In addition, the multivariate data analyses including principle component analysis (PCA) and orthogonal projections to latent structures discriminant analysis (OPLS-DA) were performed for the first time to assess the effect of HLEO-loaded LSSM edible coating on beef shelf life.
Materials and methods
Materials
L. sativum seeds were purchased from a local market (Mashhad, Iran). The plant H. lasiopetalum was collected from Kuhrang (Chaharmahal and Bakhtiari, Iran) during June–July 2018. All chemical reagents and materials used in this study were of analytical grade.
Extraction and chemical analysis of H. lasiopetalum essential oil (HLEO)
Sample dispersion (50 g powdered plant in 750 mL distilled water) was subjected to a Clevenger apparatus, based on the hydrodistillation method, for 3 h to produce EO with 0.5% v/w oil yield.
The main chemical constituents of HLEO were identified by gas chromatography-mass spectroscopy (GC–MS; Finnigant Trace MS-Thermo, USA) technique. Briefly, 0.1 µL HLEO was injected to the apparatus and the separation was performed as follows: 5 °C/min heating rate, helium gas with 1.1 mL/min rate, and an ionization energy of 70 eV. HLEO components were then identified according to the alkane spectra (C8–C28) followed by the calculation of the Kovats retention index and refer them to the international library of natural compounds (Alizadeh Behbahani and Imani Fooladi, 2018).
HLEO total phenolic and flavonoid contents
The total phenolic content (TPC) and flavonoid content of HLEO were measured based on the method described by Marinova et al. (2005). The extract (0.1 mL; 0.1 mg/mL) or gallic acid (0.1 mL; 0–0.5 mg/mL) solution was charged with Folin–Ciocalteu reagent (1 mL; 10% v/v) followed by mixing for 5 min and adding 0.3 mL Na2CO3 (10%). Next, the solution was incubated for 2 h at ambient temperature and its absorbance was recorded at 756 nm. The TPC was eventually expressed as mg gallic acid equivalent per gram HLEO.
In order to measure the total flavonoid content, 0.1 mL HLEO (0.1 mg/mL) or quercetin (0–0.5 mg/mL) solution was added to the NaNO2 solution (0.3 mL; 5%). Afterwards, the obtained solution was mixed for 5 min and charged with 0.3 mL AlCl3 (10% w/v) followed by mixing for 6 min. After adding 2 mL of 1 M NaOH, the absorbance was read at 510 nm and the total flavonoid content was expressed as mg quercetin equivalent per gram HLEO.
HLEO antioxidant activity
DPPH-radical scavenging (DPPH-RS) activity
DPPH-RS activity of HLEO was assessed using Kartal et al. (2007) method with some changes. The ethanolic DPPH solution (5 mL; 0.12 mM) was added to 50 µL HLEO (As) or control (Ac) and mixed. The solution was then stored for 30 min at room temperature and under dark conditions. In the next step, the absorbance was measured at 517 nm and antioxidant potential was calculated according to the following equation.
ABTS-radical scavenging (ABTS-RS) activity
In this assay, the same volumes of 2.45 mM potassium persulfate and 7 mM ABTS solutions were mixed and the resultant solution was kept at room temperature in a dark place for 16 h to generate ABTS radical cations. Thereafter, methanol was added to the ABTS radical solution to reach 0.7 ± 0.2 absorbance at 734 nm. The ABTS radical solution (3.9 mL) was then mixed thoroughly with 0.1 mL HLEO (As) or methanol (Ac) and after storing at room temperature for 6 min, its absorbance was read at 734 nm against methanol as blank. The ABTS-RS activity was finally determined as below (Shan et al., 2005):
β-carotene-linoleic acid assay
Dapkevicius et al. (1998) spectrophotometric method was used to monitor the bleaching of β-carotene-linoleate solution in the presence of HLEO by measuring the absorbance of the solution at 490 nm and after 120 min incubation (AA-120) against the control sample at time zero (AC-0) and after 120 min (AC-120), according to the below equation:
HLEO antibacterial activity
Antibacterial activity of HLEO was evaluated against Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Salmonella typhi ATCC 6539 and Bacillus cereus ATCC 14579.
Disk diffusion agar (DDA)
DDA test was performed based on the procedure of Hajlaoui et al. (2010) with some modifications. Sterile HLEO (20 µL) was gently added to the blank discs followed by standing them for 15 min at room temperature. The blank discs were then placed on the media contaminated with the above-mentioned bacterial strains. After incubating the petri-dishes (containing bacteria species and discs) at 37 °C for 24 h, the inhibition zone (mm) around discs was measured and reported as the antibacterial potential of HLEO.
Well diffusion agar (WDA)
The modified method of Tepe et al. (2004) was employed to conduct the WDA. For this aim, HLEO (20 µL) was added to the holes (6 mm in diameter) which were previously created on the surface of Mueller–Hinton agar in petri-dishes and contaminated with bacterial strains. The petri-dishes were kept at 37 °C for 24 h and then the inhibition zones were recorded as antibacterial effect.
Minimum inhibitory concentration (MIC)
The microdilution assay, in 96 well plates, was used to determine the MIC of HLEO with modification (El Atki et al., 2019). HLEO at sequential concentrations (1, 2, 4, 8, 16, 32, 64, 128, 256, and 512 mg/mL) in Mueller–Hinton broth medium (Merck Co., Germany) were sterilized via 0.45 µm syringe filters followed by adding them (200 µL) to the wells which were incorporated previously with microbial suspensions (20 µL; 0.5 McFarland equivalent). Afterwards, the plates were incubated at 37 °C for 24 h and then 2,3,5- triphenyltetrazolium chloride solution (20 µL; 5.0% w/v) was added to the wells. After re-incubating at 37 °C for 30 min, the lack of an amethystine or dark red color (as the indicator of microbial growth) in the wells, which was triggered by the lowest HLEO concentration, was considered as MIC of HLEO.
Minimum bactericidal concentration (MBC)
MBC of the bioactive HLEO was conducted according to the method of Alghooneh et al. (2015). Based on the results of the MIC test, 100 µL of the microbial growth-free wells was cultured on the Mueller–Hinton Agar medium. Next, the medium was incubated according to the above-mentioned conditions for the MIC antimicrobial test and the lowest HLEO concentration that resulted in bacterial death (i.e., no visible colony formation) was regarded as MBC.
Extraction of L. sativum seed mucilage (LSSM)
LSSM was extracted from the plant seeds based on the method of Karazhiyan et al. (2011). The extraction was performed at 30:1 water to seed ratio, pH 10, and 35 °C for 15 min. The mucilage was scrapped from the seed surfaces and it was then filtered and dried at 60 °C overnight. The obtained mucilage was finally milled, packaged, and stored at ambient temperature.
LSSM chemical analysis
The moisture, ash, fat, and protein contents of the LSSM were measured according to AOAC (1995). Total carbohydrate content was determined from 100% − (moisture + fat + protein + ash).
Preparation of HLEO/LSSM edible coating
To prepare the edible coating, LSSM (2 g) and Tween 80 (0.1 g) were dissolved in 100 mL sterile distilled water followed by stirring and heating for 2 h. HLEO (0, 0.5, 1, and 1.5% v/v) was then incorporated into the hydrocolloidal solution to fabricate a biologically active edible coating, HLEO-loaded LSSM edible coating, with antioxidant and antimicrobial effects.
Beef coating
Beefs were immersed in LSSM and HLEO-loaded LSSM solutions for 1 min and after air drying for 10 min at ambient temperature, they were stored at refrigerated condition (4 °C) for 9 days. The non-coated meat was regarded as the control sample. Fresh beef contained 64.53 ± 0.62% moisture, 19.52 ± 0.48% fat, 14.1 ± 0.39% protein, and 1.85 ± 0.41% ash contents (AOAC, 1995).
Physicochemical analysis of coated beef
pH value
The coated or uncoated beef (10 g) was mixed with 90 mL distilled water. The resultant slurry was homogenized at 13000 rpm for 30 s and the pH value of the homogenate was measured at room temperature by a pH meter (Hassanzadeh et al., 2017).
Moisture content
The oven drying method was applied to measure the moisture content of the beef samples (AOAC, 1995).
Peroxide value (PV) and thiobarbituric acid (TBA) value
The PV (as the indicator of primary lipid oxidation products) and TBA value measurements of coated and uncoated samples stored at 4 °C, were measured according to the methods described by Behbahani and Imani Fooladi (2018).
Textural studies
The beef samples were subjected to a Stable Micro System Texture Analyzer (TA, XT2i, UK) to monitor their hardness changes during cold storage. In brief, the samples were compressed by an aluminum cylinder probe (36 mm in diameter) to 30% of their initial heights at the test speed of 5 mm/s. The highest force applied to compress the samples was considered as the hardness (N).
Microbiological analysis of coated beef
The microbial development of the coated and uncoated beef samples during cold storage was monitored by performing the microbial assays of total viable count (TVC) bacteria, Psychrotrophic count (PTC), E. coli and S. aureus count, coliform bacteria count, and fungi (mold and yeast) count, according to the method of Alizadeh Behbahani and Imani Fooladi (2018).
Sensory evaluation of coated beef
The color, odor and overall acceptability of the beef samples (coded with 3-digit random numbers) were assessed by 10 well trained panelists through a nine-point hedonic scale test (the highest score 9: like extremely to the lowest score 1: dislike extremely), according to the Hansen et al. (1995) method. The sensory score higher than 4 was considered as acceptable.
Statistical analysis
The data were analyzed by SPSS software (version 18.0) through one-way ANOVA and the difference between the data mean was determined by the Duncan’s test at p < 0.05. All experiments were done triplicates.
Multivariate data analysis (OPLS–DA and PCA)
Correlation of pathogenic microorganisms, physicochemical changes, and sensory characteristics evaluation of beef samples were analyzed using multivariate data analysis. Multivariate data analyses including PCA and OPLS–DA were performed on mean centering and unit variance scaling of variables by Simca (Umetrics Inc., Umeå University, Sweden).
Results and discussion
HLEO characterization
Chemical composition
Twenty-one compounds were identified and HLEO was particularly rich in eucalyptol/cineol (30.75%), 1-bornyl alcohol (29.98%), camphor (17.2%), β-pinene (2.89%), α-pinene (2.67%), crypton (2.3%), 3-carene (1.96%), and α-terpineol (1.9%) with 0.5% v/w oil yield. As far as our literature could ascertain, there are very few researches on studying the chemical composition of HLEO. The compounds in HLEO in this study are different from the previous studies (Pirbalouti et al., 2013b; Sonboli et al., 2007), due to the fact that the plant growth and its EO composition are extremely affected by various factors, such as genetic constitution, development stage, temperature, moisture, and so forth (Behbahani et al., 2017a).
Total phenolic and flavonoid contents
The TPC of HLEO was determined by using Folin’s reagent. HLEO had a TPC of 44.35 ± 0.95 mg gallic acid/g plant extract. Total flavonoid content of the extract was found to be 21.65 ± 0.76 mg quercetin/g extract. According to our literature survey, there is only one report dealing with the TPC and flavonoid content of H. lasiopetalum extract. Pirbalouti et al. (2013a) found a TPC of 120 ± 2.12 mg tannic acid/g dry weight extract and total flavonoids of 7.63 ± 0.23 mg rutin/g dry weight methanolic extract H. lasiopetalum. Flavonoids astragalin and hyperoside with antioxidant activity have been isolated from Heracleum species (Park et al., 2010). Therefore, the consumption of plant extracts rich in flavonoids and other phenolic compounds could significantly reduce the extent of reactive oxygen species-related diseases, due to their strong antioxidant activity (Bahadori et al., 2016).
Antioxidant capacity
Several antioxidant assays, such as β-carotene-linoleic acid model system and free synthetic radical trapping ones like ABTS⋅+ and DPPH⋅ have been commonly employed to evaluate the total antioxidant characteristics of plant extracts and EO (Hajlaoui et al., 2010). Antioxidant compounds have the potential to reduce the stable DPPH⋅ to the yellowish diphenylpicrylhydrazine solution (Ye et al., 2013). DPPH-RS activity of the HLEO was observed to be 58%, indicating a hydrogen-donating ability of the HLEO to reduce free radicals and terminate their detrimental effects. ABTS-RS activity measures the antioxidant potential of both hydrophilic and hydrophobic antioxidants along with the extracts derived from natural sources (Erkan et al., 2008). HLEO had an ABTS-RS capacity of 61% and this considerably high antioxidant power is due to the fact the antioxidative compounds could reduce ABTS⋅ through radical trapping via hydrogen atom and electron transfer mechanisms (Kim and Lee, 2009). It is worth noting that the nearly same data obtained by the DPPH and ABTS tests are ascribed to the fact that the two procedures are based on the same principle (Ćavar et al., 2012).
In β-carotene-linoleic acid model system, antioxidant agents such as phenolics are able to inhibit the extent of rapid discoloration of β-carotene through neutralizing the formed linoleate free radicals within the system (Hajlaoui et al., 2010). β-carotene bleaching was manifestly inhibited (i.e., 66% antioxidant activity) by the bioactive HLEO, indicating its potent hydroperoxide scavenging power. The antioxidant activity of the HLEO is mainly attributed to its major constituents in conjugation with phenolic and flavonoid compounds, which their potential antioxidative powers have been ascribed to the electron and hydrogen atom donating ability (Bahadori et al., 2016; Pirbalouti et al., 2013a, b). Therefore, HLEO could be used as a natural antioxidant agent to scavenge free radicals and inhibit the oxidation of food products rich in oxidizable lipids.
Antimicrobial capacity
The antimicrobial potential of HLEO against pathogenic and spoilage bacteria was examined and the results are indicated in Table 1. Based on the results, the growth of both gram positive and negative bacteria was appreciably suppressed by the HLEO. Higher inhibition zones were observed for gram positive bacteria (B. cereus and S. aureus) in disc and well diffusion agar tests compared with gram negative microorganisms (E. coli, P. aeruginosa and S. typhi). Similarly, the gram positive bacteria were generally inhibited and killed in the presence of lower HLEO concentrations according to the MIC and MBC results. This means that HLEO is more active towards the growth of gram positive microorganisms in comparison to gram negative ones; the latter have a complex lipopolysaccharide layer on the outer cell membrane which could slow down the diffusion rate of hydrophobic constituents of HLEO across the cell membrane, whereas, the single mucopeptide layer with high diffusivity is found in gram positive bacteria which enables them to be more sensitive to bioactive extracts and EO (Alizadeh Behbahani and Imani Fooladi, 2018). It is also noteworthy that the higher inhibition zones (i.e. greater antimicrobial power) in well diffusion agar compared to disc diffusion agar for microorganisms tested could be ascribed to the fact that the HLEO is in direct contact with bacteria in well diffusion agar method, but the inhibitory effect of HLEO in disc diffusion agar technique is derived from the diffusion of its antimicrobial constituents from the surface of discs into medium containing pathogenic and spoilage bacteria (Behbahani et al., 2017c). Similar findings have been reported by Pirbalouti’s research group, who observed a relatively high antimicrobial activity of HLEO against bacterial strains (Pirbalouti et al., 2010; Pirbalouti et al., 2011).
Table 1.
In vitro antibacterial effect of Heracleum lasiopetalum essential oil (HLEO)
| Microbial strains | Antimicrobial tests | |||
|---|---|---|---|---|
| Disc diffusion agar (mm) | Well diffusion agar (mm) | MIC (mg/mL) | MBC (mg/mL) | |
| B. cereus | 25.10 ± 0.75 | 28.20 ± 0.75 | 4 | 16 |
| E. coli | 18.60 ± 0.33 | 21.90 ± 0.58 | 8 | 32 |
| P. aeruginosa | 17.90 ± 0.66 | 19.90 ± 0.82 | 16 | 32 |
| S. typhi | 19.50 ± 0.50 | 20.20 ± 0.44 | 8 | 16 |
| S. aureus | 26.60 ± 0.30 | 30.10 ± 0.44 | 4 | 8 |
As a whole, HLEO contains bioactive compounds with superb antioxidant and antimicrobial activity, which make it to be used as a natural source of preservative agents in various food products to inhibit the microbial growth and lipid oxidation, thus improving their shelf life and quality.
LSSM chemical analysis
The composition of LSSM’ main chemicals components showed that the majority of the plant extract comprised about 73% carbohydrate. It also contained low levels of proteins (6.20%) and fats (2.9%) along with 3.6% moisture and 14.7% ash contents. According to the results obtained by Karazhiyan et al. (2009), LSSM contains 77.03, 7.17, 2.45, 1.85, and 11.5% total carbohydrate, moisture, protein, fat, and ash contents, respectively. The small changes in the main chemical components of LSSM could be due to some important factors, such as different extraction processes and plant growing conditions.
Effect of HLEO-loaded LSSM coating on beef quality and shelf life
Changes in pH value
The pH values of the fresh beef coated with edible LSSM in the presence or absence of HLEO and the non-coated control are presented in Table 2. The initial pH of the fresh beef before edible coating application was 5.71 and it did not show significant change (p > 0.05) in control and LSSM groups. The control sample showed a significant increase (p < 0.05) in pH during storage period and, at day 9, the pH value reached to 6.78. The increased pH of the control sample over storage period could be due to the accumulation of volatile basic compounds produced as a consequence of bacterial and enzymatic activity (Mohan et al., 2012). LSSM coating application on the beef surface led to a significantly lower pHs compared to the control (p < 0.05), and this was more pronounced in samples wrapped with LSSM incorporated with higher level of HLEO. Lower pH changes with EO-enriched mucilage-based coating usages have been also reported by Behbahani et al. (2017b) in meat samples, which was ascribed to the inhibitory effect of EO against both gram positive and negative bacteria along with fungi strains.
Table 2.
Effect of different concentrations of Heracleum lasiopetalum essential oil added to Lepidium sativum seed mucilage-based edible coating on physicochemical changes of beef slices during 9 days of storage at 4 °C
| Parameters | Days of storage | Edible coating | ||||
|---|---|---|---|---|---|---|
| Control | LSSM + 0% HLEO | LSSM + 0.5% HLEO | LSSM + 1.0% HLEO | LSSM + 1.50% HLEO | ||
| pH | 0 | 5.71 ± 0.29Aa | 5.71 ± 0.29Aa | 5.71 ± 0.29Aa | 5.71 ± 0.29Aa | 5.71 ± 0.29Aa |
| 3 | 6.28 ± 0.50Ab | 5.69 ± 0.35Bb | 5.64 ± 0.60Cb | 5.64 ± 0.57Cb | 5.63 ± 0.41Cb | |
| 6 | 6.66 ± 0.28Ac | 5.81 ± 0.19Bc | 5.69 ± 0.57Cb | 5.67 ± 0.49Cb | 5.66 ± 0.50Cc | |
| 9 | 6.78 ± 0.35Ad | 6.11 ± 0.29Bd | 6.00 ± 0.34Cc | 5.78 ± 0.40Dc | 5.69 ± 0.50Ec | |
| Moisture content (%) | 0 | 64.53 ± 0.5Aa | 64.53 ± 0.5Aa | 64.53 ± 0.5Aa | 64.53 ± 0.5Aa | 64.53 ± 0.5Aa |
| 3 | 56.62 ± 0.44Ab | 61.08 ± 0.45Bb | 62.10 ± 0.50Cb | 63.50 ± 0.45Db | 63.79 ± 0.50Bb | |
| 6 | 52.78 ± 0.28Ac | 59.52 ± 0.50Bc | 61.14 ± 0.19Cc | 62.00 ± 0.47Dc | 62.99 ± 0.39Ec | |
| 9 | 47.00 ± 0.30Ad | 57.99 ± 0.15Bd | 58.89 ± 0.45Cd | 60.40 ± 0.79Dd | 61.51 ± 0.50Ed | |
| Peroxide value (meq O2/kg) | 0 | 1.15 ± 0.20Ad | 1.15 ± 0.20Ad | 1.15 ± 0.20Ad | 1.15 ± 0.20Ad | 1.15 ± 0.20Ad |
| 3 | 4.85 ± 0.25Ac | 3.75 ± 0.55Bc | 2.22 ± 0.30Cc | 1.95 ± 0.18Dc | 1.55 ± 0.70Ec | |
| 6 | 7.29 ± 0.50Ab | 6.00 ± 0.50Bb | 5.10 ± 0.28Cb | 3.85 ± 0.39Db | 3.85 ± 0.33Db | |
| 9 | 10.86 ± 0.65Aa | 7.80 ± 0.50Ba | 7.65 ± 0.27Ba | 6.00 ± 0.45Ca | 5.10 ± 0.62 Da | |
| TBA value (mg MDA/kg) | 0 | 0.12 ± 0.03Ad | 0.12 ± 0.03Ad | 0.12 ± 0.03Ad | 0.12 ± 0.03Ad | 0.12 ± 0.03Ad |
| 3 | 0.55 ± 0.19Ac | 0.33 ± 0.10Bc | 0.25 ± 0.05Cc | 0.20 ± 0.08Cc | 0.20 ± 0.08Cc | |
| 6 | 1.15 ± 0.21Ab | 0.80 ± 0.05Bb | 0.60 ± 0.05Cb | 0.66 ± 0.10Cb | 0.38 ± 0.08Db | |
| 9 | 1.85 ± 0.25Aa | 1.22 ± 0.35Ba | 1.05 ± 0.13Ca | 0.81 ± 0.06 Da | 0.70 ± 0.12Ea | |
| Hardness (N) | 0 | 67.10 ± 1.00Aa | 67.96 ± 1.50Aa | 68.00 ± 0.66Aa | 68.58 ± 1.28Aa | 69.10 ± 1.28Aa |
| 3 | 62.15 ± 0.95Bab | 64.88 ± 1.15ABab | 67.01 ± 0.84ABa | 67.06 ± 1.00ABa | 68.00 ± 0.95Aa | |
| 6 | 56.85 ± 0.45Bbc | 60.00 ± 0.63ABbc | 61.50 ± 1.00Ab | 62.89 ± 0.85Aab | 63.19 ± 1.00Aab | |
| 9 | 51.75 ± 1.15Bc | 56.38 ± 0.25ABc | 58.20 ± 0.75Ab | 59.69 ± 0.77Ab | 60.89 ± 1.27Ab | |
LSSM Lepidium sativum seed mucilage, HLEO Heracleum lasiopetalum essential oil
Means within the same column with different small letters differ significantly (p < 0.05). Means within the same row with different capital letters differ significantly (p < 0.05)
Changes in moisture content
Despite the fact that the moisture content of all samples was reduced over storage time, it was efficiently preserved upon edible coating application (Table 2). The non-coated sample underwent a significant decrease in moisture content (from 64.53 to 47%) as function of storage time, representing a weight loss of approximately 27.16% at the end of refrigeration period. The use of LSSM coating, especially HLEO-loaded ones, lowered the moisture losses significantly (p < 0.05); the weight losses in the meat samples wrapped with LSSM, LSSM + 0.5%HLEO, LSSM + 1.0%HLEO, and LSSM + 1.5%HLEO were found to be about 10.13%, 8.74%, 6.4%, and 4.68%, respectively, at the 9th day of storage. In accordance with the findings of the present research, Cardoso et al. (2016) found that the edible coating was able to remarkably inhibit the water losses in meat, likely due to its low permeability to water vapors in conjugation with acting as a physical barrier.
Changes in lipid oxidation progression
The effect of LSSM coating on the PV changes of beef lipids is shown in Table 2. The initial PV of the samples was 1.15 meq O2/kg. The coated and control samples experienced a marked increase (p < 0.05) in the PV with storage time; nonetheless, there were significant differences in the PV between the non-coated sample (10.86 meq O2/kg) and the samples wrapped with LSSM and HLEO-enriched LSSM by the end of storage period, indicating lower PVs of 7.8, 7.65, 6, and 5.1 meq O2/kg in LSSM, LSSM + 0.5%HLEO, LSSM + 1%HLEO, and LSSM + 1.5%HLEO samples, respectively. It is clear that the HLEO-loaded LSSM edible coating is effective in inhibiting the hydroperoxide formation in beef slices during cold storage. The inhibitory potential of EO-loaded edible coating towards the generation of primary lipid oxidation products in meat has also been reported (Bonilla et al., 2014).
In this study, the formation of secondary lipid oxidation products is also assessed by TBA assay. The initial TBA value of fresh beef was observed to be 0.12 mg malondialdehyde/kg and it was then increased significantly (p < 0.05) as the storage time increased (Table 2). By the end of refrigeration period (9-day), the LSSM wrapped beef slices had, however, significantly lower TBA contents compared to the control sample, which attained the highest level of 1.85 mg malondialdehyde/kg. In beef samples coated with LSSM enriched with HLEO, the TBA content was reduced markedly (p < 0.05) as a function of HLEO concentration, proving the antioxidant power of the EO.
It has been proposed that the highest levels of PV and TBA showing good quality of the beef are 7.0 meq O2/kg and 1.0 mg malondialdehyde/kg, respectively (Alizadeh Behbahani and Imani Fooladi, 2018). As indicated in Table 2, the PV and TBA values for coated beef samples containing 1.0% and 1.5% HLEO were lower than the permitted limits during the 9-day cold storage. The use of edible packaging coatings rich in bioactive EO could therefore ameliorate the oxidative stability of meat products in the virtue of their outstanding antioxidant effects.
Changes in meat texture
The edible coating application did not adversely affect the hardness of beef (Table 2). The slightly higher hardness of the coated beef than that of the non-coated counterpart at the first day of storage could be due to the gel-forming ability of the LSSM (Behrouzian et al., 2014) which the samples, therefore, required greater forces to be compressed. A decreasing trend was observed in the hardness of both coated and uncoated samples throughout the storage time. The LSSM coated beefs had higher hardness than the control; the higher HLEO content in LSSM coating, the higher was beef hardness. It was claimed that EO could suppress the growth of microorganisms and reduce the activity of meat endogenous enzymes collagenases, calpains, and cathepsins, and subsequently inhibit the collagen and myofibrillar protein degradation, thereby preserving the meat texture (Ghani et al., 2018). In addition, Guerrero et al. (2015) reported that lipid oxidation not only leads to food rancidity, but also affects the texture. This explains why the LSSM + 1.5%HLEO coated meat had the highest oxidative stability and hardness among other treatments.
Changes in microbial growth
The effect of LSSM coating containing different concentrations of HLEO on the microbial growth in beef during cold storage is shown in Table 3. As expected, the coated and non-coated samples had the same TVC of 2.70 log CFU/g in the first reading. It was, however, cleared that the TVC increased significantly (p < 0.05) as the time elapsed. LSSM coatings enriched with higher levels of HLEO resulted in a marked decrease in TVC over the experimental period. By the day 9 of storage, the TVC in beef wrapped with 1% and 1.5% HLEO-loaded LSSM was still below 7 log CFU/g; whilst, the control sample had a TVC of 8.8 log CFU/g at 6-day, which exceeded the maximum recommendation limit of 107 CFU/g or 7 log CFU/g TVC for fresh meat (ICMSF, 1986), representing a microbial shelf life of approximately 3–5 days for the uncoated beef.
Table 3.
Effect of different concentrations of Heracleum lasiopetalum essential oil added to Lepidium sativum seed mucilage-based edible coating on microbial load changes of beef slices during 9 days of storage at 4 °C
| Parameters | Days of storage | Edible coating | ||||
|---|---|---|---|---|---|---|
| Control | LSSM + 0% HLEO | LSSM + 0.5% HLEO | LSSM + 1.0% HLEO | LSSM + 1.50% HLEO | ||
| TVC (log CFU/g) | 0 | 2.70 ± 0.08Ad | 2.70 ± 0.08Ad | 2.70 ± 0.08Ad | 2.70 ± 0.08Ad | 2.70 ± 0.08Ad |
| 3 | 5.33 ± 0.69Ac | 4.61 ± 0.38Bc | 4.00 ± 0.71Cc | 3.80 ± 0.75Dc | 3.10 ± 0.280Ec | |
| 6 | 8.80 ± 0.50Ab | 6.60 ± 0.91Bb | 5.70 ± 0.39Cb | 5.00 ± 0.40Db | 4.80 ± 0.67Db | |
| 9 | 11.90 ± 0.50Aa | 8.80 ± 0.19Ba | 7.90 ± 0.80Ca | 6.80 ± 0.46 Da | 6.00 ± 0.59Ea | |
| PTC (log CFU/g) | 0 | 1.45 ± 0.10Ad | 1.45 ± 0.10Ad | 1.45 ± 0.10Ad | 1.45 ± 0.10Ad | 1.45 ± 0.10Ad |
| 3 | 3.63 ± 0.84Ac | 3.00 ± 0.67Bc | 2.80 ± 0.20Cc | 2.20 ± 0.61Dc | 2.00 ± 0.15Dc | |
| 6 | 5.30 ± 0.28Ab | 4.72 ± 0.43Bb | 3.80 ± 0.61Cb | 2.95 ± 0.27Db | 2.86 ± 0.50Db | |
| 9 | 8.20 ± 0.41Aa | 6.29 ± 0.55Ba | 5.00 ± 0.68Ca | 4.33 ± 0.33 Da | 4.27 ± 0.12 Da | |
| E. coli (log CFU/g) | 0 | 0.22 ± 0.05Ad | 0.22 ± 0.05Ad | 0.22 ± 0.05Ad | 0.22 ± 0.05Ad | 0.22 ± 0.05Ad |
| 3 | 0.95 ± 0.15Ac | 0.55 ± 0.10Bc | 0.41 ± 0.12Cc | 0.40 ± 0.09Cc | 0.38 ± 0.06Cc | |
| 6 | 1.70 ± 0.26Ab | 1.00 ± 0.20Bb | 1.00 ± 0.18Bb | 0.85 ± 0.07Cb | 0.62 ± 0.10Db | |
| 9 | 2.20 ± 0.41Aa | 1.60 ± 0.35Ba | 1.55 ± 0.33Ba | 1.02 ± 0.27Ca | 1.00 ± 0.15Ca | |
| S. aureus (log CFU/g) | 0 | 0.33 ± 0.08Ad | 0.33 ± 0.08Ad | 0.33 ± 0.08Ad | 0.33 ± 0.08Ad | 0.33 ± 0.08Ad |
| 3 | 1.29 ± 0.29Ac | 1.00 ± 0.31Bc | 0.83 ± 0.20Bc | 0.66 ± 0.10Cc | 0.50 ± 0.09Dc | |
| 6 | 2.20 ± 0.66Ab | 2.05 ± 0.39Ab | 1.63 ± 0.28Bb | 1.12 ± 0.19Cb | 1.08 ± 0.15Cb | |
| 9 | 3.30 ± 0.56Aa | 3.11 ± 0.58Aa | 2.20 ± 0.60Ba | 1.50 ± 0.63Ca | 1.47 ± 0.64Ca | |
| Coliforms (log CFU/g) | 0 | 0.55 ± 0.11Ad | 0.55 ± 0.11Ad | 0.55 ± 0.11Ad | 0.55 ± 0.11Ad | 0.55 ± 0.11Ad |
| 3 | 1.22 ± 0.21Ac | 1.05 ± 0.27Bc | 1.00 ± 0.10Bc | 0.89 ± 0.09Cc | 0.84 ± 0.02Cc | |
| 6 | 2.25 ± 0.36Ab | 2.00 ± 0.50Bb | 1.88 ± 0.2Cb | 1.56 ± 0.17Db | 1.54 ± 0.24Db | |
| 9 | 3.10 ± 0.50Aa | 2.78 ± 0.44Ba | 2.33 ± 0.28Ca | 2.10 ± 0.20Da | 2.10 ± 0.16Da | |
| Fungi (log CFU/g) | 0 | 0.57 ± 0.05Ad | 0.57 ± 0.05Ad | 0.57 ± 0.05Ad | 0.57 ± 0.05Ad | 0.57 ± 0.05Ad |
| 3 | 1.66 ± 0.29Ac | 1.00 ± 0.21Bc | 0.88 ± 0.16Cc | 0.71 ± 0.10Dc | 0.68 ± 0.01Dc | |
| 6 | 2.78 ± 0.60Ab | 1.80 ± 0.31Bb | 1.45 ± 0.34Cb | 1.20 ± 0.16Db | 1.11 ± 0.11Eb | |
| 9 | 3.51 ± 0.50Aa | 2.50 ± 0.31Ba | 2.08 ± 0.29Ca | 2.00 ± 0.26Ca | 1.60 ± 0.30Da | |
LSSM Lepidium sativum seed mucilage, HLEO Heracleum lasiopetalum essential oil
Means within the same column with different small letters differ significantly (p < 0.05). Means within the same row with different capital letters differ significantly (p < 0.05)
PTC increased significantly (p < 0.05) with increasing cold storage time, but the PTC increased faster for the uncoated sample (Table 3). The PTC showed the same growth pattern as that of TVC, with uncoated sample also being the highest on the 9th day (8.2 log CFU/g) followed by the beefs wrapped with LSSM (6.29 log CFU/g), LSSM + 0.5%HLEO (5 log CFU/g), LSSM + 1.0%HLEO (4.33 log CFU/g), and LSSM + 1.5%HLEO (4.27 log CFU/g). The EO-rich edible coating could therefore function as an oxygen barrier and, in turn, limit the growth of most important and aerobic psychrotrophic bacteria, i.e. Pseudomonas species, which are mainly responsible for the fresh beef spoilage under aerobic conditions (Behbahani et al., 2017b).
Similar behaviors were observed for the growth pattern of E. coli, S. aureus and coliforms. Although, all the samples underwent an increase in the bacterial growth, edible coating application led to a significantly lower count of the bacterial species over the experimental cold storage period, compared to the control (Table 3). The shelf life increasing effect of HLEO-loaded edible coating for beef could be attributed to the presence of bioactive compounds in HLEO (Pirbalouti et al., 2010; Pirbalouti et al., 2011).
It is also worth noting that the addition of HLEO to the LSSM-based edible coatings significantly (p < 0.05) lowered the growth of fungi strains on the beef surface (Table 3). The uncoated and LSSM + 1.5%HLEO-enriched coated samples showed the highest and lowest fungi counts of 3.51 and 1.60 log CFU/g, respectively, at the end of cold storage. The decreased fungi growth in the coated samples was ascribed to the oxygen barrier characteristics of the EO-loaded edible coatings, as the fungi are aerobic microorganisms and they could not therefore grow under anaerobic conditions of the coated meats (Behbahani and Imani Fooladi, 2018). Indeed, phenolic compounds in EO could sensitize the cell membrane phospholipidic bilayer and lead to an increase in cell permeability and subsequent key intracellular compounds leakage, or inhibit the normal activity of bacterial enzyme systems (Ojagh et al., 2010).
Changes in sensory properties
Table 4 indicates the results of sensory properties of the coated and uncoated beefs over the experimental period. The beef sample with sensory score higher than 4 is considered to be acceptable for human consumption (Behbahani et al., 2017a). Color, odor, and overall acceptance of the uncoated beef were perceived unacceptable scores by the 9th, 6th, and 6th days, respectively. It appears that the results of sensory evaluation are well correlated with those of the chemical and microbial analyses. The non-coated sample became unacceptable in the terms of overall acceptability after 3 days’ cold storage, due to its high microbial growth and lipid oxidation. The antimicrobial, antioxidant, and gas-barrier characteristics by HLEO-loaded LSSM coating have been shown to significantly (p < 0.05) increase the meat oxidability and microbial safety, thereby improving the product quality and shelf life. Incorporating 1.5% HLEO into LSSM coating significantly (p < 0.05) increased the odor, color, and overall acceptance of the beef by the end of refrigerated storage period. In general, the edible coating enriched with 1.5% essential oil could retain the shelf life of beef till the end of cold storage time (9 days) without leading to significant losses in sensory characteristics and increase in microbial spoilage; whilst, the uncoated sample had an only 3-day shelf life.
Table 4.
Effect of different concentrations of Heracleum lasiopetalum essential oil added to Lepidium sativum seed mucilage-based edible coating on sensory properties of beef slices during 9 days of storage at 4 °C
| Sensory attribute | Days of storage | Edible coating | ||||
|---|---|---|---|---|---|---|
| Control | LSSM + 0% HLEO | LSSM + 0.5% HLEO | LSSM + 1.0% HLEO | LSSM + 1.50% HLEO | ||
| Color | 0 | 8.70 ± 0.43Aa | 8.70 ± 0.43Aa | 8.70 ± 0.43Aa | 8.70 ± 0.43Aa | 8.70 ± 0.43Aa |
| 3 | 6.20 ± 0.56Ab | 6.90 ± 1.00Bb | 7.00 ± 0.35Bb | 7.60 ± 0.50Cb | 7.60 ± 0.62Cb | |
| 6 | 5.05 ± 0.39Ac | 5.90 ± 0.32Bc | 6.00 ± 0.20Bc | 6.30 ± 0.40Bc | 6.80 ± 0.40Cc | |
| 9 | 2.00 ± 0.40Ad | 3.80 ± 0.19Bd | 4.40 ± 0.36Cd | 5.20 ± 0.50Dd | 6.10 ± 0.25Ed | |
| Odor | 0 | 8.40 ± 0.42Aa | 8.40 ± 0.42Aa | 8.40 ± 0.42Aa | 8.40 ± 0.42Aa | 8.40 ± 0.42Aa |
| 3 | 4.10 ± 0.85Ab | 5.00 ± 0.44Bb | 5.90 ± 0.70Cb | 7.10 ± 0.33Db | 7.20 ± 0.35Db | |
| 6 | 2.60 ± 1.05Ac | 3.90 ± 0.95Bc | 4.90 ± 0.60Cc | 6.30 ± 0.50Dc | 6.90 ± 0.55Db | |
| 9 | 1.50 ± 0.28Ad | 2.60 ± 0.52Bd | 3.60 ± 0.40Cd | 5.10 ± 0.75Dd | 5.40 ± 0.46Dc | |
| Overall acceptance | 0 | 8.00 ± 0.49Aa | 8.00 ± 0.49Aa | 8.00 ± 0.49Aa | 8.00 ± 0.49Aa | 8.00 ± 0.49Aa |
| 3 | 5.00 ± 0.26Ab | 5.70 ± 0.50Bb | 6.60 ± 0.46Bb | 7.80 ± 0.50Ca | 7.90 ± 0.50Ca | |
| 6 | 3.80 ± 0.30Ac | 4.10 ± 0.33Ac | 5.50 ± 0.30Bc | 6.00 ± 0.46Cb | 6.10 ± 0.55Cb | |
| 9 | 2.00 ± 0.11Ad | 3.00 ± 0.19Bd | 4.10 ± 0.30Cd | 5.10 ± 0.15Dc | 6.00 ± 0.22Eb | |
LSSM Lepidium sativum seed mucilage, HLEO Heracleum lasiopetalum essential oil
Means within the same column with different small letters differ significantly (p < 0.05). Means within the same row with different capital letters differ significantly (p < 0.05)
Multivariate data analysis (OPLS–DA and PCA)
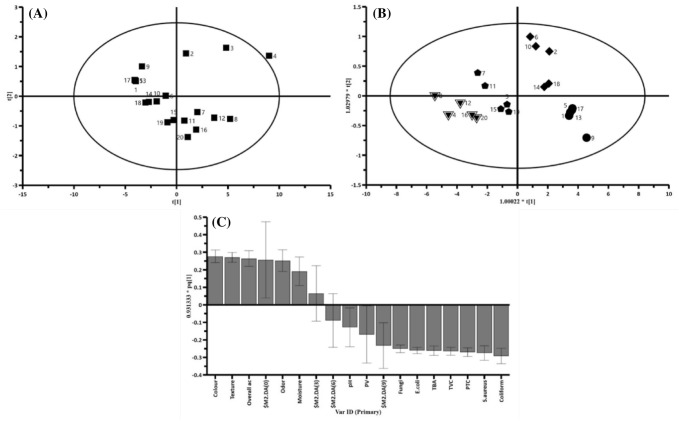
PCA is known as a conversion in the vector space capable of reducing dataset dimensions, which can analyze the responses as a consequence of the exerted treatments on the samples in the vector space in a dataset, according to the correlation between the extracted data (Jiang et al., 2016; Ying et al., 2015; Zheng et al., 2016). As shown in Fig. 1A, the different treatments (control, LSSM, and LSSM containing different concentrations of EO) were well separated and homogenously distributed. Although, the uncoated (control) sample was out of the range on the 9th day of storage, it did not show a significant effect on the model. Similar results were reported by Behbahani et al. (2017b) and Behbahani and Imani Fooladi (2018).
Fig. 1.
Principal component analysis (PCA): The distribution and correlation between the exerted treatments on the beef samples (A); OPLS–DA score plot of Samples during storage time (B); The VIP discriminative parameter for OPLS-DA Model for comparison of Samples during storage time (C)
Figure 1B, C reveal that the OPLS-DA model was able to well separate the data according to the storage days. The model parameters were R2X = 0.543, R2Y = 0.667, and Q2 = 0.213. Despite the fact that a large variation was found between the data, it had the highest and lowest effects on the odor and pH parameters. Based on the different treatments, the highest variations were observed in color and coliform count parameters in OPLS-DA loading plot. As well, the storage day and treatment type were considered as Y and X variables in OPLS-DA model, respectively. Although, the model was able to separate the data according to the cold storage day, no other model and variable could detect a significant difference between the date. Color parameter decreased and coliform count increased in all treatments through a treatment-dependent mode. For example, although the coliform count increased as a function of storage time, the growth rate was lower in treatments compared to the control sample. The use of this biologically active edible coating with outstanding antioxidant and antimicrobial effects, could be considered as a good strategy and safe preservative to substitute the synthetic preservatives in meat products under refrigeration storage.
Acknowledgements
The authors wish to express their profound gratitude sincerely to the Research Deputy of Agricultural Sciences and Natural Resources University of Khuzestan for funding this project.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hassan Barzegar, Email: hbarzegar@asnrukh.ac.ir.
Behrooz Alizadeh Behbahani, Email: B.alizadeh@asnrukh.ac.ir.
Mohammad Amin Mehrnia, Email: Mehrnia@asnrukh.ac.ir.
References
- Alghooneh A, Behbahani BA, Noorbakhsh H, Yazdi FT. Application of intelligent modeling to predict the population dynamics of Pseudomonas aeruginosa in Frankfurter sausage containing Satureja bachtiarica extracts. Microb. Pathog. 2015;85:58–65. doi: 10.1016/j.micpath.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Alizadeh Behbahani B, Imani Fooladi AA. Development of a novel edible coating made by Balangu seed mucilage and Feverfew essential oil and investigation of its effect on the shelf life of beef slices during refrigerated storage through intelligent modeling. J. Food Saf. 2018;38:1–16. doi: 10.1111/jfs.12443. [DOI] [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International. 16th ed. Association of Official Analytical Chemists, Arlington, VA, USA (1995)
- Bahadori MB, Dinparast L, Zengin G. The genus Heracleum: a comprehensive review on its phytochemistry, pharmacology, and ethnobotanical values as a useful herb. Compr. Rev. Food Sci. Food Saf. 2016;15:1018–1039. doi: 10.1111/1541-4337.12222. [DOI] [PubMed] [Google Scholar]
- Behbahani BA, Imani Fooladi AA. Shirazi balangu (Lallemantia royleana) seed mucilage: chemical composition, molecular weight, biological activity and its evaluation as edible coating on beefs. Int. J. Biol. Macromol. 2018;114:882–889. doi: 10.1016/j.ijbiomac.2018.03.177. [DOI] [PubMed] [Google Scholar]
- Behbahani BA, Shahidi F, Yazdi FT, Mortazavi SA, Mohebbi M. Use of Plantago major seed mucilage as a novel edible coating incorporated with Anethum graveolens essential oil on shelf life extension of beef in refrigerated storage. Int. J. Biol. Macromol. 2017;94:515–526. doi: 10.1016/j.ijbiomac.2016.10.055. [DOI] [PubMed] [Google Scholar]
- Behbahani BA, Yazdi FT, Shahidi F, Mortazavi SA, Mohebbi M. Principle component analysis (PCA) for investigation of relationship between population dynamics of microbial pathogenesis, chemical and sensory characteristics in beef slices containing Tarragon essential oil. Microb. Pathog. 2017;105:37–50. doi: 10.1016/j.micpath.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Behbahani BA, Shahidi F, Yazdi FT, Mortazavi SA, Mohebbi M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J. Food Meas. Charact. 2017;11:847–863. doi: 10.1007/s11694-016-9456-3. [DOI] [Google Scholar]
- Behrouzian F, Razavi SM, Phillips GO. Cress seed (Lepidium sativum) mucilage, an overview. Bioact. Carbohydr. Diet. Fibre. 2014;3:17–28. doi: 10.1016/j.bcdf.2014.01.001. [DOI] [Google Scholar]
- Bonilla J, Vargas M, Atarés L, Chiralt A. Effect of chitosan essential oil films on the storage-keeping quality of pork meat products. Food Bioprocess Tech. 2014;7:2443–2450. doi: 10.1007/s11947-014-1329-3. [DOI] [Google Scholar]
- Cardoso GP, Dutra MP, Fontes PR, Ramos ADLS, de Miranda Gomide LA, Ramos EM. Selection of a chitosan gelatin-based edible coating for color preservation of beef in retail display. Meat Sci. 2016;114:85–94. doi: 10.1016/j.meatsci.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Ćavar S, Maksimović M, Vidic D, Parić A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crop. Prod. 2012;37:479–485. doi: 10.1016/j.indcrop.2011.07.024. [DOI] [Google Scholar]
- Dapkevicius A, Venskutonis R, van Beek TA, Linssen JP. Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J. Sci. Food Agr. 1998;77:140–146. doi: 10.1002/(SICI)1097-0010(199805)77:1<140::AID-JSFA18>3.0.CO;2-K. [DOI] [Google Scholar]
- El Atki Y, Aouam I, El Kamari F, Taroq A, Nayme K, Timinouni M, Lyoussi B, Abdellaoui A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019;10:63–67. doi: 10.4103/japtr.JAPTR_366_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008;110:76–82. doi: 10.1016/j.foodchem.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Ghani S, Barzegar H, Noshad M, Hojjati M. The preparation, characterization and in vitro application evaluation of soluble soybean polysaccharide films incorporated with cinnamon essential oil nanoemulsions. Int. J. Biol. Macromol. 2018;112:197–202. doi: 10.1016/j.ijbiomac.2018.01.145. [DOI] [PubMed] [Google Scholar]
- Guerrero P, O’Sullivan MG, Kerry JP, de la Caba K. Application of soy protein coatings and their effect on the quality and shelf life stability of beef patties. RSC Adv. 2015;5:8182–8189. doi: 10.1039/C4RA13421D. [DOI] [Google Scholar]
- Hajlaoui H, Mighri H, Noumi E, Snoussi M, Trabelsi N, Ksouri R, Bakhrouf A. Chemical composition and biological activities of Tunisian Cuminum cyminum L. essential oil: A high effectiveness against Vibrio spp. strains. Food Chem. Toxicol. 48: 2186-2192 (2010) [DOI] [PubMed]
- Hansen LT, Gill T, Hussa HH. Effects of salt and storage temperature on chemical, microbiological and sensory changes in cold-smoked salmon. Food Res. Int. 1995;28:123–130. doi: 10.1016/0963-9969(95)90795-C. [DOI] [Google Scholar]
- Hassanzadeh P, Tajik H, Rohani SMR, Moradi M, Hashemi M, Aliakbarlu J. Effect of functional chitosan coating and gamma irradiation on the shelf life of chicken meat during refrigerated storage. Radiat. Phys. Chem. 2017;141:103–109. doi: 10.1016/j.radphyschem.2017.06.014. [DOI] [Google Scholar]
- Huang Z, Li J, Zhang J, Gao Y, Hui G. Physicochemical properties enhancement of Chinese kiwi fruit (Actinidia chinensis Planch) via chitosan coating enriched with salicylic acid treatment. J. Food Meas. Charact. 2017;11:184–191. doi: 10.1007/s11694-016-9385-1. [DOI] [Google Scholar]
- Hui G, Liu W, Feng H, Li J, Gao Y. Effects of chitosan combined with nisin treatment on storage quality of large yellow croaker (Pseudosciaena crocea) Food Chem. 2016;203:276–282. doi: 10.1016/j.foodchem.2016.01.122. [DOI] [PubMed] [Google Scholar]
- ICMSF. International Commission on Microbiological Specification for Foods. Microorganisms in Foods. 2. Sampling for Microbiological Analysis: Principles and Specific Applications. 2nd ed. University of Toronto Press, Toronto, Canada (1986)
- Jiang J, Li J, Zheng F, Lin H, Hui G. Rapid freshness analysis of mantis shrimps (Oratosquilla oratoria) by using electronic nose. J. Food Meas. Charact. 2016;10:48–55. doi: 10.1007/s11694-015-9275-y. [DOI] [Google Scholar]
- Karazhiyan H, Razavi SM, Phillips GO, Fang Y, Al-Assaf S, Nishinari K, Farhoosh R. Rheological properties of Lepidium sativum seed extract as a function of concentration, temperature and time. Food hydrocolloid. 2009;23:2062–2068. doi: 10.1016/j.foodhyd.2009.03.019. [DOI] [Google Scholar]
- Karazhiyan H, Razavi SM, Phillips GO, Fang Y, Al-Assaf S, Nishinari K. Physicochemical aspects of hydrocolloid extract from the seeds of Lepidium sativum. Int. J. Food Sci. Tech. 2011;46:1066–1072. doi: 10.1111/j.1365-2621.2011.02583.x. [DOI] [Google Scholar]
- Kartal N, Sokmen M, Tepe B, Daferera D, Polissiou M, Sokmen A. Investigation of the antioxidant properties of Ferula orientalis L. using a suitable extraction procedure. Food Chem. 100: 584-589 (2007)
- Kim JS, Lee YS. Antioxidant activity of Maillard reaction products derived from aqueous glucose/glycine, diglycine, and triglycine model systems as a function of heating time. Food Chem. 2009;116:227–232. doi: 10.1016/j.foodchem.2009.02.038. [DOI] [Google Scholar]
- Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005;40:255–260. [Google Scholar]
- Mohan CO, Ravishankar CN, Lalitha KV, Gopal TS. Effect of chitosan edible coating on the quality of double filleted Indian oil sardine (Sardinella longiceps) during chilled storage. Food Hydrocoll. 2012;26:167–174. doi: 10.1016/j.foodhyd.2011.05.005. [DOI] [Google Scholar]
- Ojagh SM, Rezaei M, Razavi SH, Hosseini SMH. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010;120:193–198. doi: 10.1016/j.foodchem.2009.10.006. [DOI] [Google Scholar]
- Park HJ, Nugroho A, Jung BR, Won YH, Jung YJ, Kim WB, Choi JS. Isolation and Quantitative Analysis of Flavonoids with Peroxynitrite-scavenging Effect from the Young Leaves of Heracleum moellendorffii. Korean J. Plant Res. 2010;23:393–398. [Google Scholar]
- Pirbalouti AG, Broujeni VN, Momeni M, Poor FM, Hamedi B. Antibacterial activity of Iranian medicinal plants against Streptococcus iniae isolated from rainbow trout (Oncorhynchus mykiss) Arch. Biol. Sci. 2011;63:59–66. doi: 10.2298/ABS1101059P. [DOI] [Google Scholar]
- Pirbalouti AG, Malekpoor F, Enteshari S, Yousefi M, Momtaz H, Hamedi B. Antibacterial activity of some folklore medicinal plants used by Bakhtiari tribal in Southwest Iran. Int. J. Biol. 2010;2:55–63. [Google Scholar]
- Pirbalouti AG, Setayesh M, Siahpoosh A, Mashayekhi H. Antioxidant activity, total phenolic and flavonoids contents of three herbs used as condiments and additives in pickles products. Herba Pol. 2013;59:51–62. doi: 10.2478/hepo-2013-0016. [DOI] [Google Scholar]
- Pirbalouti AG, Sedaghat L, Hamedi B, Tirgir F. Chemical composition and antioxidant activity of essential oils of three endemic medicinal plants of Iran. Bangladesh J. Bot. 2013;42:327–332. doi: 10.3329/bjb.v42i2.18038. [DOI] [Google Scholar]
- Sánchez-González L, Vargas M, González-Martínez C, Chiralt A, Cháfer M. Use of essential oils in bioactive edible coatings: a review. Food Eng. Rev. 2011;3:1–16. doi: 10.1007/s12393-010-9031-3. [DOI] [Google Scholar]
- Sapper M, Palou L, Pérez-Gago MB, Chiralt A. Antifungal Starch-Gellan Edible Coatings with Thyme Essential Oil for the Postharvest Preservation of Apple and Persimmon. Coatings. 2019;9:333. doi: 10.3390/coatings9050333. [DOI] [Google Scholar]
- Shan B, Cai YZ, Sun M, Corke H. Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005;53:7749–7759. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- Sonboli A, Azizian D, Yousefzadi M, Kanani MR, Mehrabian AR. Volatile constituents and antimicrobial activity of the essential oil of Tetrataenium lasiopetalum (Apiaceae) from Iran. Flavour Frag. J. 2007;22:119–122. doi: 10.1002/ffj.1767. [DOI] [Google Scholar]
- Tepe B, Donmez E, Unlu M, Candan F, Daferera D, Vardar-Unlu G, Polissiou M, Sokmen A. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucher ex Benth.) and Salvia multicaulis (Vahl). Food Chem. 84: 519-525 (2004)
- Wu Z, Zhou W, Pang C, Deng W, Xu C, Wang X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019;295:16–25. doi: 10.1016/j.foodchem.2019.05.114. [DOI] [PubMed] [Google Scholar]
- Ye CL, Dai DH, Hu WL. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa L.). Food Control 30: 48-53 (2013)
- Ying X, Liu W, Hui G. Litchi freshness rapid non-destructive evaluating method using electronic nose and non-linear dynamics stochastic resonance model. Bioengineered. 2015;6:218–221. doi: 10.1080/21655979.2015.1011032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Gao Y, Zhang J, Li J, Yu Y, Hui G. Chinese quince (Cydonia oblonga Miller) freshness rapid determination method using surface acoustic wave resonator combined with electronic nose. Int. J. Food Prop. 2016;19:2623–2634. doi: 10.1080/10942912.2016.1169285. [DOI] [Google Scholar]